Abstract 抽象的
Transforming growth factor (TGF)-β is a multifunctional cytokine expressed by almost every tissue and cell type. The signal transduction of TGF-β can stimulate diverse cellular responses and is particularly critical to embryonic development, wound healing, tissue homeostasis, and immune homeostasis in health. The dysfunction of TGF-β can play key roles in many diseases, and numerous targeted therapies have been developed to rectify its pathogenic activity. In the past decades, a large number of studies on TGF-β signaling have been carried out, covering a broad spectrum of topics in health, disease, and therapeutics. Thus, a comprehensive overview of TGF-β signaling is required for a general picture of the studies in this field. In this review, we retrace the research history of TGF-β and introduce the molecular mechanisms regarding its biosynthesis, activation, and signal transduction. We also provide deep insights into the functions of TGF-β signaling in physiological conditions as well as in pathological processes. TGF-β-targeting therapies which have brought fresh hope to the treatment of relevant diseases are highlighted. Through the summary of previous knowledge and recent updates, this review aims to provide a systematic understanding of TGF-β signaling and to attract more attention and interest to this research area.
转化生长因子 (TGF)-β 是一种多功能细胞因子,几乎所有组织和细胞类型均表达。 TGF-β 的信号转导可以刺激多种细胞反应,对健康中的胚胎发育、伤口愈合、组织稳态和免疫稳态尤其重要。 TGF-β 的功能障碍在许多疾病中发挥着关键作用,并且已经开发出许多靶向疗法来纠正其致病活性。在过去的几十年里,人们对 TGF-β 信号传导进行了大量的研究,涵盖了健康、疾病和治疗领域的广泛主题。因此,需要对 TGF-β 信号传导进行全面概述,以了解该领域研究的概况。在这篇综述中,我们回顾了TGF-β的研究历史,并介绍了其生物合成、激活和信号转导的分子机制。我们还深入了解 TGF-β 信号传导在生理条件和病理过程中的功能。重点介绍了TGF-β靶向疗法,为相关疾病的治疗带来了新的希望。通过对以往知识和近期更新的总结,本综述旨在提供对TGF-β信号传导的系统了解,并引起更多人对该研究领域的关注和兴趣。
Similar content being viewed by others
其他人正在查看类似内容
Introduction 介绍
The studies on TGF-β started as early as the 1980s and have developed rapidly ever since. Although TGF-β was first found to be secreted by transformed cells,1 it is widely produced by non-neoplastic tissues such as salivary glands, muscles, kidneys, liver, heart, brain, and embryos as well.2,3,4 In fact, platelets have been identified as one of the most abundant sources of TGF-β among all normal tissues.5 The ubiquitous expression of TGF-β in health strongly indicates its critical and multiple roles in physiological conditions.
TGF-β的研究早在20世纪80年代就开始了,此后发展迅速。尽管 TGF-β 最初被发现是由转化细胞分泌的1 ,但它也广泛由非肿瘤组织产生,如唾液腺、肌肉、肾脏、肝脏、心脏、大脑和胚胎。 2 , 3 , 4事实上,血小板已被确定为所有正常组织中最丰富的 TGF-β 来源之一。 5 TGF-β 在健康中的普遍表达强烈表明其在生理条件下的关键和多重作用。
Accumulating evidence has suggested that TGF-β functions diversely among different cell types in a context-dependent manner. Generally, cell survival, metabolism, growth, proliferation, differentiation, adhesion, migration, and death are all under the regulation of TGF-β. Proper TGF-β signaling is critical to the normal functioning and homeostasis of healthy bodies while aberrant TGF-β signaling can lead to diseases of various categories. For this reason, numerous targeted therapies that can remedy dysregulated TGF-β activity have been developed with some demonstrating encouraging safety and efficacy in clinical trials.
越来越多的证据表明,TGF-β 在不同细胞类型中以环境依赖性方式发挥不同的功能。一般而言,细胞的存活、代谢、生长、增殖、分化、粘附、迁移和死亡均受TGF-β的调控。正确的 TGF-β 信号传导对于健康身体的正常功能和体内平衡至关重要,而异常的 TGF-β 信号传导则可能导致各种类型的疾病。出于这个原因,已经开发了许多可以纠正 TGF-β 活性失调的靶向疗法,其中一些疗法在临床试验中显示出令人鼓舞的安全性和有效性。
In this review, we focus on the mechanism, physiology, pathology, as well as therapeutics of TGF-β signaling, aiming to provide historical, current, and future perspectives on relevant topics.
在这篇综述中,我们重点关注 TGF-β 信号传导的机制、生理学、病理学以及治疗学,旨在提供相关主题的历史、当前和未来观点。
History of research on TGF-β signaling
TGF-β信号研究的历史
TGF-β was first reported in 1978 when De Larco and Todaro discovered the ‘sarcoma growth factors’ which were produced by transformed murine fibroblasts and were able to transform normal fibroblasts to anchorage-independent growth.1 In 1981, Roberts et al. successfully isolated and purified TGF-β from non-neoplastic murine tissues,3 while at about the same time, Moses et al. independently accomplished the purification and characterization of the cytokine as well.6 Both groups also noticed that this relatively acid- and heat-stable polypeptide required disulfide bonds for activity and was sensitive to disulfide-reducing agent dithiothreitol. In 1983, studies by electrophoresis on sodium dodecyl sulfate-polyacrylamide gels indicated that the 25,000-dalton TGF-β molecule in humans was actually composed of two 12,500-dalton subunits cross-linked by disulfide bonds.7,8 Two years later, the amino-acid sequence of human TGF-β1, the first known TGF-β isoform, was revealed by Derynck et al. through direct protein sequencing and complementary deoxyribonucleic acid (DNA) cloning.2 The sequencing established that the 112-amino-acid-long TGF-β1 monomer is initially synthesized as the C-terminal segment of a 390-amino-acid-long precursor polypeptide.2 By the time of 1988, researchers had realized that TGF-β generally remained non-covalently associated with the N-terminal segment of its precursor when it was secreted.9,10 TGF-β cannot bind to its receptors with its receptor-binding site being masked in this inactive form, however, certain treatments such as acidification could convert latent TGF-β complex into active TGF-β ligand.11 In addition, the other two TGF-β isoforms in mammals, TGF-β2 and TGF-β3, were respectively identified in 198712 and 1988.13,14 Although the three TGF-β isoforms are encoded by three different genes, their mature ligands show strong conservation of amino acid sequences.
TGF-β 于 1978 年首次报道,当时 De Larco 和 Todaro 发现了由转化的小鼠成纤维细胞产生的“肉瘤生长因子”,并且能够将正常成纤维细胞转化为不依赖贴壁的生长。 1 1981 年,罗伯茨等人。成功地从非肿瘤性鼠组织中分离和纯化了 TGF-β, 3大约在同一时间,Moses 等人。并独立完成了细胞因子的纯化和表征。 6两个小组还注意到,这种对酸和热相对稳定的多肽需要二硫键才能发挥活性,并且对二硫键还原剂二硫苏糖醇敏感。 1983年,十二烷基硫酸钠-聚丙烯酰胺凝胶电泳研究表明,人体中25,000道尔顿的TGF-β分子实际上是由两个通过二硫键交联的12,500道尔顿的亚基组成。 7 , 8两年后,Derynck 等人揭示了人类 TGF-β1(第一个已知的 TGF-β 同工型)的氨基酸序列。通过直接蛋白质测序和互补脱氧核糖核酸(DNA)克隆。 2测序确定,112 个氨基酸长的 TGF-β1 单体最初是作为 390 个氨基酸长的前体多肽的 C 端片段合成的。 2到 1988 年,研究人员意识到 TGF-β 在分泌时通常与其前体的 N 末端片段保持非共价结合。9 , 10 TGF-β 不能与其受体结合,其受体结合位点以这种非活性形式被掩盖,但是,某些治疗(例如酸化)可以将潜在的 TGF-β 复合物转化为活性 TGF-β 配体。 11此外,哺乳动物中的另外两种 TGF-β 同工型 TGF-β2 和 TGF-β3 分别于 1987 年12和 1988 年被鉴定。 13 , 14尽管这三种 TGF-β 同工型由三个不同的基因编码,但它们的成熟配体显示出氨基酸序列的强烈保守性。
The effects of TGF-β signaling in cell proliferation,15,16 cell differentiation,17,18 embryonic development,19 wound healing,20 immune regulation,21,22 tissue fibrosis,23,24 and tumor development25,26 have been studied shortly after the discovery of the cytokine. Meanwhile, the receptors in TGF-β signaling known as TGF-β receptor I (TβRI) and TβRII were also identified and characterized in the 1980s.27,28,29 But it was not until the discovery of signaling mediators small (Sma) in Caenorhabditis elegans and mothers against decapentaplegic (Mad) in Drosophila melanogaster that the homologous small mothers against decapentaplegic (SMAD) proteins were identified as the canonical signal transducers of TGF-β signaling in humans in 1996.30,31,32 Since then, the development of TGF-β research has been largely accelerated. In recent times, as studies on TGF-β signaling in both health and disease going deeper and further, a lot of TGF-β-targeting therapies have been developed and assessed for the treatment of various diseases,33,34,35,36,37,38,39 revealing a promising future for the studies in this area (Fig. 1).
TGF-β信号传导对细胞增殖、 15、16细胞分化、 17、18胚胎发育、 19伤口愈合、 20免疫调节、 21、22组织纤维化、 23、24和肿瘤发展25、26的影响已被短期研究细胞因子被发现后。与此同时,TGF-β 信号传导中的受体,即 TGF-β 受体 I (TβRI) 和 TβRII 也在 20 世纪 80 年代被鉴定和表征。 27 , 28 , 29但直到发现了秀丽隐杆线虫中的小信号介导物(Sma)和果蝇中的抗十五麻痹母蛋白(Mad),同源的小母抗十五麻痹蛋白(SMAD)才被确定为典型的信号转导子。 1996 年人类中 TGF-β 信号转导的研究进展。 30 , 31 , 32从那时起,TGF-β 研究的发展大大加速。近年来,随着对健康和疾病中 TGF-β 信号传导的研究越来越深入,许多 TGF-β 靶向疗法已被开发和评估用于治疗各种疾病, 33 , 34 , 35 , 36 , 37、38、39揭示了该领域研究的广阔前景(图1 ) 。
Biosynthesis and activation of TGF-β
TGF-β的生物合成和激活
During the biosynthesis of TGF-β, the precursor undergoes post-translational processing to become a latent complex which is the secretory form of TGF-β. The latent TGF-β complex still requires further activation to eventually become a mature cytokine before it can trigger signal transduction in cells (Fig. 2).
在 TGF-β 的生物合成过程中,前体经过翻译后加工成为潜在复合物,即 TGF-β 的分泌形式。潜在的TGF-β复合物仍需要进一步激活才能最终成为成熟的细胞因子,然后才能触发细胞内的信号转导(图2 )。
Biosynthesis and activation of TGF-β. Each TGF-β monomer is initially synthesized as a precursor polypeptide. In the endoplasmic reticulum, TGF-β precursors lose their signal peptides and dimerize through disulfide bonds. The dimers then transit into the Golgi where they are cleaved by protease furin into mature cytokine segments and latency-associated peptides (LAPs) to form small latent complexes (SLCs). The secreted SLCs can further link to latent TGF-β-binding proteins (LTBPs) which target them into the extracellular matrix (ECM) for storage, or they can link to glycoprotein-A repetitions predominant protein (GARP) or leucine-rich repeat-containing protein 33 (LRRC33) which tethers them to the cell surface. Numerous factors such as acids, bases, reactive oxygen species (ROS), thrombospondin-1 (TSP-1), certain proteases, and integrins can release the mature cytokines from the latent complexes and thus are known as TGF-β activators
TGF-β 的生物合成和激活。每个 TGF-β 单体最初都是作为前体多肽合成的。在内质网中,TGF-β前体失去信号肽并通过二硫键形成二聚体。然后二聚体进入高尔基体,在那里它们被弗林蛋白酶裂解成成熟的细胞因子片段和潜伏相关肽(LAP),从而形成小的潜伏复合物(SLC)。分泌的 SLC 可以进一步连接到潜在的 TGF-β 结合蛋白 (LTBP),将其靶向细胞外基质 (ECM) 进行储存,或者它们可以连接到糖蛋白 A 重复优势蛋白 (GARP) 或富含亮氨酸的重复蛋白含有将它们束缚在细胞表面的蛋白质 33 (LRRC33)。许多因子,如酸、碱、活性氧 (ROS)、血小板反应蛋白-1 (TSP-1)、某些蛋白酶和整合素,可以从潜在复合物中释放成熟的细胞因子,因此被称为 TGF-β 激活剂
TGF-β biosynthesis and latency
TGF-β生物合成和潜伏期
Each TGF-β monomer is initially synthesized as a precursor polypeptide composed of a mature cytokine as its C-terminal segment, a signal peptide at the N-terminus, and a latency-associated peptide (LAP) in between.2 The signal peptide leads the precursor into the endoplasmic reticulum lumen and promptly gets removed. The remainder of the precursor then dimerizes through three disulfide bonds and transits into the Golgi where it gets cleaved between the mature cytokine and LAP by protease furin.40 However, the cytokine segment is still unable to bind its receptors after the cleavage, for it remains associated with LAP in a non-covalent way that masks its receptor-binding site and forms a small latent complex (SLC).41 In most cases, LAP is linked to latent TGF-β-binding protein (LTBP) through a disulfide bond, making the SLC into a large latent complex (LLC) when secreted.42 LTBP can further bind to fibrillin to target the LLC into the extracellular matrix (ECM) for storage.43 Alternatively, LAP can also form disulfide linkage with leucine-rich repeat-containing protein 32 (LRRC32) or LRRC33 to tether SLC to the cell surface. Unlike LTBP which is widely expressed by many cell types, LRRC32, also known as glycoprotein-A repetitions predominant protein (GARP), is specifically detected in regulatory T cells (Tregs), platelets, and endothelium,44 whereas high expression of LRRC33 is found in macrophages, dendritic cells (DCs), and B cells.45
每个 TGF-β 单体最初合成为前体多肽,由成熟细胞因子作为其 C 端片段、N 端信号肽和其间的潜伏相关肽 (LAP) 组成。 2信号肽引导前体进入内质网腔并迅速被去除。然后前体的其余部分通过三个二硫键二聚化并转移到高尔基体中,在那里它被蛋白酶弗林蛋白酶在成熟细胞因子和 LAP 之间裂解。 40然而,细胞因子片段在裂解后仍然无法结合其受体,因为它仍然以非共价方式与 LAP 结合,从而掩盖其受体结合位点并形成小的潜在复合物 (SLC)。 41在大多数情况下,LAP 通过二硫键与潜在的 TGF-β 结合蛋白 (LTBP) 连接,使 SLC 在分泌时形成一个大的潜在复合物 (LLC)。 42 LTBP 可以进一步与原纤维蛋白结合,将 LLC 靶向细胞外基质 (ECM) 进行储存。 43另外,LAP 还可以与富含亮氨酸重复序列的蛋白 32 (LRRC32) 或 LRRC33 形成二硫键,将 SLC 束缚在细胞表面。与在多种细胞类型中广泛表达的 LTBP 不同,LRRC32(也称为糖蛋白 A 重复优势蛋白 (GARP))在调节性 T 细胞 (Treg)、血小板和内皮细胞中特异性检测到, 44而 LRRC33 则高表达存在于巨噬细胞、树突状细胞 (DC) 和 B 细胞中。 45
TGF-β activation TGF-β激活
The bioactivity of TGF-β is based on ligand-receptor interaction which requires the exposure of its receptor-binding site. Thus, the activation of TGF-β represents the release of mature cytokine from the latent complex. Numerous factors have been identified as TGF-β activators as introduced below. Notably, integrin-dependent activation is so far the best described and likely the most important mechanism, while TGF-β activation mediated by acids, bases, reactive oxygen species (ROS), thrombospondin-1 (TSP-1), proteases, and other TGF-β activators is collectively known as integrin-independent activation.
TGF-β的生物活性基于配体-受体相互作用,这需要暴露其受体结合位点。因此,TGF-β的激活代表成熟细胞因子从潜在复合物中的释放。如下所述,许多因子已被鉴定为 TGF-β 激活剂。值得注意的是,整合素依赖性激活是迄今为止描述最好的,也可能是最重要的机制,而 TGF-β 激活则由酸、碱、活性氧 (ROS)、血小板反应蛋白-1 (TSP-1)、蛋白酶和其他酶介导。 TGF-β激活剂统称为整合素非依赖性激活。
TGF-β activation by integrins
整合素激活 TGF-β
Integrins are heterodimeric transmembrane receptors each consisting of an α-subunit and a β-subunit. TGF-β activation by integrins requires the binding of the integrins to an RGD sequence in the LAP of TGF-β1 and TGF-β3. Therefore, latent TGF-β2 without the RGD motif is excluded from integrin-dependent activation.46
整合素是异二聚体跨膜受体,每个受体由α亚基和β亚基组成。整联蛋白激活 TGF-β 需要整联蛋白与 TGF-β1 和 TGF-β3 的 LAP 中的 RGD 序列结合。因此,没有 RGD 基序的潜在 TGF-β2 被排除在整合素依赖性激活之外。 46
Among all integrins, αVβ6 and αVβ8 integrins are the best studied TGF-β activators. The expression of αVβ6 integrin is nearly restricted to epithelial cells and is upregulated in response to morphogenesis, wounding, inflammation, and tumorigenesis.47 In contrast, αVβ8 integrin is widely expressed by epithelial cells,48 fibroblasts,49 macrophages,50 DCs,51 Tregs,52 and different kinds of tumor cells.53 The lack of αVβ6 and αVβ8 integrin activity reproduces the phenotypes of TGF-β1- and TGF-β3-null mice, indicating the central importance of integrin-dependent activation.54,55
在所有整合素中,αVβ6 和 αVβ8 整合素是研究最多的 TGF-β 激活剂。 αVβ6 整合素的表达几乎仅限于上皮细胞,并且在形态发生、受伤、炎症和肿瘤发生时上调。 47相比之下,αVβ8 整合素广泛表达于上皮细胞、 48成纤维细胞、 49巨噬细胞、 50 种DC、 51 种Tregs、 52 种和不同种类的肿瘤细胞。 53缺乏 αVβ6 和 αVβ8 整合素活性会重现 TGF-β1 和 TGF-β3 缺失小鼠的表型,表明整合素依赖性激活的核心重要性。 54 , 55
Upon binding to the RGD motif in LAP, the mechanisms by which αVβ6 and αVβ8 integrins activate TGF-β are quite different. With latent TGF-β being tethered to ECM or cell membrane (through the binding of LAP to LTBP, GARP, or LRRC33 as mentioned before) and the cytoplasmic domain of integrin β6 subunit linking to the actin cytoskeleton, αVβ6 integrin can transmit contractile force which changes the conformation of LAP to release TGF-β ligand.56,57 However, the cytoplasmic domain of integrin β8 subunit does not link to the actin cytoskeleton. One effective mechanism for αVβ8 integrin-mediated TGF-β activation requires the proteolytic activity of membrane type 1-matrix metalloproteinase (MT1-MMP, also known as MMP14).48 Alternatively, membrane molecules such as GARP and LRRC33 which bind and present latent TGF-β on the surface of one cell can cooperate with the αVβ8 integrin expressed on a different cell to activate TGF-β in trans.45,58,59 A recent study reveals that upon binding to αVβ8 integrin, the flexible membrane-presented latent complex can expose the active domain of the TGF-β ligand to its receptors for binding and signaling without the need to release diffusible cytokine.60
与 LAP 中的 RGD 基序结合后,αVβ6 和 αVβ8 整合素激活 TGF-β 的机制截然不同。由于潜在的 TGF-β 被束缚在 ECM 或细胞膜上(通过前面提到的 LAP 与 LTBP、GARP 或 LRRC33 的结合)以及整合素 β6 亚基的胞质结构域与肌动蛋白细胞骨架相连,αVβ6 整合素可以传递收缩力,改变 LAP 的构象以释放 TGF-β 配体。 56 , 57然而,整合素 β8 亚基的胞质结构域并不与肌动蛋白细胞骨架相连。 αVβ8 整合素介导的 TGF-β 激活的一种有效机制需要 1 型膜基质金属蛋白酶(MT1-MMP,也称为 MMP14)的蛋白水解活性。 48另外,GARP 和 LRRC33 等膜分子在一个细胞表面结合并呈递潜在的 TGF-β,可以与不同细胞上表达的 αVβ8 整合素配合,反式激活 TGF-β。 45 , 58 , 59最近的一项研究表明,与 αVβ8 整合素结合后,柔性膜呈递的潜在复合物可以将 TGF-β 配体的活性结构域暴露于其受体,进行结合和信号传导,而无需释放可扩散的细胞因子。 60
TGF-β activation by acids and bases
酸和碱激活 TGF-β
It has long been noticed that acidification can unmask the activity of freshly secreted TGF-β.61 Sharply defined parameters for human TGF-β activation by acids and bases show that the transition from latency of all three isoforms occurred between pH 2.5 and 4, and between pH 10 and 12.62 Thus, extremely acidic environments such as the microenvironments in tumor tissues and the resorption lacunae of osteoclasts are possibly conducive to local TGF-β activation.63,64 A study on lung fibrosis even suggests that physiologic concentrations of lactic acid are sufficient enough to activate TGF-β in a pH-dependent manner.65
人们早就注意到酸化可以揭示新分泌的 TGF-β 的活性。 61酸和碱对人 TGF-β 激活的明确定义的参数表明,所有三种亚型的潜伏期转变发生在 pH 2.5 至 4 之间以及 pH 10 至 12 之间。 62因此,极端酸性环境(例如肿瘤中的微环境)组织和破骨细胞的吸收腔隙可能有利于局部 TGF-β 的激活。 63 , 64一项关于肺纤维化的研究甚至表明,乳酸的生理浓度足以以 pH 依赖性方式激活 TGF-β。 65
TGF-β activation by ROS ROS 激活 TGF-β
TGF-β1 is the only isoform that can be directly activated by ROS, for a unique methionine residue at the amino acid position 253 of its LAP is required for oxidation-triggered conformational change.66 However, ROS can induce other TGF-β activators such as TSP-167 and MMPs68 to activate all three isoforms in an indirect manner. ROS-mediated TGF-β activation prevails in tissues exposed to asbestos,69,70 ultraviolet,68 and ionizing radiation.71 High glucose intake can also induce ROS production and consequentially increase TGF-β activation to play roles in the development of fibrotic diseases and inflammatory diseases.72,73 Moreover, in T cells, ROS can be elevated during apoptosis or upon stimulation by T cell receptor (TCR) and cluster of differentiation 28 (CD28) to contribute to the immunosuppression mediated by activated TGF-β.74,75
TGF-β1 是唯一可以被 ROS 直接激活的异构体,因为其 LAP 253 位氨基酸上的独特蛋氨酸残基是氧化触发的构象变化所必需的。 66然而,ROS 可以诱导其他 TGF-β 激活剂,例如 TSP-1 67和 MMP 68以间接方式激活所有三种亚型。 ROS 介导的 TGF-β 激活在暴露于石棉、 69 、 70紫外线、 68和电离辐射的组织中普遍存在。 71高葡萄糖摄入还可以诱导 ROS 产生,从而增加 TGF-β 激活,从而在纤维化疾病和炎症性疾病的发展中发挥作用。 72 , 73此外,在 T 细胞中,ROS 在细胞凋亡期间或在受到 T 细胞受体 (TCR) 和分化簇 28 (CD28) 刺激后会升高,从而有助于激活的 TGF-β 介导的免疫抑制。 74 , 75
TGF-β activation by TSP-1
TSP-1 激活 TGF-β
TSP-1 is a multi-functional ECM protein not only abundant in platelet α-granules but also secreted by fibroblasts, endothelial cells, macrophages, T cells, and many other cell types.76 The KRFK sequence in TSP-1 can recognize the LSKL sequence in LAP to competitively disrupt its interaction with the receptor-binding site of the TGF-β ligand. Since the LSKL sequence in LAP is conserved among TGF-β isoforms, it is suggested that the direct binding of TSP-1 to latent complex is capable of activating all three TGF-β isoforms through this protease- and cell-independent mechanism.77 Interestingly, TSP-1 can also bind to the mature TGF-β ligand to form a complex that retains the biological activity of the cytokine.78 ROS,67 glucose,79 angiotensin II,80 hypoxia,81 wounding,82 inflammation,83 pathogens,84,85,86 and many other factors can all induce TSP-1 to function as a TGF-β activator in wound healing,67,82 cardiovascular diseases,81,86 renal diseases,79 fibrotic diseases,87,88 inflammatory diseases,83 infectious diseases,89 and tumors.90
TSP-1是一种多功能ECM蛋白,不仅在血小板α颗粒中丰富,而且由成纤维细胞、内皮细胞、巨噬细胞、T细胞和许多其他细胞类型分泌。 76 TSP-1 中的 KRFK 序列可以识别 LAP 中的 LSKL 序列,从而竞争性破坏其与 TGF-β 配体的受体结合位点的相互作用。由于 LAP 中的 LSKL 序列在 TGF-β 同工型中是保守的,因此表明 TSP-1 与潜在复合物的直接结合能够通过这种独立于蛋白酶和细胞的机制激活所有三种 TGF-β 同工型。 77有趣的是,TSP-1 还可以与成熟的 TGF-β 配体结合形成复合物,保留细胞因子的生物活性。 78 ROS、 67葡萄糖、 79血管紧张素 II、 80缺氧、 81受伤、 82炎症、 83病原体、 84、85、86和许多其他因素都可以诱导 TSP-1 在伤口愈合中充当 TGF-β 激活剂, 67 、 82心血管疾病、 81 、 86肾脏疾病、 79纤维化疾病、 87 、 88炎症性疾病、 83传染病、 89和肿瘤。 90
TGF-β activation by proteases
蛋白酶激活 TGF-β
Many proteases have been proved capable of directly activating TGF-β in vitro. However, the function of an individual protease seems redundant in vivo, as deficiency of a single species generally leads to no significant signs of impaired TGF-β activation.91 Among these proteases, MMPs such as MMP-2, MMP-9, and MMP-13 are conducive to the TGF-β activation in wound healing,92 cardiovascular diseases,93 renal diseases,94 fibrotic diseases,95 and tumors.96 Interestingly, although the activation by MMPs works for all three TGF-β isoforms, latent TGF-β2 and TGF-β3 appear much more sensitive to MMP-9 treatment than latent TGF-β1.96 Moreover, a serine protease known as plasmin plays an important role in the TGF-β activation mediated by macrophages97,98 and endothelial cells.99,100
许多蛋白酶已被证明能够在体外直接激活TGF-β。然而,单个蛋白酶的功能在体内似乎是多余的,因为单个物种的缺陷通常不会导致 TGF-β 激活受损的明显迹象。 91在这些蛋白酶中,MMP-2、MMP-9 和 MMP-13 等 MMP 有利于伤口愈合、 92心血管疾病、 93肾脏疾病、 94纤维化疾病、 95和肿瘤中的 TGF-β 激活。 96有趣的是,尽管 MMP 的激活作用适用于所有三种 TGF-β 同工型,但潜在的 TGF-β2 和 TGF-β3 似乎对 MMP-9 治疗比潜在的 TGF-β1 更敏感。 96此外,一种称为纤溶酶的丝氨酸蛋白酶在巨噬细胞97、98和内皮细胞介导的 TGF-β 激活中发挥着重要作用。 99 , 100
Signal transduction of TGF-β
TGF-β的信号转导
TGF-β signal is transmitted into the cells by TβRI (also known as activin receptor-like kinase 5, ALK5) and TβRII both of which are enzyme-linked receptors with dual specificity of serine/threonine kinase and tyrosine kinase. Studies have revealed that TGF-β1 and TGF-β3 bind TβRII prior to TβRI due to higher affinity, while TGF-β2 binds poorly to both receptors.12,101,102 TβRIII, also known as β-glycan, lacks the motifs to directly mediate TGF-β signal transduction. However, TβRIII is able to bind TGF-β especially TGF-β2 with high affinity and thus acts as a co-receptor that presents the ligand to the receptors and further enhances their binding.101,103,104,105,106,107 The ligand-receptor interaction subsequently activates the intracellular signaling of TGF-β through a canonical pathway and several non-canonical pathways.
TGF-β信号通过TβRI(也称为激活素受体样激酶5,ALK5)和TβRII传递到细胞中,这两种受体都是具有丝氨酸/苏氨酸激酶和酪氨酸激酶双重特异性的酶联受体。研究表明,由于亲和力较高,TGF-β1 和 TGF-β3 在 TβRII 之前先与 TβRII 结合,而 TGF-β2 与这两种受体的结合都很差。 12 , 101 , 102 TβRIII,也称为β-聚糖,缺乏直接介导TGF-β信号转导的基序。然而,TβRIII能够以高亲和力结合TGF-β尤其是TGF-β2,因此充当共受体,将配体呈递给受体并进一步增强它们的结合。 101 , 103 , 104 , 105 , 106 , 107配体-受体相互作用随后通过经典途径和几个非经典途径激活TGF-β的细胞内信号传导。
Canonical TGF-β signaling
典型的 TGF-β 信号传导
The canonical TGF-β signaling is mediated by transcription factors SMADs and thus is also known as the SMAD signaling. Notably, the canonical pathway is under the regulation of various factors that can control the intensity and manner of cellular responses at different levels (Fig. 3).
典型的 TGF-β 信号传导由转录因子 SMAD 介导,因此也称为 SMAD 信号传导。值得注意的是,经典途径受到多种因素的调节,这些因素可以在不同水平上控制细胞反应的强度和方式(图3 )。
Canonical TGF-β signaling. TGF-β can initially bind to its co-receptor TGF-β receptor III (TβRIII) or directly bind to its receptor TβRII which subsequently recruits TβRI to form a TGF-β-TβRI-TβRII complex. TβRII then actives TβRI through phosphorylation, leading to its dissociation with signaling inhibitor FK506-binding protein 1A (FKBP12) and interaction with signaling effectors receptor-activated SMADs (R-SMADs). R-SMADs which are presented to TβRI by adaptor protein SMAD anchor for receptor activation (SARA) get activated through phosphorylation and undergo oligomerization with common-partner SMAD (co-SMAD). The SMAD oligomers then translocate into the nucleus where they function as transcription factors (TFs), mediating the transcriptional activation or repression of target genes by binding to specific DNA sequences known as SMAD-binding elements (SBEs) and generally in cooperation with other TFs as well as transcriptional cofactors. In this way, TGF-β signaling can activate the expression of inhibitory SMADs (I-SMADs) which in turn function to attenuate the transcriptional regulation mediated by TGF-β signaling through several mechanisms. Moreover, many protein kinases (PKs), protein phosphatases (PPs), and (E3) ubiquitin ligases can also modulate canonical TGF-β signaling through various post-translational modifications of SMADs. (TFBS, TF-binding site)
典型的 TGF-β 信号传导。 TGF-β 最初可以与其共受体 TGF-β 受体 III (TβRIII) 结合,或直接与其受体 TβRII 结合,后者随后招募 TβRI 形成 TGF-β-TβRI-TβRII 复合物。然后,TβRII 通过磷酸化激活 TβRI,导致其与信号传导抑制剂 FK506 结合蛋白 1A (FKBP12) 解离,并与信号传导效应器受体激活的 SMAD (R-SMAD) 相互作用。 R-SMAD 通过接头蛋白 SMAD 受体激活锚 (SARA) 呈递给 TβRI,通过磷酸化被激活,并与共同伙伴 SMAD (co-SMAD) 发生寡聚化。然后 SMAD 寡聚体易位到细胞核中,在细胞核中充当转录因子 (TF),通过与称为 SMAD 结合元件 (SBE) 的特定 DNA 序列结合并通常与其他 TF 配合来介导靶基因的转录激活或抑制,如以及转录辅助因子。通过这种方式,TGF-β信号传导可以激活抑制性SMADs (I-SMADs)的表达,而抑制性SMADs进而通过多种机制减弱TGF-β信号传导介导的转录调节。此外,许多蛋白激酶 (PK)、蛋白磷酸酶 (PP) 和 (E3) 泛素连接酶也可以通过 SMAD 的各种翻译后修饰来调节经典 TGF-β 信号传导。 (TFBS,TF 结合位点)
TGF-β-activated SMAD signaling
TGF-β 激活 SMAD 信号传导
TGF-β ligand initially binds to TβRII monomer to promote its homodimerization or directly binds to pre-existing TβRII homodimer to recruit TβRI for assembly.108,109,110,111 This forms a heteromeric TGF-β-TβRI-TβRII complex in which low-affinity TβRI requires high-affinity TβRII to bind TGF-β ligand and constitutively active TβRII requires phosphorylating TβRI to transduce intracellular signal.112 The phosphorylation of TβRI occurs in its juxtamembrane GS domain at several serine and threonine residues, triggering conformational changes that transform the GS domain from a site that binds the signaling inhibitor known as immunophilin FK506-binding protein 1A (FKBP12) into a binding site for the signaling effectors known as receptor-activated SMADs (R-SMADs).113
TGF-β配体最初与TβRII单体结合以促进其同二聚化,或直接与预先存在的TβRII同二聚体结合以募集TβRI进行组装。 108 , 109 , 110 , 111这形成异聚 TGF-β-TβRI-TβRII 复合物,其中低亲和力 TβRI 需要高亲和力 TβRII 才能结合 TGF-β 配体,而组成型活性 TβRII 需要磷酸化 TβRI 来转导细胞内信号。 112 TβRI 的磷酸化发生在其近膜 GS 结构域的几个丝氨酸和苏氨酸残基处,引发构象变化,将 GS 结构域从结合信号抑制剂(称为亲免素 FK506 结合蛋白 1A (FKBP12))的位点转变为亲免素 FK506 结合蛋白 1A (FKBP12) 的结合位点。信号传导效应器称为受体激活 SMAD (R-SMAD)。 113
R-SMADs, including SMAD2 and SMAD3, consist of a globular Mad homology 1 (MH1) domain at the N-terminus, a globular MH2 domain at the C-terminus, and a highly flexible long linker region in between. R-SMADs are retained in cytoplasm and presented to TβRI by the adaptor protein known as SMAD anchor for receptor activation (SARA).114 The R-SMAD MH2 domain then gets phosphorylated at two serine residues in the extreme C-terminal SXS motif by the TβRI kinase domain which is located immediately downstream of the TβRI GS domain.113 Activated R-SMADs undergo homo-oligomerization or hetero-oligomerization through their MH2 domains upon phosphorylation, and they can also oligomerize with SMAD4, the common-partner SMAD (co-SMAD) which lacks the SXS motif for phosphorylation by TβRI kinase. Notably, studies have suggested that SMAD heterotrimers containing two R-SMADs and one SMAD4 are likely more common and stable than other SMAD oligomers.115,116,117,118,119 Although different SMAD oligomers can vary in function, they all act to regulate the transcription of target genes by binding to DNA after translocating into the nucleus. The MH1 domains of SMAD4, SMAD3, and a specific SMAD2 splicing variant recognize the nucleic acid sequence GTCT or its reverse complement AGAC in double-stranded DNA which are known as the canonical SMAD-binding elements (SBEs).120 Other SBEs such as the 5GC SBEs including GGCGC and GGCCG have also been discovered, indicating a relatively loose DNA-binding specificity of the SMAD oligomers.121 However, the binding to a single SBE is so weak that SMAD oligomers generally require interacting with replications of SBE copies as well as other DNA-binding sequence-specific transcription factors to function.119,120,122 In fact, many SBE repeats are enriched at the binding sites for SMAD-interacting transcription factors, exactly increasing the binding accessibility, specificity, and affinity of SMAD oligomers associated with specific transcription factors.123,124,125 Despite a large number of SMAD-interacting transcription factors indicating a huge amount of potential gene targets for canonical TGF-β signaling, the dominant effects are generally determined by the master transcription factors in specific cell types and contexts which contribute to the complexity and variability of cellular responses to TGF-β.125 重试 错误原因
Regulation of SMAD signaling by inhibitory SMADs (I-SMADs) 重试 错误原因
TGF-β and many other factors can induce the expression of SMAD6 and SMAD7 which function to inhibit TGF-β signaling and thus are known as I-SMADs.126,127 Unlike R-SMADs, I-SMADs lack the N-terminal MH1 domain and the C-terminal SXS motif, however, they retain the C-terminal MH2 domain which can competitively bind to activated receptor TβRI to inhibit the phosphorylation of R-SMADs.128,129 Through some extra mechanisms, SMAD7 confers greater abilities in suppressing TGF-β signaling than SMAD6 does.130 For example, SMAD7 recruits E3 ubiquitin ligases such as SMAD ubiquitination regulatory factors (SMURFs) and neural precursor cell expressed, developmentally downregulated 4-like (NEDD4L) to TβRI, R-SMADs, and co-SMAD to mediate the proteasomal and lysosomal degradation of these TGF-β signaling components.131,132,133,134,135 SMAD7 can also trigger the dephosphorylation of TβRI by recruiting protein phosphatase 1 (PP1) to the receptor.136 Moreover, with its MH2 domain, SMAD7 can oligomerize with R-SMADs to compete with co-SMAD133 and can bind to specific DNA sequences to disrupt the formation of the transcriptional SMAD-DNA complex.137 Taken together, TGF-β signaling induces I-SMADs to form a negative feedback loop of itself.
TGF-β和许多其他因子可以诱导SMAD6和SMAD7的表达,其功能是抑制TGF-β信号传导,因此被称为I-SMAD。 126 , 127与 R-SMAD 不同,I-SMAD 缺乏 N 端 MH1 结构域和 C 端 SXS 基序,但保留了 C 端 MH2 结构域,可以竞争性地与激活的受体 TβRI 结合,抑制 R 的磷酸化-SMAD。 128 , 129通过一些额外的机制,SMAD7 比 SMAD6 具有更强的抑制 TGF-β 信号传导的能力。 130例如,SMAD7 招募 E3 泛素连接酶,例如 SMAD 泛素化调节因子 (SMURF) 和神经前体细胞表达的、发育下调的 4-like (NEDD4L) 至 TβRI、R-SMAD 和 co-SMAD,以介导蛋白酶体和溶酶体降解这些 TGF-β 信号传导成分。 131 , 132 , 133 , 134 , 135 SMAD7 还可以通过将蛋白磷酸酶1 (PP1)募集至受体来触发TβRI的去磷酸化。 136此外,凭借其 MH2 结构域,SMAD7 可以与 R-SMAD 寡聚,与 co-SMAD 竞争133 ,并且可以结合特定的 DNA 序列,破坏转录 SMAD-DNA 复合物的形成。 137总之,TGF-β 信号传导诱导 I-SMAD 形成自身的负反馈循环。
Regulation of SMAD signaling by transcriptional cofactors
转录辅助因子对 SMAD 信号传导的调节
Transcriptional cofactors are actively recruited to the transcriptional SMAD complex to regulate its activity. Notably, many of these transcriptional cofactors have histone modification activity and thus enable TGF-β signaling to trigger epigenetic changes. Histone acetyltransferases (HATs) such as p300, cyclic adenosine monophosphate (cAMP) response element-binding protein (CREB)-binding protein (CBP), p300/CBP-associated factor (PCAF), and general control non‐repressed protein 5 (GCN5) act as the transcriptional coactivators of SMADs by increasing the accessibility to DNA.138,139,140,141 The interaction between p300/CBP and doubly phosphorylated R-SMADs requires SMAD4 for stabilization and is critical for SMAD-mediated transcriptional activation. Other SMAD coactivators include melanocyte-specific gene 1 (MSG1),142 zinc finger E-box-binding homeobox 1 (ZEB1),143,144 and the histone methyltransferase (HMT) known as SET domain-containing protein 7 (SETD7).145 Contrary to HATs, histone deacetylases (HDACs) generally act as the transcriptional corepressors of SMADs by decreasing the accessibility to DNA. SMAD3 can directly recruit HDAC4 and HDAC5 to gene promoters to inhibit the function of transcription factors via histone deacetylation.146 SMADs can also associate with HDACs through interaction with other corepressors such as TGF-β-induced factor (TGIF),147 ecotropic viral integration site 1 (EVI1),148,149 Sloan-Kettering Institute proto-oncogene (SKI),150,151,152 as well as SKI-related novel gene N (SNO).153 Other transcriptional corepressors of SMADs include cellular-myelocytomatosis viral oncogene (MYC),154 SMAD nuclear-interacting protein 1 (SNIP1),155 ZEB2,143,156 and HMTs such as suppressor of variegation 3-9 homolog 1 (SUV39H1) and SET domain bifurcated 1 (SETDB1) which can both trigger the methylation of histone 3 lysine 9 (H3K9) at gene promoters.157,158
转录辅助因子被积极招募到转录 SMAD 复合物中以调节其活性。值得注意的是,许多转录辅助因子具有组蛋白修饰活性,从而使 TGF-β 信号传导能够触发表观遗传变化。组蛋白乙酰转移酶 (HAT),例如 p300、环磷酸腺苷 (cAMP) 反应元件结合蛋白 (CREB) 结合蛋白 (CBP)、p300/CBP 相关因子 (PCAF) 和一般控制非抑制蛋白 5 (GCN5) )通过增加 DNA 的可及性,充当 SMAD 的转录共激活剂。 138 , 139 , 140 , 141 p300/CBP 和双磷酸化 R-SMAD 之间的相互作用需要 SMAD4 来稳定,并且对于 SMAD 介导的转录激活至关重要。其他 SMAD 共激活剂包括黑素细胞特异性基因 1 (MSG1)、 142锌指 E 盒结合同源框 1 (ZEB1) 、 143、144和被称为含 SET 结构域的蛋白 7 (SETD7) 的组蛋白甲基转移酶 (HMT)。 145与 HAT 相反,组蛋白脱乙酰酶 (HDAC) 通常通过降低 DNA 的可及性来充当 SMAD 的转录辅阻遏物。 SMAD3可以直接将HDAC4和HDAC5招募到基因启动子处,通过组蛋白脱乙酰化来抑制转录因子的功能。第146章151、152以及SKI相关新基因N(SNO)。第153章SMAD 的其他转录辅阻遏物包括细胞骨髓细胞瘤病毒癌基因 (MYC)、第 154 章SMAD 核相互作用蛋白 1 (SNIP1)、第 155 章ZEB2、第 143 章、第 156 章和HMT,例如杂色抑制因子 3-9 同源物 1 (SUV39H1) 和 SET结构域分叉 1 (SETDB1) 均可触发基因启动子处组蛋白 3 赖氨酸 9 (H3K9) 的甲基化。157 , 158
Regulation of SMAD signaling by SMAD modifications
通过 SMAD 修饰调节 SMAD 信号传导
Post-translational modifications can also regulate the functions of SMADs. Apart from TβRI kinase which phosphorylates R-SMADs in their C-terminal SXS motif to mediate their activation, many other protein kinases such as mitogen-activated protein kinase kinase kinase 1 (MAPKKK1),159 p38 MAPK,160 c-Jun N-terminal kinase (JNK),161 extracellular signal-regulated kinase (ERK),162,163,164 rat sarcoma (RAS) homolog (Rho)-associated coiled-coil-containing protein kinase (ROCK),160 glycogen synthase kinase (GSK)-3β,165,166,167 calcium/calmodulin-dependent protein kinase II (CAMK2),168 protein kinase C (PKC),169 PKG,170 and several cyclin-dependent kinases (CDKs)167,171,172 can phosphorylate R-SMADs as well as co-SMAD at many different sites to enhance or attenuate SMAD activity. Meanwhile, the various phosphorylation of SMADs can be reversed by phosphatases. Several nuclear phosphatases known as the small C-terminal domain phosphatases (SCPs) can specifically dephosphorylate the linker region and MH1 domain of R-SMADs,173,174 whereas protein phosphatase, magnesium/manganese-dependent 1A (PPM1A),175 myotubularin-related protein 4 (MTMR4),176 and protein phosphatase 2A (PP2A)177 catalyze the dephosphorylation of the C-terminal SXS motif to terminate the signaling and promote the dissociation and cytoplasmic localization of SMADs.
翻译后修饰也可以调节 SMAD 的功能。除了磷酸化 R-SMAD C 端 SXS 基序以介导其激活的 TβRI 激酶外,还有许多其他蛋白激酶,如丝裂原激活蛋白激酶激酶 1 (MAPKKK1)、 159 p38 MAPK、 160 c-Jun N 端第161章细胞外信号调节激酶(ERK),第162章,第163章,第164章大鼠肉瘤(RAS)同源物(Rho)相关卷曲螺旋蛋白激酶(ROCK),第160章糖原合酶激酶(GSK)- 3β 、 165、166、167钙/钙调蛋白依赖性蛋白激酶 II (CAMK2)、 168蛋白激酶 C (PKC)、 169 PKG、 170和几种细胞周期蛋白依赖性激酶 ( CDK ) 167、171、172可以磷酸化 R-SMAD以及在许多不同位点的 co-SMAD 以增强或减弱 SMAD 活性。同时,SMADs的各种磷酸化可以被磷酸酶逆转。 几种被称为小 C 端结构域磷酸酶 (SCP) 的核磷酸酶可以特异性地使 R-SMAD 的接头区域和 MH1 结构域去磷酸化, 173 , 174而蛋白磷酸酶,镁/锰依赖性 1A (PPM1A), 175肌管蛋白相关蛋白 4 (MTMR4)、 176和蛋白磷酸酶 2A (PP2A) 177催化 C 端 SXS 基序的去磷酸化,以终止信号传导并促进 SMAD 的解离和细胞质定位。
Furthermore, SMADs can be ubiquitinated and deubiquitinated respectively by E3 ubiquitin ligases and deubiquitylating enzymes (DUBs). The E3 ubiquitin ligases that can mediate SMAD ubiquitination include SMURFs,135,178,179,180 NEDD4L,134,181 WW domain-containing proteins (WWPs),182,183,184 really interesting new gene (RING) finger protein 111 (RNF111),185 C-terminus of heat shock protein (HSP) 70-interacting protein (CHIP),186 itchy (ITCH) E3 ubiquitin ligase,187 and S-phase kinase-associated protein (SKP)-cullin-F-box (SCF) E3 ubiquitin ligase complex.188,189 The ubiquitination generally leads to the proteasomal degradation of SMADs, but in some cases, it also exerts non-degradative effects on SMAD activity.190 Notably, the degradative ubiquitination of R-SMADs by NEDD4L requires the phosphorylation of the R-SMAD linker by CDK8/9 and GSK-3 in sequence to create binding sites for the E3 ubiquitin ligase.171,181,191
此外,SMAD 可以分别被 E3 泛素连接酶和去泛素化酶 (DUB) 泛素化和去泛素化。能够介导 SMAD 泛素化的 E3 泛素连接酶包括 SMURF 、 135、178、179、180 NEDD4L 、 134、181含WW 结构域的蛋白(WWP) 、 182、183、184真正有趣的新基因(RING)指蛋白 111(RNF111) )、 185热休克蛋白 (HSP) C 末端 70 相互作用蛋白 (CHIP)、 186痒 (ITCH) E3 泛素连接酶、 187和 S 期激酶相关蛋白 (SKP)-cullin-F-box (SCF) ) E3 泛素连接酶复合物。 188 , 189泛素化通常会导致 SMAD 的蛋白酶体降解,但在某些情况下,它也会对 SMAD 活性产生非降解作用。 190值得注意的是,NEDD4L 对 R-SMAD 的降解性泛素化需要 CDK8/9 和 GSK-3 按顺序磷酸化 R-SMAD 接头,以创建 E3 泛素连接酶的结合位点。 171 , 181 , 191
Non-canonical TGF-β signaling
非典型 TGF-β 信号传导
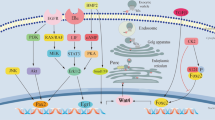
Apart from the SMAD-dependent pathway, TGF-β can also signal through SMAD-independent pathways to activate ERK signaling, Rho guanosine triphosphatase (GTPase) signaling, p38 MAPK signaling, JNK signaling, nuclear factor-κB (NF-κB) signaling, phosphatidylinositol 3-kinase (PI3K)/AKR mouse thymoma proto-oncogene (AKT) signaling, as well as Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling. These non-canonical TGF-β signaling pathways are involved in an extensive range of cellular events, greatly expanding the participation of TGF-β signaling in health and disease (Fig. 4).
除了 SMAD 依赖性途径外,TGF-β 还可以通过 SMAD 独立途径发出信号,激活 ERK 信号、Rho 鸟苷三磷酸酶 (GTPase) 信号、p38 MAPK 信号、JNK 信号、核因子-κB (NF-κB) 信号、磷脂酰肌醇 3-激酶 (PI3K)/AKR 小鼠胸腺瘤原癌基因 (AKT) 信号传导,以及 Janus 激酶 (JAK)/信号转导器和转录激活子 (STAT) 信号传导。这些非常规的TGF-β信号通路参与了广泛的细胞事件,极大地扩展了TGF-β信号传导在健康和疾病中的参与(图4 )。
Non-canonical TGF-β signaling. TGF-β can signal through non-canonical pathways to activate extracellular signal-regulated kinase (ERK) signaling, rat sarcoma (RAS) homolog (Rho)-guanosine triphosphatase (GTPase) signaling, p38 mitogen-activated protein kinase (MAPK) signaling, c-Jun N-terminal kinase (JNK) signaling, nuclear factor-κB (NF-κB) signaling, phosphatidylinositol 3-kinase (PI3K)/AKR mouse thymoma proto-oncogene (AKT) signaling, as well as Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling. These non-canonical TGF-β signaling pathways are actively involved in an extensive range of cellular events. (RAF, RAS-associated factor; MEK, MAPK/ERK kinase; ROCK1, Rho-associated coiled-coil-containing protein kinase 1; LIMK2, LIM domain kinase 2; TRAF, tumor necrosis factor (TNF) receptor-associated factor; TAK1, TGF-β-activated kinase 1; MKK, MAPK kinase; IKK, NF-κB inhibitor (IκB) kinase; GSK-3β, glycogen synthase kinase-3β; MTOR, mechanistic target of rapamycin; FOXO, forkhead box O; S6K, S6 kinase; 4EBP1, 4E-binding protein 1)
非典型 TGF-β 信号传导。 TGF-β 可以通过非经典途径发出信号,激活细胞外信号调节激酶 (ERK) 信号、大鼠肉瘤 (RAS) 同源物 (Rho)-鸟苷三磷酸酶 (GTPase) 信号、p38 丝裂原激活蛋白激酶 (MAPK) 信号、 c-Jun N 末端激酶 (JNK) 信号传导、核因子-κB (NF-κB) 信号传导、磷脂酰肌醇 3-激酶 (PI3K)/AKR 小鼠胸腺瘤原癌基因 (AKT) 信号传导以及 Janus 激酶 (JAK) /信号转导子和转录激活子 (STAT) 信号传导。这些非经典 TGF-β 信号通路积极参与广泛的细胞事件。 (RAF,RAS 相关因子;MEK,MAPK/ERK 激酶;ROCK1,Rho 相关卷曲螺旋蛋白激酶 1;LIMK2,LIM 结构域激酶 2;TRAF,肿瘤坏死因子 (TNF) 受体相关因子;TAK1 、 TGF-β 激活激酶 1;IKK、NF-κB 抑制剂 (IκB) 激酶;MTOR、雷帕霉素的机制靶点; S6 激酶;4EBP1、4E 结合蛋白 1)
TGF-β-activated ERK signaling
TGF-β 激活的 ERK 信号传导
As a dual-specificity kinase, TβRI can phosphorylate at its tyrosine residues to activate ERK signaling upon TGF-β stimulation.192 In this case, TβRI with tyrosine kinase activity initially phosphorylates the adapter protein known as sarcoma (SRC) homology and collagen A (SHCA) which subsequently forms a complex with growth factor receptor-bound protein 2 (GRB2) and son of sevenless homolog (SOS). The SHCA-GRB2-SOS complex then initiates a canonical MAPK signaling cascade which involves the sequential activation of RAS, the MAPKKK known as RAS-associated factor (RAF), the MAPKK known as MAPK/ERK kinase (MEK), and eventually, the ERK MAPK. Activated ERK is known to regulate various biological events including cell survival, proliferation, differentiation, adhesion, migration, as well as metabolism, and is implicated in a spectrum of diseases such as developmental disorders, chronic inflammation, neurodegeneration, obesity, and cancers.193,194
作为一种双特异性激酶,TβRI 可以在其酪氨酸残基处磷酸化,从而在 TGF-β 刺激下激活 ERK 信号传导。 192在这种情况下,具有酪氨酸激酶活性的 TβRI 首先磷酸化称为肉瘤 (SRC) 同源物的接头蛋白和胶原蛋白 A (SHCA),随后与生长因子受体结合蛋白 2 (GRB2) 和七同源物的儿子形成复合物 (求救)。然后,SHCA-GRB2-SOS 复合物启动典型的 MAPK 信号级联,其中依次激活 RAS、称为 RAS 相关因子 (RAF) 的 MAPKKK、称为 MAPK/ERK 激酶 (MEK) 的 MAPKK,以及最终激活ERK 映射。已知激活的 ERK 可调节各种生物事件,包括细胞存活、增殖、分化、粘附、迁移以及代谢,并与发育障碍、慢性炎症、神经退行性疾病、肥胖和癌症等一系列疾病有关。 193 , 194
TGF-β-activated Rho GTPase signaling
TGF-β 激活的 Rho GTPase 信号转导
Rho GTPases such as RHO, RAS-related C3 botulinum toxin substrate 1 (RAC1), and cell division cycle 42 (CDC42) play a central role in the organization and dynamics of the actin cytoskeleton. They are activated by guanine nucleotide exchange factors (GEFs) through the exchange of a bound GDP for GTP.195 TGF-β can trigger RHO activation in a rapid SMAD-independent manner or by inducing a GEF known as neuroepithelial cell transforming 1 (NET1) through SMAD and MEK/ERK pathways.196,197,198,199,200 RHO then activates its key effector ROCK1 which further mediates the phosphorylation of LIM domain kinase 2 (LIMK2). Activated LIMK2 subsequently phosphorylates cofilin to inhibit its function as a constitutive actin-depolymerizing factor, leading to the reorganization of the actin cytoskeleton in the end.201,202,203 Additionally, TGF-β-triggered RHO/ROCK1 signaling can contribute to ERK phosphorylation,204,205 and besides RHO, TGF-β can also activate the signaling of other Rho GTPases such as RAC1202 and CDC42.206 Besides the regulation of cell morphogenesis, adhesion, and movement, Rho GTPase signaling is also known to participate in transcriptional regulation, cell cycle progression, vesicular trafficking, and pathological processes such as fibrosis, inflammation, wound repair, and tumor development.207,208
RHO GTP 酶,例如 RHO、RAS 相关的 C3 肉毒毒素底物 1 (RAC1) 和细胞分裂周期 42 (CDC42) 在肌动蛋白细胞骨架的组织和动力学中发挥着核心作用。它们通过鸟嘌呤核苷酸交换因子 (GEF) 将结合的 GDP 交换为 GTP 来激活。 195 TGF-β 可以以不依赖 SMAD 的方式快速触发 RHO 激活,或者通过 SMAD 和 MEK/ERK 途径诱导称为神经上皮细胞转化 1 (NET1) 的 GEF。 196 , 197 , 198 , 199 , 200 RHO 然后激活其关键效应子 ROCK1 ,进一步介导 LIM 结构域激酶 2 (LIMK2) 的磷酸化。激活的 LIMK2 随后磷酸化 cofilin,抑制其作为组成型肌动蛋白解聚因子的功能,最终导致肌动蛋白细胞骨架的重组。 201 、 202 、 203此外,TGF-β 触发的 RHO/ROCK1 信号传导可促进 ERK 磷酸化, 204 、 205并且除了 RHO 之外,TGF-β 还可激活其他 Rho GTP 酶(例如 RAC1 202和 CDC42)的信号传导。 206除了调节细胞形态发生、粘附和运动外,Rho GTPase 信号传导还参与转录调节、细胞周期进程、囊泡运输以及纤维化、炎症、伤口修复和肿瘤发展等病理过程。 207 , 208
TGF-β-activated p38, JNK, and NF-κB signaling
TGF-β 激活的 p38、JNK 和 NF-κB 信号转导
TGF-β can activate the signaling of another two MAPKs known as p38 and JNK through a receptor kinase-independent mechanism which is different from that of ERK signaling. TGF-β-activated TβR complex can recruit tumor necrosis factor (TNF) receptor-associated factor 4 (TRAF4) and TRAF6 to trigger their lysine 63 (K63)-linked polyubiquitination. With E3 ubiquitin ligase activity, polyubiquitinated TRAF then attaches the polyubiquitin chain on the MAPKKK known as TGF-β-activated kinase 1 (TAK1) which subsequently gets activated and phosphorylates several MAPKKs (MKKs).209,210,211 As a result, MKK3 and MKK6 specifically trigger the activation of p38 while MKK4 mediates the phosphorylation of both p38 and JNK. TGF-β-activated Rho GTPases such as RHOA, RAC1, and CDC42 can also contribute to p38 and JNK activation.204,212,213,214,215,216 Both the two MAPKs regulate a series of biological events to respond to all kinds of environmental and intracellular stresses, meanwhile, they engage actively in embryonic development, metabolic regulation, neuronal functions, immunological actions, as well as tumor development.217,218,219,220
TGF-β可以通过与ERK信号传导不同的受体激酶独立机制激活另外两种MAPK(p38和JNK)的信号传导。 TGF-β 激活的 TβR 复合物可以招募肿瘤坏死因子 (TNF) 受体相关因子 4 (TRAF4) 和 TRAF6,以触发其赖氨酸 63 (K63) 连接的多聚泛素化。借助 E3 泛素连接酶活性,多聚泛素化 TRAF 将多聚泛素链附着在称为 TGF-β 激活激酶 1 (TAK1) 的 MAPKKK 上,随后该激酶被激活并磷酸化多个 MAPKK (MKK)。 209 , 210 , 211因此,MKK3 和 MKK6 特异性触发 p38 的激活,而 MKK4 介导 p38 和 JNK 的磷酸化。 TGF-β 激活的 Rho GTP 酶(例如 RHOA、RAC1 和 CDC42)也可促进 p38 和 JNK 激活。 204 , 212 , 213 , 214 , 215 , 216两种MAPK均调节一系列生物事件以应对各种环境和细胞内应激,同时积极参与胚胎发育、代谢调节、神经元功能、免疫作用、以及肿瘤的发展。 217 , 218 , 219 , 220
Additionally, TGF-β-activated TRAF/TAK1 signaling, RHO/ROCK1 signaling, and PI3K/AKT signaling can also lead to the phosphorylation of NF-κB inhibitor (IκB) kinase (IKK).221,222,223,224 Activated IKK then triggers the phosphorylation of IκB which subsequently gets polyubiquitinated and degraded while releasing active NF-κB for nuclear translocation.221 NF-κB as a transcription factor can regulate hundreds of genes involved in cell survival, proliferation, metabolism, and immunity in particular.225,226,227
此外,TGF-β激活的TRAF/TAK1信号传导、RHO/ROCK1信号传导和PI3K/AKT信号传导也可导致NF-κB抑制剂(IκB)激酶(IKK)的磷酸化。 221 , 222 , 223 , 224激活的 IKK 然后触发 IκB 的磷酸化,随后 IκB 被多泛素化并降解,同时释放活性 NF-κB 以进行核易位。 221 NF-κB 作为转录因子可以调节数百个与细胞存活、增殖、代谢、特别是免疫相关的基因。 225 , 226 , 227
TGF-β-activated PI3K/AKT signaling
TGF-β 激活 PI3K/AKT 信号转导
The TβR complex can activate the lipid kinase PI3K upon TGF-β stimulation, either via the kinase activity of TβRI or through the recruitment of TRAF6, which polyubiquitylates PI3K regulatory subunit p85α independent of the receptor kinase.228,229 Activated PI3K then phosphorylates phosphoinositide phosphatidylinositol-4,5-bisphosphate (PIP2) into phosphatidylinositol-3,4,5-trisphosphate (PIP3) which further triggers the phosphorylation of AKT.228,230 Activated AKT targets plenty of substrates, including mechanistic target of rapamycin (MTOR),231,232 GSK-3β,233 and several forkhead box O (FOXO) transcription factors.234Among them, MTOR is the most common downstream effector of AKT, and ribosomal protein S6 kinase (S6K) and eukaryotic initiation factor 4E-binding protein 1 (4EBP1) are the best-characterized downstream effectors of MTOR. In general, the consequences of PI3K/AKT signaling include diverse cellular responses such as survival, metabolism, growth, proliferation, and differentiation.235
TβR 复合物可以在 TGF-β 刺激下通过 TβRI 的激酶活性或通过 TRAF6 的募集来激活脂质激酶 PI3K,TRAF6 不依赖于受体激酶而多泛素化 PI3K 调节亚基 p85α。 228 , 229激活的 PI3K 然后将磷酸肌醇磷脂酰肌醇-4,5-二磷酸 (PIP2) 磷酸化为磷脂酰肌醇-3,4,5-三磷酸 (PIP3),这进一步触发 AKT 的磷酸化。 228 、 230激活的 AKT 靶向大量底物,包括雷帕霉素 (MTOR) 的机械靶标、 231 、 232 GSK-3β、 233和几种叉头盒 O (FOXO) 转录因子。 234其中,MTOR 是 AKT 最常见的下游效应子,核糖体蛋白 S6 激酶 (S6K) 和真核起始因子 4E 结合蛋白 1 (4EBP1) 是特征最明显的 MTOR 下游效应子。一般来说,PI3K/AKT 信号传导的后果包括多种细胞反应,例如生存、代谢、生长、增殖和分化。 235
TGF-β-activated JAK/STAT signaling
TGF-β 激活的 JAK/STAT 信号传导
TGF-β is found to induce JAK1 and JAK2 activation respectively in hepatic stellate cells (HSCs) and fibroblasts. In these cases, activated JAK triggers the phosphorylation of STAT3 which functions to mediate the fibrogenic effects of TGF-β, including increased cell proliferation, myofibroblast (MF) differentiation, ECM production, α-smooth muscle actin (α-SMA) expression, and stress fiber formation.236,237,238 Like other signaling pathways, JAK/STAT signaling can also drive many physiological and pathological events, including development, metabolism, immunity, wounding, and cancers.239
研究发现 TGF-β 分别在肝星状细胞 (HSC) 和成纤维细胞中诱导 JAK1 和 JAK2 激活。在这些情况下,激活的 JAK 会触发 STAT3 的磷酸化,STAT3 的作用是介导 TGF-β 的纤维化作用,包括增加细胞增殖、肌成纤维细胞 (MF) 分化、ECM 产生、α-平滑肌肌动蛋白 (α-SMA) 表达和应力纤维的形成。 236 , 237 , 238与其他信号传导途径一样,JAK/STAT 信号传导也可以驱动许多生理和病理事件,包括发育、代谢、免疫、受伤和癌症。 239
TGF-β signaling in health
健康中的 TGF-β 信号传导
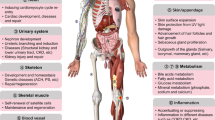
In physiological conditions, TGF-β signaling is greatly required by multiple biological processes and is particularly critical to embryonic development, wound healing, tissue homeostasis, and immune homeostasis (Fig. 5).
在生理条件下,多种生物过程非常需要TGF-β信号传导,并且对于胚胎发育、伤口愈合、组织稳态和免疫稳态尤其重要(图5 )。
TGF-β signaling in health. TGF-β signaling plays a critical role in physiological conditions. a During embryonic development, TGF-β regulates cell differentiation, epithelial/endothelial-mesenchymal transition (EMT/EndMT), and apoptosis to ensure proper histogenesis and organogenesis. b TGF-β promotes wound healing by participating in inflammation, re-epithelialization, angiogenesis, and fibroblast activation. c TGF-β is indispensable for tissue homeostasis as it generally suppresses cell proliferation and induces cell apoptosis through various mechanisms. d TGF-β functions to suppress the activity of multiple immunocompetent cells while inducing the phenotypes of several immune immunosuppressive cells to maintain immune homeostasis. (SMC, smooth muscle cell; VEGF, vascular endothelial growth factor; MMP, matrix metalloproteinase; TIMP tissue inhibitor of MMP, PAI plasminogen activator inhibitor, CDK cyclin-dependent kinase, CKI CDK inhibitor, ID inhibitor of DNA binding, MYC cellular-myelocytomatosis viral oncogene, CDC25A cell division cycle 25A, BCL-2 B-cell lymphoma-2, BAX BCL-2-associated X protein, BIM BCL-2-interacting mediator of cell death, BCL-XL BCL-extra-large, GADD45β growth arrest and DNA damage-inducible β, SHIP sarcoma (SRC) homology 2 (SH2) domain-containing inositol 5’-phosphatase, TIEG TGF-β-inducible early gene, CTL cytotoxic T lymphocyte, Th T helper, Treg regulatory T cell, Breg regulatory B cell, NK natural killer, DC dendritic cell)
健康中的 TGF-β 信号传导。 TGF-β信号传导在生理条件下发挥着关键作用。 a在胚胎发育过程中,TGF-β 调节细胞分化、上皮/内皮-间质转化 (EMT/EndMT) 和细胞凋亡,以确保正确的组织发生和器官发生。 b TGF-β 通过参与炎症、上皮再生、血管生成和成纤维细胞激活来促进伤口愈合。 c TGF-β 对于组织稳态是不可或缺的,因为它通常通过各种机制抑制细胞增殖并诱导细胞凋亡。 d TGF-β 的作用是抑制多种免疫活性细胞的活性,同时诱导多种免疫免疫抑制细胞的表型,以维持免疫稳态。 (SMC,平滑肌细胞;VEGF,血管内皮生长因子;MMP,基质金属蛋白酶;TIMP MMP 组织抑制剂,PAI 纤溶酶原激活剂抑制剂,CDK 细胞周期蛋白依赖性激酶,CKI CDK 抑制剂,ID DNA 结合抑制剂,MYC 细胞骨髓细胞增多症病毒癌基因、CDC25A 细胞分裂周期 25A、BCL-2 B 细胞淋巴瘤-2、BAX BCL-2 相关 X 蛋白、BIM BCL-2 细胞死亡相互作用介体、BCL-XL BCL-超大、GADD45β 生长阻滞和 DNA 损伤诱导型 β、SHIP 肉瘤 (SRC) 同源性 2 (SH2) 结构域含肌醇 5'-磷酸酶、TIEG TGF-β 诱导型早期基因、CTL 细胞毒性 T 淋巴细胞、Th T 辅助细胞、Treg 调节性 T 细胞、 Breg 调节性 B 细胞、NK 自然杀伤细胞、DC 树突状细胞)
Embryonic development 胚胎发育
In situ hybridization and immunohistochemical staining reveal overlapping but distinct expression patterns of the three TGF-β isoforms at different developmental stages of murine embryos. TGF-β is expressed in nearly all kinds of embryonic tissues such as heart, vessels, lungs, kidneys, liver, gut, bones, teeth, cartilages, muscles, skin, thymus, thyroid, suprarenal glands, salivary glands, nervous system, and craniofacial tissues.19,240,241,242,243,244 In particular, mesenchymal and epithelial components undergoing organogenesis and morphogenesis which involve active cell differentiation and epithelial-mesenchymal interactions generally express high levels of TGF-β.19,240,241,242,243
原位杂交和免疫组织化学染色揭示了小鼠胚胎不同发育阶段三种 TGF-β 亚型重叠但不同的表达模式。 TGF-β在几乎所有类型的胚胎组织中表达,如心脏、血管、肺、肾、肝脏、肠道、骨骼、牙齿、软骨、肌肉、皮肤、胸腺、甲状腺、肾上腺、唾液腺、神经系统和颅面组织。 19、240、241、242、243、244特别地,经历涉及活跃细胞分化和上皮-间质相互作用的器官发生和形态发生的间充质和上皮成分通常表达高水平的TGF-β。 19、240、241、242、243
TGF-β has a significant impact on cell differentiation. Studies on Xenopus embryos reveal that TGF-β can induce mesoderm formation which is a primary patterning event in early vertebrate development.245,246 TGF-β can further regulate the development of hemangioblasts from mesoderm as well as subsequent differentiation of hematopoietic stem and progenitor cells (HSPCs) to participate in hematopoiesis and vasculogenesis.240,247,248,249,250 Mesenchymal stem cells (MSCs) which are derived from the mesoderm as well also respond actively to TGF-β signaling during their differentiation into several connective tissue cell lineages such as osteocytes, chondrocytes, myocytes, and adipocytes.251,252 TGF-β inhibits osteogenic differentiation by inducing the nuclear translocation of β-catenin and repressing the transcriptional activity of core-binding factor subunit α-1 (CBFA1) in a SMAD3-dependent manner.252,253 TGF-β-induced SMAD signaling also inhibits myogenesis and adipogenesis by respectively repressing the transcriptional activity of myogenic differentiation (MYOD) family members254,255,256,257 and CCAAT/enhancer-binding proteins (C/EBPs).17,258,259 However, the differentiation of MSCs into smooth muscle cells (SMCs) is promoted by TGF-β through mechanisms involving the activation of SMAD signaling, RHO signaling, and NOTCH signaling.260 Moreover, TGF-β stimulates chondrogenesis by inducing mesenchymal cells to differentiate into chondrocytes and produce cartilage-specific proteoglycan and type II collagen.18,261,262 As for other cell types, TGF-β signaling also regulates the differentiation and development in epidermis,263 lungs,264,265 kidneys,266 pancreas,267,268 teeth,269 and nervous system.270,271,272,273,274,275,276
TGF-β对细胞分化有显着影响。对非洲爪蟾胚胎的研究表明,TGF-β 可以诱导中胚层形成,这是早期脊椎动物发育中的主要模式事件。 245 , 246 TGF-β可以进一步调节中胚层成血管细胞的发育以及随后的造血干细胞和祖细胞(HSPC)的分化以参与造血和血管生成。 240 , 247 , 248 , 249 , 250源自中胚层的间充质干细胞 (MSC) 在分化为骨细胞、软骨细胞、肌细胞和脂肪细胞等多种结缔组织细胞谱系的过程中也会对 TGF-β 信号做出积极反应。 251 , 252 TGF-β通过诱导β-连环蛋白的核转位并以SMAD3依赖性方式抑制核心结合因子亚基α-1 (CBFA1)的转录活性来抑制成骨分化。 252、253 TGF -β诱导的 SMAD 信号传导还通过分别抑制生肌分化 (MYOD )家族成员254、255、256、257和 CCAAT/增强子结合蛋白 (C/EBP) 的转录活性来抑制肌生成和脂肪生成。 17 , 258 , 259然而,TGF-β 通过涉及 SMAD 信号传导、RHO 信号传导和 NOTCH 信号传导的激活机制来促进 MSC 分化为平滑肌细胞 (SMC)。260此外,TGF-β 通过诱导间充质细胞分化为软骨细胞并产生软骨特异性蛋白多糖和 II 型胶原来刺激软骨形成。 18 , 261 , 262与其他细胞类型一样,TGF-β信号还调节表皮、 263肺、 264、265肾、 266胰腺、 267、268牙齿、 269和神经系统的分化和发育。270、271、272、273、274、275、276
Especially for epithelial cells, TGF-β can induce a reversible de-differentiation process known as epithelial-mesenchymal transition (EMT) which is critical to embryonic development.277 During EMT, epithelial cells lose their cellular polarity, intercellular junctions, and epithelial markers such as E-cadherin, but turn to acquire mesenchymal or fibroblastic phenotype with increased cell migratory motility, ECM proteolytic activity, and expression of mesenchymal markers such as fibronectin.278 This process is generally mediated by transcription factors such as SNAIL, SLUG, ZEB, and TWIST, involving both SMAD-dependent and SMAD-independent pathways in the case of TGF-β signaling.198,200,219,230,231,232,279,280 The developmental functions of TGF-β-induced EMT have been well studied in embryonic palate formation during which the expression of TGF-β is significantly elevated.19,243 Among the three TGF-β isoforms expressed in developing murine palate,281,282 only TGF-β3 is indispensable to the fusion of palatal shelves which is a crucial step during palatogenesis.283 Mechanically, TGF-β3 induces the EMT of palatal midline epithelial seam (MES) cells, leading to the disintegration of the epithelium and subsequent confluence of the mesenchyme.279,280 Interestingly, endothelial cells can undergo a similar process known as endothelial-mesenchymal transition (EndMT) which is crucial for cardiovascular development. In humans, TGF-β2 is the most potent inducer of EndMT, while TGF-β1 and TGF-β3 at least partially rely on the induction of TGF-β2 to trigger this process.284 Consistently, although all three TGF-β isoforms are differentially expressed during murine cardiogenesis,19,240,242,243,285,286,287 only TGF-β2 is obligatory to the EndMT during the endocardial cushion development in the atrioventricular canal which is necessary to valvular formation.288,289,290,291 Moreover, TGF-β1 and TGF-β2 can trigger EndMT in the epicardium to contribute to coronary vessel formation.292,293 In fact, TGF-β signaling is essential to vasculogenesis in many developing tissues by promoting the proliferation and migration of endothelial cells.19,294
特别是对于上皮细胞,TGF-β可以诱导可逆的去分化过程,称为上皮间质转化(EMT),这对胚胎发育至关重要。 277在 EMT 期间,上皮细胞失去细胞极性、细胞间连接和上皮标记物(如 E-钙粘蛋白),但转而获得间充质或成纤维细胞表型,并增加细胞迁移运动、ECM 蛋白水解活性和间充质标记物(如纤连蛋白)的表达。 278这一过程通常由 SNAIL、SLUG、ZEB 和 TWIST 等转录因子介导,在 TGF-β 信号转导中涉及 SMAD 依赖性和 SMAD 独立途径。 198 , 200 , 219 , 230 , 231 , 232 , 279 , 280 TGF-β 诱导的 EMT 的发育功能已在胚胎腭形成过程中得到充分研究,在此期间 TGF-β 的表达显着升高。 19 , 243在发育中的小鼠上颚中表达的三种 TGF-β 亚型中, 281 , 282只有 TGF-β3 对于腭架融合是不可或缺的,而腭架融合是腭发育过程中的关键步骤。 283从机械角度来看,TGF-β3 诱导腭中线上皮缝 (MES) 细胞发生 EMT,导致上皮崩解以及随后间质的汇合。 279 , 280有趣的是,内皮细胞可以经历类似的过程,称为内皮间质转化 (EndMT),这对心血管发育至关重要。 在人类中,TGF-β2 是 EndMT 最有效的诱导剂,而 TGF-β1 和 TGF-β3 至少部分依赖 TGF-β2 的诱导来触发这一过程。 284一致地,尽管所有三种 TGF-β 亚型在小鼠心脏发生过程中都有差异表达, 19、240、242、243、285、286、287 ,但在房室管心内膜垫发育过程中,只有 TGF-β2 对 EndMT 是必需的。瓣膜形成所必需的。 288 , 289 , 290 , 291此外,TGF-β1 和 TGF-β2 可以触发心外膜中的 EndMT,促进冠状血管形成。 292 , 293事实上,TGF-β 信号传导通过促进内皮细胞的增殖和迁移,对于许多发育组织中的血管生成至关重要。19 , 294
Furthermore, TGF-β can induce apoptosis of unnecessary cells during embryonic development to ensure proper histogenesis and organogenesis. During murine palatogenesis, the disintegration of MES not only relies on TGF-β3-induced EMT as introduced above but also requires TGF-β3-induced apoptosis of MES cells to complete palatal confluency.295 In murine limb buds, highly expressed TGF-β triggers massive cell death in the mesenchyme of interdigital spaces to induce the regression of interdigital webs and the formation of free digits.19,243,296 Endogenous TGF-β also mediates the apoptotic death of certain neuron types in chick embryos to contribute to nervous system development.297 Notably, TGF-β2 and TGF-β3 presenting in the central part of the developing chick retina are essentially required to trigger retinal cell apoptosis, which can create space for incoming axons of retinal ganglion cells to form optic nerve.298,299 In mice, however, TGF-β signaling also protects retinal neurons from excessive apoptosis to ensure proper development of eyes.300
此外,TGF-β可以诱导胚胎发育过程中不必要的细胞凋亡,以确保适当的组织发生和器官发生。在小鼠腭发育过程中,MES的解体不仅依赖于上文介绍的TGF-β3诱导的EMT,而且还需要TGF-β3诱导MES细胞凋亡才能完成腭融合。 295在小鼠肢芽中,高表达的 TGF-β 会触发指间间隙间充质的大量细胞死亡,从而诱导指间网的退化和游离指的形成。 19 , 243 , 296内源性 TGF-β 还可介导鸡胚胎中某些神经元类型的细胞凋亡,从而有助于神经系统发育。 297值得注意的是,发育中的小鸡视网膜中央部分存在的 TGF-β2 和 TGF-β3 本质上是触发视网膜细胞凋亡所必需的,这可以为视网膜神经节细胞的传入轴突形成视神经创造空间。 298 , 299然而,在小鼠中,TGF-β 信号传导还可以保护视网膜神经元免于过度凋亡,以确保眼睛的正常发育。 300
Wound healing 伤口愈合
Wound healing which happens after tissue injuries generally involves four orderly and overlapping stages known as hemostasis, inflammation, proliferation, and remodeling.301 Throughout the healing of cutaneous wounds, all TGF-β isoforms and TβR types are induced in a distinct spatial and temporal pattern.302,303 During hemostasis, platelets provide an immediate and abundant supply of TGF-β after wounding, contributing largely to subsequent healing stages by promoting the influx of inflammatory cells and fibroblasts into the wounds due to its chemotactic activity.302,304,305,306,307 Interestingly, many of the cell types recruited by TGF-β are also active in secreting TGF-β, leading to even higher TGF-β concentrations in the wounds. In ovine skin, all three TGF-β isoforms increase dramatically only one day after wounding, attributed to the expression by epithelial cells, endothelial cells, fibroblasts, and inflammatory cells such as neutrophils, macrophages, and lymphocytes.302 During the stage of proliferation and remodeling, TGF-β is implicated in wound re-epithelialization, tissue angiogenesis, and fibroblast activation.308,309 Upon cutaneous injury, TGF-β1 is initially expressed by all epidermal keratinocytes adjacent to the wounds but gradually gets excluded from the basal keratinocytes, corresponding to the transient block and subsequent burst of basal keratinocyte proliferation after wounding.310 TGF-β1 also contributes to the migration of epithelial sheets at the leading edges of cutaneous wounds through the regulation of integrins and the activation of PI3K.310,311,312 Other TGF-β isoforms such as TGF-β3 can have similar impacts on cell migration during cutaneous wound healing.313 As for angiogenesis, TGF-β regulates the proliferation and migration of endothelial cells in vitro and shows potent angiogenic activity when overexpressed or directly applied in vivo.307,314,315,316,317,318,319,320,321 A possible mechanism of TGF-β-induced angiogenesis involves the induction of vascular endothelial growth factor (VEGF) in epithelial cells and fibroblasts.322,323 Moreover, TGF-β can stimulate fibroblasts to proliferate and produce bioactive factors such as collagen, fibronectin, MMPs, tissue inhibitor of MMPs (TIMPs), and plasminogen activator inhibitor 1 (PAI-1) which contribute to the deposition and remodeling of wound ECM.304,306,307,315,317,321,324,325,326,327,328,329,330,331,332,333,334 It can also promote fibroblast-mediated wound contraction through MF differentiation and RHO activation.335,336,337
组织损伤后发生的伤口愈合通常涉及四个有序且重叠的阶段,即止血、炎症、增殖和重塑。 301在皮肤伤口的愈合过程中,所有 TGF-β 同工型和 TβR 类型均以独特的空间和时间模式诱导。 302 , 303在止血过程中,血小板在受伤后立即提供充足的 TGF-β 供应,由于其趋化活性,促进炎症细胞和成纤维细胞流入伤口,从而在很大程度上促进后续的愈合阶段。 302 , 304 , 305 , 306 , 307有趣的是,TGF-β 招募的许多细胞类型也积极分泌 TGF-β ,导致伤口中的 TGF-β 浓度甚至更高。在绵羊皮肤中,所有三种 TGF-β 亚型在受伤后仅一天就急剧增加,归因于上皮细胞、内皮细胞、成纤维细胞和炎症细胞(如中性粒细胞、巨噬细胞和淋巴细胞)的表达。 302在增殖和重塑阶段,TGF-β 参与伤口上皮再形成、组织血管生成和成纤维细胞活化。 308 , 309皮肤损伤时,TGF-β1 最初由伤口附近的所有表皮角质形成细胞表达,但逐渐从基底角质形成细胞中排除,对应于受伤后基底角质形成细胞增殖的短暂阻断和随后的爆发。310 TGF-β1 还通过整合素的调节和 PI3K 的激活,促进皮肤伤口前缘上皮片的迁移。 310、311、312其他TGF -β 同工型(例如 TGF-β3)对皮肤伤口愈合过程中的细胞迁移也有类似的影响。 313至于血管生成,TGF-β 在体外调节内皮细胞的增殖和迁移,并且在体内过表达或直接应用时显示出有效的血管生成活性。 307、314、315、316、317、318、319、320、321 TGF - β诱导的血管生成的可能机制涉及上皮细胞和成纤维细胞中血管内皮生长因子(VEGF)的诱导。 322 , 323此外,TGF-β 可以刺激成纤维细胞增殖并产生生物活性因子,如胶原蛋白、纤连蛋白、MMP、MMP 组织抑制剂 (TIMPs) 和纤溶酶原激活剂抑制剂 1 (PAI-1),这些因子有助于沉积和重塑伤口 ECM。 304、306、307、315、317、321、324、325、326、327、328、329、330、331、332、333、334还可以通过MF分化和RHO激活促进成纤维细胞介导的伤口收缩。335 , 336 , 337
Apart from the skin, TGF-β also functions in the repair and regeneration of many other tissues. During rat liver regeneration, all TGF-β isoforms are induced in non-parenchymal cells rather than hepatocytes, which however, exhibit upregulation of all TβR types to enhance the responsiveness to TGF-β, which may help to prevent uncontrolled cell proliferation.338,339,340,341,342 Similarly, the marked increase in TGF-β and TβR expression following acute pancreatitis suggests the role of TGF-β signaling in pancreatic repair.343,344,345 Upon vascular injury, TGF-β mobilizes MSCs to peripheral blood and further recruits them to the injured sites for vascular repair.346 As for cardiac repair after myocardial injury, TGF-β triggers the EndMT of epicardial cells, which then migrate into the injured myocardium to generate various cardiac cell types.347 TGF-β also plays a role in cartilage repair by stimulating proteoglycan synthesis in chondrocytes.348,349 Moreover, after injury in the nervous system, neurons, astrocytes, microglia, as well as recruited macrophages all upregulate the expression of TGF-β which may contribute to the healing process of the nervous tissues.350,351
除皮肤外,TGF-β 还参与许多其他组织的修复和再生。在大鼠肝脏再生过程中,所有 TGF-β 亚型均在非实质细胞而非肝细胞中诱导,然而,肝细胞表现出所有 TβR 类型的上调,以增强对 TGF-β 的反应性,这可能有助于防止不受控制的细胞增殖。 338 , 339 , 340 , 341 , 342同样,急性胰腺炎后 TGF-β 和 TβR 表达显着增加表明 TGF-β 信号传导在胰腺修复中的作用。 343 , 344 , 345血管损伤后,TGF-β 将 MSC 动员到外周血,并进一步将它们募集到损伤部位进行血管修复。 346至于心肌损伤后的心脏修复,TGF-β 会触发心外膜细胞的 EndMT,然后迁移到受损的心肌中,生成各种心肌细胞类型。 347 TGF-β 还通过刺激软骨细胞中的蛋白多糖合成而在软骨修复中发挥作用。 348 , 349此外,神经系统损伤后,神经元、星形胶质细胞、小胶质细胞以及招募的巨噬细胞都会上调 TGF-β 的表达,这可能有助于神经组织的愈合过程。 350 , 351
Tissue homeostasis 组织稳态
Tissue homeostasis is maintained by the balance between cell proliferation and cell death in which TGF-β acts as a key regulator.
组织稳态是通过细胞增殖和细胞死亡之间的平衡来维持的,其中 TGF-β 起着关键的调节作用。
Cell proliferation is generally driven by CDKs through a series of events collectively known as the cell cycle. For most cells, TGF-β inhibits their proliferation, or in other words, triggers their cytostasis by inducing cell cycle arrest in the gap 1 (G1) phase. In epithelial cells and glial cells, TGF-β suppresses the activity of CDKs by activating the transcription of CDK inhibitors (CKIs) such as p15 and p21 to induce cytostasis.352,353,354,355 The transcriptional activation of CKIs in response to TGF-β is likely mediated by SMADs in cooperation with transcription factor FOXO355,356 or specificity protein 1 (SP1).357,358 Notably, the SMAD-FOXO complex additionally requires transcription factor C/EBPβ for the induction of p15 but not of p21.356 In epithelial cells, TGF-β-mediated upregulation of p15 also prevents the non-inhibitory binding of CKI p27 to CDK4. As a result, p15 and p27 turn to bind their own targets which are respectively CDK4 and CDK2 to exert their inhibitory effects.359,360 Interestingly, in murine B cells, TGF-β increases the expression of p27 instead of p21 to trigger cytostasis,361 while in human hematopoietic cells, p57 is likely the only TGF-β-induced CKI for cell cycle arrest.362 Besides CKIs, TGF-β can also target other proliferative factors such as MYC, inhibitors of DNA binding (IDs), and CDC25A to inhibit cell proliferation as mostly shown in epithelial cells. TGF-β induces the transcriptional repression of MYC through a complex containing SMADs, transcription factors E2F4/5 and C/EBPβ, as well as transcriptional corepressor p107.356,363,364 It also inhibits ID1 expression through SMADs which mediate the induction and recruitment of transcriptional repressor activating transcription factor 3 (ATF3) to target ID1 promoter.365 As for ID2 which can be induced by MYC at the transcriptional level, its suppression by TGF-β is attributed to the downregulation of MYC or the upregulation of antagonistic MYC repressors known as MYC-associated factor X (MAX) dimerization proteins (MADs).366,367 By these means, TGF-β is able to relieve the transcriptional repression on CKIs exerted by MYC and IDs to facilitate the induction of cytostasis.368,369,370,371 Furthermore, TGF-β can downregulate the activity of the CDK-activating phosphatase CDC25A through several mechanisms such as the transcriptional repression by E2F4-p130-HDAC1 complex,372 the inhibitory phosphorylation by RHOA/ROCK1 signaling,373 as well as the SMAD3-dependent degradative ubiquitination by E3 ubiquitin ligase complex SCF.374 Notably, TGF-β can also stimulate the proliferation of certain cell types, including SMCs, fibroblasts, and chondrocytes, likely due to the induction of autocrine growth factors such as fibroblast growth factor (FGF) and platelet-derived growth factor (PDGF).324,325,375
细胞增殖通常由 CDK 通过一系列统称为细胞周期的事件驱动。对于大多数细胞来说,TGF-β 会抑制其增殖,或者换句话说,通过诱导细胞周期停滞在间隙 1 (G1) 期来触发细胞停滞。在上皮细胞和神经胶质细胞中,TGF-β 通过激活 CDK 抑制剂 (CKI)(如 p15 和 p21)的转录来抑制 CDK 的活性,从而诱导细胞停滞。 352 , 353 , 354 , 355 CKI 响应 TGF-β 的转录激活可能是由 SMAD 与转录因子 FOXO 355 , 356或特异性蛋白 1 (SP1) 协同介导的。 357 , 358值得注意的是,SMAD-FOXO 复合物还需要转录因子 C/EBPβ 来诱导 p15,但不需要诱导 p21。 356在上皮细胞中,TGF-β 介导的 p15 上调也会阻止 CKI p27 与 CDK4 的非抑制性结合。结果,p15和p27转而结合各自的靶标CDK4和CDK2来发挥抑制作用。 359 , 360有趣的是,在鼠 B 细胞中,TGF-β 增加 p27 而不是 p21 的表达来触发细胞停滞, 361而在人类造血细胞中,p57 可能是唯一 TGF-β 诱导的细胞周期停滞 CKI。 362除 CKI 外,TGF-β 还可以靶向其他增殖因子,例如 MYC、DNA 结合抑制剂 (ID) 和 CDC25A,以抑制细胞增殖,这主要在上皮细胞中表现出来。 TGF-β 通过包含 SMAD、转录因子 E2F4/5 和 C/EBPβ 以及转录辅阻遏物 p107 的复合物诱导 MYC 的转录抑制。 356 , 363 , 364它还通过 SMAD 抑制 ID1 表达,SMAD 介导转录抑制因子激活转录因子 3 (ATF3) 的诱导和募集以靶向 ID1 启动子。 365至于可由 MYC 在转录水平诱导的 ID2,其被 TGF-β 抑制的原因是 MYC 的下调或称为 MYC 相关因子 X (MAX) 二聚化蛋白 (MAD) 的拮抗 MYC 阻遏物的上调。 366 , 367通过这些方式,TGF-β 能够解除 MYC 和 ID 对 CKI 的转录抑制,从而促进细胞抑制的诱导。 368、369、370、371此外, TGF -β 可以通过多种机制下调 CDK 激活磷酸酶 CDC25A 的活性,例如 E2F4-p130-HDAC1 复合物的转录抑制, 372 RHOA/ROCK1 信号传导的抑制性磷酸化, 373以及 E3 泛素连接酶复合体 SCF 的 SMAD3 依赖性降解泛素化。 374值得注意的是,TGF-β 还可以刺激某些细胞类型的增殖,包括 SMC、成纤维细胞和软骨细胞,这可能是由于诱导了自分泌生长因子,如成纤维细胞生长因子 (FGF) 和血小板衍生生长因子 (PDGF) 。324 , 325 , 375
As for cell death, TGF-β can trigger apoptosis which is one of the most common forms of cell death in a wide range of cell types including lymphocytes, hepatocytes, podocytes, glial cells, hematopoietic cells, and epithelial cells. Such effect is generally attributed to SMAD-dependent regulation of B-cell lymphoma-2 (BCL-2) family members. More specifically, TGF-β can upregulate pro-apoptotic BCL-2 family members such as BCL-2-associated X protein (BAX) and BCL-2-interacting mediator of cell death (BIM),376,377,378,379 meanwhile, it can also downregulate anti-apoptotic BCL-2 family members such as BCL-2 and BCL-extra-large (BCL-XL).378,380,381 Apart from BCL-2 family members, many other effectors and pathways are also involved in TGF-β-induced cell apoptosis. A septin-like protein known as apoptosis-related protein in the TGF-β signaling pathway (ARTS) undergoes mitochondrial-to-nuclear translocation to promote cell apoptosis in response to TGF-β.382 Death domain-associated protein (DAXX) interacts with TβRII as an intermediary to convey pro-apoptotic TGF-β signal to downstream machinery.383 In B cells and hepatocytes, TGF-β triggers the transient activation of TAK1/IKK/NF-κB pathway, sequentially leading to the transcriptional activation of IκB-α, the post-repression of NF-κB, the upregulation of JNK signaling, the increase of activator protein 1 (AP-1) complex activity, and finally, the apoptotic death of cells.384,385,386 In hepatocytes, TGF-β also promotes the expression of growth arrest and DNA damage-inducible β (GADD45β), which functions as a positive mediator of cell apoptosis by acting upstream of p38 MAPK.387 As for podocytes, TGF-β can activate both pro-apoptotic p38 signaling and anti-apoptotic PI3K/AKT signaling to regulate their survival and death.379,388 In fact, AKT, especially when phosphorylated, can bind to unphosphorylated SMAD3 to inhibit its activity and thus protect several cell types from SMAD-dependent apoptosis. In contrast, TGF-β can prevent the AKT-SMAD3 interaction by triggering SMAD3 phosphorylation to facilitate the cell death program.389,390 Moreover, in hematopoietic cells, SMAD-dependent TGF-β signaling induces the expression of a central regulator of phospholipid metabolism known as SRC homology 2 (SH2) domain-containing inositol 5’-phosphatase (SHIP) to inhibit AKT phosphorylation as well as cell survival.391 Furthermore, TGF-β triggers the apoptosis of oligodendrocytes and epithelial cells by inducing transcription factors TGF-β-inducible early genes (TIEGs) to downregulate BCL-XL expression.392,393,394 Notably, TGF-β is also found to promote cell survival in certain cases.300,395,396,397,398 Related mechanisms involve the AKT-dependent inhibition of FOXO3 as in epithelial cells,399 the suppression of AKT and the induction of BCL-2 as in pre-B lymphocytes,400 the early induction and phosphorylation of c-Jun and consequential attenuation of JNK as in lung carcinoma cells,401 the downregulation of CD95L and p53 as well as the upregulation of NF-κB, BCL-XL, and p21 as in HSCs.402
至于细胞死亡,TGF-β可以引发细胞凋亡,这是多种细胞类型中最常见的细胞死亡形式之一,包括淋巴细胞、肝细胞、足细胞、神经胶质细胞、造血细胞和上皮细胞。这种效应通常归因于 B 细胞淋巴瘤 2 (BCL-2) 家族成员的 SMAD 依赖性调节。更具体地说,TGF-β 可以同时上调促凋亡 BCL-2 家族成员,例如 BCL-2 相关 X 蛋白 (BAX) 和 BCL-2 相互作用细胞死亡介质 ( BIM ) , 376、377、378、379 ,它还可以下调抗凋亡 BCL-2 家族成员,如 BCL-2 和 BCL-extra-large (BCL-XL)。 378 , 380 , 381除 BCL-2 家族成员外,许多其他效应子和途径也参与 TGF-β 诱导的细胞凋亡。 TGF-β 信号通路 (ARTS) 中的一种类似脓毒症的蛋白,称为凋亡相关蛋白,会经历线粒体到核的易位,以响应 TGF-β 促进细胞凋亡。 382死亡结构域相关蛋白 (DAXX) 作为中介与 TβRII 相互作用,将促凋亡 TGF-β 信号传递至下游机制。 383在 B 细胞和肝细胞中,TGF-β 触发 TAK1/IKK/NF-κB 通路瞬时激活,依次导致 IκB-α 转录激活、NF-κB 后抑制、JNK 信号传导上调,激活蛋白 1 (AP-1) 复合物活性增加,最后导致细胞凋亡。384 , 385 , 386在肝细胞中,TGF-β 还促进生长停滞和 DNA 损伤诱导型 β (GADD45β) 的表达,后者通过作用于 p38 MAPK 的上游,充当细胞凋亡的正介体。 387对于足细胞,TGF-β 可以激活促凋亡 p38 信号传导和抗凋亡 PI3K/AKT 信号传导,以调节其生存和死亡。 379 , 388事实上,AKT,尤其是磷酸化时,可以与未磷酸化的 SMAD3 结合,抑制其活性,从而保护多种细胞类型免受 SMAD 依赖性细胞凋亡。相反,TGF-β 可以通过触发 SMAD3 磷酸化来阻止 AKT-SMAD3 相互作用,从而促进细胞死亡程序。 389 , 390此外,在造血细胞中,SMAD 依赖性 TGF-β 信号转导诱导磷脂代谢中央调节因子(称为 SRC 同源 2 (SH2) 结构域)的表达,其中含有肌醇 5'-磷酸酶 (SHIP),以抑制 AKT 磷酸化,如以及细胞的存活率。 391此外,TGF-β 通过诱导转录因子 TGF-β 诱导早期基因 (TIEG) 下调 BCL-XL 表达,从而引发少突胶质细胞和上皮细胞凋亡。 392 , 393 , 394值得注意的是,TGF-β 还被发现在某些情况下可以促进细胞存活。300、395、396、397、398相关机制涉及上皮细胞中 FOXO3 的 AKT 依赖性抑制、 399 AKT 抑制和前 B 淋巴细胞中 BCL-2 的诱导、 400早期诱导和磷酸化c-Jun 和随之而来的 JNK 减弱(如肺癌细胞中), 401 CD95L 和 p53 下调以及 NF-κB、BCL-XL 和 p21(如 HSC 中)上调。第402章
Immune homeostasis 免疫稳态
Generally, TGF-β functions to suppress the activity of multiple immunocompetent cells while inducing the phenotypes of several immune immunosuppressive cells. For this reason, it is regarded as one of the most potent immunosuppressive cytokines which are of vital importance to the maintenance of immune homeostasis and self-immune tolerance.403
一般来说,TGF-β的作用是抑制多种免疫活性细胞的活性,同时诱导多种免疫免疫抑制细胞的表型。因此,它被认为是最有效的免疫抑制细胞因子之一,对于维持免疫稳态和自身免疫耐受至关重要。 403
Cytotoxic T lymphocytes (CTLs), T helper type 1 (Th1), and Th2 cells
细胞毒性 T 淋巴细胞 (CTL)、1 型辅助 T (Th1) 和 Th2 细胞
TGF-β prevents naïve T cells from differentiating into classical effecter T cells through numerous mechanisms. For CD8+ T cells which can develop into CTLs upon activation, TGF-β inhibits their functions by suppressing the expression of cytolytic factors such as perforin, granzyme A, granzyme B, Fas ligand, and interferon (IFN)-γ. Mechanically, the encoding genes of granzyme B and IFN-γ are directly recognized by SMADs and transcription factor ATF1 which both bind to the gene promoter regions to mediate transcriptional repression in response to TGF-β signaling.404 The suppression of IFN-γ release is also correlated to the reduction of transcription factor T-box expressed in T cells (T-BET)405 while the decrease in Fas ligand expression is partially attributed to the downregulation of MYC.406 In CD4+ T cells, TGF-β inhibits the phosphorylation of T-cell kinase (ITK) to decrease the influx of calcium ion and subsequent activation of nuclear factor of activated T cells (NFATC) which are both critical events for Th1 and Th2 cell differentiation.407 TGF-β also suppresses the expression of transcription factors T-BET and GATA-3 in CD4+ T cells which act as master transcriptional activators during Th1 and Th2 cell development respectively.408,409,410
TGF-β 通过多种机制阻止幼稚 T 细胞分化为经典效应 T 细胞。对于激活后可发育为 CTL 的 CD8+ T 细胞,TGF-β 通过抑制穿孔素、颗粒酶 A、颗粒酶 B、Fas 配体和干扰素 (IFN)-γ 等溶细胞因子的表达来抑制其功能。从机制上讲,颗粒酶 B 和 IFN-γ 的编码基因直接被 SMAD 和转录因子 ATF1 识别,它们都与基因启动子区域结合,介导响应 TGF-β 信号传导的转录抑制。 404 IFN-γ 释放的抑制也与 T 细胞中表达的转录因子 T-box (T-BET) 的减少相关。405而 Fas 配体表达的减少部分归因于 MYC 的下调。 406在 CD4+ T 细胞中,TGF-β 抑制 T 细胞激酶 (ITK) 的磷酸化,以减少钙离子的流入以及随后激活的 T 细胞核因子 (NFATC) 的激活,这对于 Th1 和 Th2 细胞来说都是关键事件差异化。 407 TGF-β 还抑制 CD4+ T 细胞中转录因子 T-BET 和 GATA-3 的表达,这些转录因子分别在 Th1 和 Th2 细胞发育过程中充当主转录激活剂。 408 , 409 , 410
Tregs, Th9, and Th17 cells
Tregs、Th9 和 Th17 细胞
TGF-β induces the expression of transcription factor forkhead box P3 (FOXP3) in an interleukin (IL)-2-dependent manner in CD4+ CD25− naïve T cells to convert them into CD4+ CD25+ Tregs which can express TGF-β and inhibit other T cell proliferation with potent immunosuppressive activity.411,412,413,414 Similarly, TGF-β can induce the generation of Tregs from CD8+ T cells through the expression of FOXP3.415,416 Interestingly, IL-4 inhibits the induction of FOXP3 by TGF-β in naïve CD4+ T cells, instead, both cytokines cooperate to drive the differentiation of another Th cell subset known as Th9 cells by inducing the expression of transcription factor purine-rich box-1 (PU.1).417,418,419 Unlike the immunosuppressive Tregs, these IL-9- and IL-10-secreting cells can potently promote tissue inflammation.417,418,419,420 In addition, inflammatory cytokines such as IL-1β, IL-6, IL-21, and IL-23 also suppress TGF-β-induced FOXP3 in naïve CD4+ T cells, meanwhile, they elevate the activity of a TGF-β-induced transcription factor known as retinoic acid receptor-related orphan receptor γt (RORγt) to contribute to the generation of Th17 cells. This pro-inflammatory Th cell subset characterized by IL-17 expression plays important roles in anti-microbial defense and autoimmunity.421,422
TGF-β 在 CD4+ CD25− 幼稚 T 细胞中以白细胞介素 (IL)-2 依赖性方式诱导转录因子叉头框 P3 (FOXP3) 的表达,将其转化为 CD4+ CD25+ Tregs,后者可以表达 TGF-β 并抑制其他 T 细胞细胞增殖,具有有效的免疫抑制活性。 411、412、413、414类似地, TGF-β 可以通过 FOXP3 的表达诱导 CD8+ T 细胞产生 Tregs。 415 , 416有趣的是,IL-4 抑制幼稚 CD4+ T 细胞中 TGF-β 对 FOXP3 的诱导,相反,两种细胞因子通过诱导转录因子嘌呤的表达,协同驱动另一个 Th 细胞亚群(称为 Th9 细胞)的分化丰富的盒子-1 (PU.1)。 417 , 418 , 419与免疫抑制性 Tregs 不同,这些 IL-9 和 IL-10 分泌细胞可以有效促进组织炎症。 417 , 418 , 419 , 420此外,IL-1β、IL-6、IL-21 和 IL-23 等炎性细胞因子也在幼稚 CD4+ T 细胞中抑制 TGF-β 诱导的 FOXP3,同时提高其活性TGF-β 诱导的转录因子(称为视黄酸受体相关孤儿受体 γt (RORγt))有助于 Th17 细胞的生成。这种以 IL-17 表达为特征的促炎 Th 细胞亚群在抗微生物防御和自身免疫中发挥着重要作用。 421 , 422
B cells B细胞
As critical effectors of humoral immune responses, B cells mainly function by secreting antibodies which are also known as immunoglobulins (Igs). TGF-β decreases B cell Ig secretion by inhibiting the synthesis and the switch from the membrane form to the secreted form of Ig messenger ribonucleic acids (mRNAs).423 More specifically, TGF-β selectively inhibits the expression of Ig λ light chains while inducing less pronounced reductions in Ig κ light chains,423,424 moreover, it suppresses the production of isotypes IgM and IgG but enhances the class switching to isotype IgA.423,425,426 Notably, TGF-β-induced IgA with poor specificity is considered insufficient to mediate immune responses such as antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP).427,428 Furthermore, TGF-β can convert B cells into regulatory B cells (Bregs) which produce numerous factors such as TGF-β, IL-10, IL-35, Fas-L, and programmed death-ligand 1 (PD-L1) to mediate immunosuppression.429,430,431,432
作为体液免疫反应的关键效应器,B 细胞主要通过分泌抗体(也称为免疫球蛋白 (Igs))发挥作用。 TGF-β 通过抑制 Ig 信使核糖核酸 (mRNA) 的合成和从膜形式到分泌形式的转换来减少 B 细胞 Ig 分泌。第 423更具体地说,TGF-β 选择性抑制 Ig λ 轻链的表达,同时诱导 Ig κ 轻链不太明显的减少,第 423 ,第 424 章此外,它抑制同种型 IgM 和 IgG 的产生,但增强向同种型 IgA 的类别转换。 423 , 425 , 426值得注意的是,特异性较差的 TGF-β 诱导的 IgA 被认为不足以介导抗体依赖性细胞毒性 (ADCC) 和抗体依赖性细胞吞噬作用 (ADCP) 等免疫反应。 427 , 428此外,TGF-β 可以将 B 细胞转化为调节性 B 细胞 (Breg),从而产生多种因子,例如 TGF-β、IL-10、IL-35、Fas-L 和程序性死亡配体 1 (PD- L1) 介导免疫抑制。 429、430、431、432
Natural killer (NK) cells
自然杀伤 (NK) 细胞
NK cells are cytotoxic lymphocytes of the innate immunity. TGF-β suppresses NK cell development by downregulating transcription factor E4 promoter-binding protein 4 (E4BP4) in a SMAD3-dependent manner.433 The SMAD3 also decreases NK cell IFN-γ secretion through the inhibition of E4BP4 and T-BET.433,434 Moreover, TGF-β downregulates the surface expression of NK triggering receptors such as NKP30 and NK group 2 member D (NKG2D) which are responsible for the recognition and killing of target cells.435,436 It also negatively regulates the expression of cytolytic factors such as granzyme A, granzyme B, and perforin through SMAD signaling to further impair NK cytotoxicity.434,436
NK 细胞是先天免疫的细胞毒性淋巴细胞。 TGF-β 通过以 SMAD3 依赖性方式下调转录因子 E4 启动子结合蛋白 4 (E4BP4) 来抑制 NK 细胞发育。 433 SMAD3 还通过抑制 E4BP4 和 T-BET 减少 NK 细胞 IFN-γ 分泌。 433 , 434此外,TGF-β 下调 NK 触发受体的表面表达,例如负责识别和杀死靶细胞的 NKP30 和 NK 2 组成员 D (NKG2D)。 435 , 436它还通过 SMAD 信号传导负调节细胞溶解因子(例如颗粒酶 A、颗粒酶 B 和穿孔素)的表达,以进一步削弱 NK 细胞毒性。 434 , 436
DCs, macrophages, and neutrophils
DC、巨噬细胞和中性粒细胞
DCs, macrophages, and neutrophils can function as antigen-presenting cells (APCs), which are the keys to the activation of adaptive immune responses. TGF-β can impair antigen presentation through the downregulation of major histocompatibility complex (MHC) molecules.437,438,439 It also reduces the expression of IL-12 and co-stimulatory molecules such as CD40 in macrophages and CD80, CD83, and CD86 in DCs to interfere in APC-mediated immune cell activation.440,441 Apart from antigen presentation, TGF-β also inhibits the cytotoxicity of macrophages, on one hand, through the downregulation of cytotoxic factors, such as TNF-α and nitric oxide (NO),442,443,444,445,446 on the other hand, by suppressing the activity of Fcγ receptors (FcγRs) which function to mediate the ADCC and ADCP of macrophages.447 Moreover, TGF-β can trigger the polarization of macrophages and neutrophils from classical M1 macrophages and N1 neutrophils to alternative M2 macrophages and N2 neutrophils which are characterized by multiple immunosuppressive properties.439,448,449,450
DC、巨噬细胞和中性粒细胞可以充当抗原呈递细胞(APC),是激活适应性免疫反应的关键。 TGF-β 可以通过下调主要组织相容性复合体 (MHC) 分子来损害抗原呈递。 437 , 438 , 439它还降低 IL-12 和共刺激分子(例如巨噬细胞中的 CD40 和 DC 中的 CD80、CD83 和 CD86 )的表达,以干扰 APC 介导的免疫细胞激活。 440 , 441除了抗原呈递之外,TGF-β 一方面还通过下调细胞毒性因子,如 TNF-α 和一氧化氮 (NO) 来抑制巨噬细胞的细胞毒性, 442 , 443 , 444 , 445 , 446另一方面,通过抑制 Fcγ 受体 (FcγR) 的活性,FcγR 的功能是介导巨噬细胞的 ADCC 和 ADCP。 447此外,TGF-β 可以触发巨噬细胞和中性粒细胞从经典 M1 巨噬细胞和 N1 中性粒细胞极化为具有多种免疫抑制特性的替代 M2 巨噬细胞和 N2 中性粒细胞。 439、448、449、450
TGF-β signaling in disease
疾病中的 TGF-β 信号传导
Dysfunctional TGF-β signaling can play key roles in numerous pathological processes, contributing to the disorders of developmental defects, aberrant healing, fibrotic diseases, inflammatory diseases, infectious diseases, as well as tumors (Fig. 6).
功能失调的TGF-β信号传导可在许多病理过程中发挥关键作用,导致发育缺陷、异常愈合、纤维化疾病、炎症性疾病、传染病以及肿瘤等疾病(图6 )。
TGF-β signaling in disease. Dysfunctional TGF-β signaling is involved in numerous pathological processes. a Mutations that lead to decreased or increased TGF-β signaling can cause various developmental defects. b Deficient TGF-β signaling contributes to wound chronicity while excess TGF-β signaling leads to wound scarring and tissue fibrosis by stimulating ECM deposition through fibroblast activation and EMT/EndMT. c Dysfunctional TGF-β signaling exacerbates tissue injuries in inflammatory diseases and infectious diseases by promoting inflammation, pathogen infection, and tissue remodeling. d Aberrant TGF-β signaling is implicated in all aspects of tumor development including tumorigenesis, tumor growth, tumor invasion, tumor metastasis, as well as tumor microenvironment (TME) remodeling. (CTGF, connective tissue growth factor; IFN-γ, interferon-γ; IL-6, interleukin-6; solid arrows from TGF-β indicate excessive TGF-β signaling, dashed arrows from TGF-β indicate deficient TGF-β signaling)
疾病中的 TGF-β 信号传导。功能失调的 TGF-β 信号传导参与许多病理过程。 a导致 TGF-β 信号传导减少或增加的突变可能导致各种发育缺陷。 b TGF-β 信号传导不足会导致伤口慢性化,而过多的 TGF-β 信号传导则通过成纤维细胞激活和 EMT/EndMT 刺激 ECM 沉积,导致伤口疤痕和组织纤维化。 c功能失调的 TGF-β 信号传导通过促进炎症、病原体感染和组织重塑,加剧炎症性疾病和传染病中的组织损伤。 d异常的 TGF-β 信号传导涉及肿瘤发展的各个方面,包括肿瘤发生、肿瘤生长、肿瘤侵袭、肿瘤转移以及肿瘤微环境 (TME) 重塑。 (CTGF,结缔组织生长因子;IFN-γ,干扰素-γ;IL-6,白介素-6;TGF-β 的实线箭头表示 TGF-β 信号传导过多,TGF-β 的虚线箭头表示 TGF-β 信号传导不足)
Developmental defects 发育缺陷
Loss of TβRI or TβRII functions due to homozygous mutations generally results in embryonic lethality in mice due to defects in the hematopoiesis and vasculogenesis of yolk sac.451,452 However, the lack of different TGF-β isoforms can lead to distinct phenotypes in mice, consistent with the isoform-specific roles of TGF-β in embryonic development. TGF-β1-knockout mice show no gross developmental abnormalities in spite of the defective hematopoiesis and vasculogenesis in yolk sac during embryonic development.452,453,454 In contrast, TGF-β2-knockout mice exhibit perinatal mortality and a wide range of developmental defects in heart, lungs, bones, eyes, inner ears, craniofacial structures, urogenital organs, and hair follicles.290,455,456,457,458 TGF-β3-knockout mice also die shortly after birth but show no detectable abnormalities except for cleft palate and abnormal lung development.459,460 Notably, palatal shelves that fail to elevate in TGF-β2-knockout mice undergo elevation in TGF-β3-knockout mice but still fail in fusion.455,459,460 Also, branching morphogenesis and respiratory epithelial cell differentiation which appear normal in the lungs of TGF-β2-knockout mice are defective in TGF-β3-knockout mice.455,459 In humans, loss-of-function mutations of a single TGF-β signaling component such as TGF-β2,461,462,463 TGF-β3,464,465,466 TβRI,467,468,469 TβRII,470,471,472 SMAD2,473,474,475 or SMAD3476,477,478 can cause Loeys-Dietz syndrome (LDS), an autosomal dominant connective tissue disorder with a range of cardiovascular, skeletal, craniofacial, and cutaneous manifestations. LDS patients typically present with features including congenital heart defects, aneurysms, arterial tortuosity and dissections, skeletal overgrowth, cervical spine instability, clubfoot deformity, craniosynostosis, hypertelorism, bifid uvula, cleft palate, thin skin, and mental retardation. Dermal fibroblasts derived from LDS patients demonstrate impaired deposition of extracellular collagen and elastin, suggesting a possible mechanism of the connective tissue defects of the patients.479,480 However, the aortic tissues of LDS patients show increased accumulation of collagen, elevated expression of connective tissue growth factor (CTGF), and enhanced activity of non-mutant TGF-β signaling components.461,462,463,465,467,468,475,476,481,482 Therefore, primary downregulation and compensatory upregulation of TGF-β signaling are both responsible for the abnormalities of LDS.
由于纯合突变而导致的 TβRI 或 TβRII 功能丧失通常会因卵黄囊的造血和血管生成缺陷而导致小鼠胚胎死亡。 451 , 452然而,缺乏不同的 TGF-β 同工型可导致小鼠出现不同的表型,这与 TGF-β 在胚胎发育中的同工型特异性作用一致。尽管胚胎发育过程中卵黄囊的造血和血管生成存在缺陷,但 TGF-β1 敲除小鼠并未表现出明显的发育异常。 452、453、454相反, TGF-β2 敲除小鼠表现出围产期死亡率以及心脏、肺、骨骼、眼睛、内耳、颅面结构、泌尿生殖器官和毛囊的广泛发育缺陷。 290 , 455 , 456 , 457 , 458 TGF-β3 敲除小鼠也在出生后不久死亡,但除了腭裂和肺部发育异常外,没有显示出可检测到的异常。 459 , 460值得注意的是,在 TGF-β2 敲除小鼠中未能升高的腭架在 TGF-β3 敲除小鼠中经历升高,但仍然无法融合。 455 , 459 , 460此外,在 TGF-β2 敲除小鼠的肺部中表现正常的分支形态发生和呼吸道上皮细胞分化在 TGF-β3 敲除小鼠中却存在缺陷。455 , 459在人类中,单一 TGF-β 信号成分的功能丧失突变,例如TGF - β2、461、462、463 TGF- β3、464、465、466 TβRI 、 467、468、469 TβRII 、 470 、 471、472 SMAD2、473、474、475或SMAD3 476、477、478可引起Loeys-Dietz 综合征(LDS),一种常染色体显性结缔组织疾病,具有一系列心血管、骨骼、颅面和皮肤表现。 LDS 患者的典型特征包括先天性心脏缺陷、动脉瘤、动脉迂曲和夹层、骨骼过度生长、颈椎不稳定、马蹄内翻足畸形、颅缝早闭、距离过远、悬雍垂裂、腭裂、皮肤薄和智力低下。来自 LDS 患者的真皮成纤维细胞表现出细胞外胶原蛋白和弹性蛋白沉积受损,这表明患者结缔组织缺陷的可能机制。 479 , 480然而,LDS 患者的主动脉组织表现出胶原蛋白积累增加、结缔组织生长因子 (CTGF) 表达升高以及非突变 TGF-β 信号成分活性增强。461 , 462 , 463 , 465 , 467 , 468 , 475 , 476 , 481 , 482因此,TGF-β信号的原发性下调和代偿性上调均是LDS异常的原因。
Excessive TGF-β signaling can also act as a primary pathogenic factor in developmental defects. In mice, overexpression of TGF-β or SMAD can lead to developmental abnormalities in several tissues, such as skin,483,484 bones,485 eyes,486 lungs,487,488 mammary glands,489,490,491 salivary glands,492 and central nervous system.493 In humans, Camurati-Engelmann disease (CED), a progressive bone dysplasia inherited in an autosomal dominant manner, is ascribed to mutations of TGF-β1, which lead to increased TGF-β1 activation and signaling.494,495 This disease is characterized by hyperostosis and sclerosis of the long bones and the skull.496,497 Studies on CED have suggested that hyperactive TGF-β1 in the bone microenvironment can induce osteoclasts and osteoblasts to increase but cluster in separated areas, uncoupling bone resorption and formation to cause bone remodeling defects.494,498,499
过度的 TGF-β 信号传导也可能是发育缺陷的主要致病因素。在小鼠中,TGF-β 或 SMAD 的过度表达可导致多种组织发育异常,例如皮肤、 483、484骨骼、 485眼睛、 486肺、 487、488乳腺、 489、490、491唾液腺、 492和中枢神经系统。 493在人类中,卡穆拉蒂-恩格尔曼病 (CED) 是一种以常染色体显性遗传方式遗传的进行性骨发育不良,归因于 TGF-β1 突变,导致 TGF-β1 激活和信号转导增加。 494 , 495这种疾病的特点是长骨和颅骨骨质增生和硬化。 496 , 497 CED 研究表明,骨微环境中过度活跃的 TGF-β1 可诱导破骨细胞和成骨细胞增加,但聚集在不同的区域,使骨吸收和形成脱钩,导致骨重塑缺陷。 494 , 498 , 499
Aberrant healing and fibrotic diseases
异常愈合和纤维化疾病
Dysregulated TGF-β signaling can contribute to the tissue damage in aberrant healing and fibrotic diseases which are caused by all kinds of injuries such as wounding, burns, radiation, infection, and inflammation.
TGF-β信号传导失调可导致异常愈合和纤维化疾病中的组织损伤,这些损伤是由各种损伤(如受伤、烧伤、辐射、感染和炎症)引起的。
Aberrant healing 异常愈合
The lack of TGF-β and TβR expression is commonly found in the chronic wounds in patients, indicating that deficient TGF-β signaling may lead to wound chronicity and even unhealing.500,501,502,503,504,505 However, in vivo studies in mice have reported quite complicated findings. An activating mutation of TβRI can lead to a regenerative healing phenotype which enables rapid regeneration of normal tissues with differentiated structures instead of scar formation in ear punch wounds.506 Paradoxically, overexpression of TGF-β1 in keratinocytes accelerates the re-epithelialization in partial-thickness cutaneous wounds but slows that of full-thickness cutaneous wounds.507,508 In TGF-β1-deficient mice, the healing of full-thickness cutaneous wounds is initially normal but ultimately damaged by severe inflammatory diseases.509 In immunodeficient mice without inflammatory diseases, the lack of TGF-β1 still leads to significant delays in each healing stage of full-thickness cutaneous wounds.510 However, loss of TGF-β signaling in keratinocytes due to expression of dominant negative TβRII leads to increased proliferation and reduced apoptosis, thus facilitating the re-epithelialization in full-thickness cutaneous wounds.511 Furthermore, cutaneous wound healing is accelerated in mice lacking SMAD3 but is aberrant in mice lacking SMAD4 exclusively in keratinocytes.512,513
患者的慢性伤口中普遍存在TGF-β和TβR表达缺失,表明TGF-β信号传导缺陷可能导致伤口慢性化甚至不愈合。 500、501、502、503、504、505然而,小鼠体内研究报告了相当复杂的发现。 TβRI 的激活突变可导致再生愈合表型,使具有分化结构的正常组织能够快速再生,而不是在耳部穿孔伤口中形成疤痕。 506矛盾的是,角质形成细胞中 TGF-β1 的过度表达会加速部分皮层皮肤伤口的再上皮化,但会减慢全层皮肤伤口的再上皮化。 507 , 508在 TGF-β1 缺陷小鼠中,全层皮肤伤口的愈合最初是正常的,但最终会受到严重炎症性疾病的损害。 509在没有炎症性疾病的免疫缺陷小鼠中,TGF-β1 的缺乏仍然会导致全层皮肤伤口的每个愈合阶段显着延迟。 510然而,由于显性失活 TβRII 的表达,角质形成细胞中 TGF-β 信号传导丧失,导致增殖增加和细胞凋亡减少,从而促进全层皮肤伤口的上皮化。 511此外,在缺乏 SMAD3 的小鼠中,皮肤伤口愈合加速,但在角质形成细胞中完全缺乏 SMAD4 的小鼠中,皮肤伤口愈合却异常。 512 , 513
In contrast to chronic wounds, hypertrophic scars and keloids both characterized by overabundant ECM deposition are the results of hyperactive cutaneous wound healing. In fact, the expression of TGF-β and TβR which decreases eventually in normal cutaneous wounds remains elevated in hypertrophic scars and keloids.514,515,516,517,518 In contrast to normal cutaneous fibroblasts, both keloid fibroblasts and hypertrophic scar fibroblasts are significantly higher in collagen production, however, only keloid fibroblasts exhibit increased sensitivity to TGF-β stimulation.519 For keloid fibroblasts, overexpressed TGF-β can promote the resistance to apoptosis, the ability of proliferation, the conversion to MFs, and the expression of CTGF and VEGF, thus contributing to the ECM deposition, focal adhesion, fibrous growth, and angiogenesis in keloid tissues.518,520,521,522,523
与慢性伤口相反,肥厚性疤痕和疤痕疙瘩均以过量的 ECM 沉积为特征,是皮肤伤口愈合过度活跃的结果。事实上,在正常皮肤伤口中最终降低的 TGF-β 和 TβR 表达在肥厚性疤痕和疤痕疙瘩中仍然升高。 514、515、516、517、518与正常皮肤成纤维细胞相比,疤痕疙瘩成纤维细胞和肥厚性疤痕成纤维细胞的胶原蛋白生成显着较高,然而,只有疤痕疙瘩成纤维细胞表现出对 TGF-β 刺激的敏感性增加。 519对于瘢痕疙瘩成纤维细胞来说,过表达的 TGF-β 可促进其抗凋亡、增殖能力、向 MF 的转化以及 CTGF 和 VEGF 的表达,从而促进 ECM 沉积、粘斑、纤维生长和血管生成。疤痕组织。 518、520、521、522、523
Fibrotic diseases 纤维化疾病
Besides wounding, other forms of injurious stimulation can also cause excessive ECM deposition in different kinds of tissues, leading to fibrotic diseases, which are closely associated with the hyperactivity of TGF-β signaling.
除创伤外,其他形式的伤害性刺激也会导致不同组织中ECM过度沉积,导致纤维化疾病,这与TGF-β信号传导的过度活跃密切相关。
TGF-β expression is significantly elevated in fibrotic lungs in various cases such as idiopathic pulmonary fibrosis (IPF) and cystic fibrosis (CF).524,525,526,527,528 In situ hybridization and immunohistochemical staining suggest that alveolar macrophages and epithelial cells are likely the major sources of TGF-β which contribute to the fibrosis of lungs.526,527,528 In vitro studies show that TGF-β1 can trigger the EMT of alveolar epithelial cells and enhance the activity of lung fibroblasts to mediate fibrogenic effects.529,530,531,532 Transgenic expression of TGF-β1 in murine and rat lungs induces pulmonary fibrosis which is accompanied by alveolar EMT, MF differentiation, and mononuclear-rich inflammation.532,533,534,535 Interestingly, the suppression of TGF-β1, the deletion of TβRII, the ablation of SMAD3, the upregulation of SMAD7, but the administration of TGF-β3 can all significantly protect mice from experimentally induced pulmonary fibrosis.535,536,537,538,539
在各种情况下,例如特发性肺纤维化(IPF)和囊性纤维化(CF),TGF-β表达在纤维化肺中显着升高。 524、525、526、527、528原位杂交和免疫组织化学染色表明,肺泡巨噬细胞和上皮细胞可能是导致肺纤维化的 TGF-β 的主要来源。 526 , 527 , 528体外研究表明TGF-β1可以触发肺泡上皮细胞的EMT并增强肺成纤维细胞的活性以介导纤维化作用。 529 , 530 , 531 , 532小鼠和大鼠肺中 TGF-β1 的转基因表达会诱导肺纤维化,并伴有肺泡 EMT、MF 分化和富含单核细胞的炎症。 532、533、534、535有趣的是,TGF-β1 的抑制、TβRII 的缺失、SMAD3 的消除、SMAD7 的上调,但 TGF-β3 的施用都可以显着保护小鼠免受实验诱导的肺纤维化。 535、536、537、538、539
Similarly, the fibrotic kidneys of human glomerulonephritis, IgA nephropathy, diabetic nephropathy, lupus nephritis, as well as renal allografts in chronic rejection all show significant increases in three TGF-β isoforms in the glomeruli and tubulointerstitium where ECM deposition and PAI-1 production is closely related to the expression of TGF-β1 isoform in particular.540,541,542,543 In vitro, TGF-β1 stimulates kidney fibroblasts, mesangial cells, glomerular epithelial cells, and tubular epithelial cells to produce several ECM components and remodelers such as collagen, fibronectin, laminin, proteoglycan, MMP, and TIMP.544,545,546,547,548,549,550 TGF-β1 also contributes to the EMT induction and MF differentiation in renal fibrosis.550 Transgenic mice that have increased levels of TGF-β1 in plasma develop progressive renal disease characterized by glomerulosclerosis and tubulointerstitial fibrosis with TIMP overexpression and ECM deposition in sub-endothelial and mesangial locations.551,552
同样,人类肾小球肾炎、IgA 肾病、糖尿病肾病、狼疮性肾炎的纤维化肾脏以及慢性排斥反应中的肾同种异体移植物都显示出肾小球和肾小管间质中三种 TGF-β 同工型的显着增加,其中 ECM 沉积和 PAI-1 的产生是尤其与TGF-β1亚型的表达密切相关。 540、541、542、543在体外, TGF-β1 刺激肾成纤维细胞、系膜细胞、肾小球上皮细胞和肾小管上皮细胞产生多种 ECM 成分和重塑剂,例如胶原、纤连蛋白、层粘连蛋白、蛋白聚糖、MMP 和 TIMP。 544 , 545 , 546 , 547 , 548 , 549 , 550 TGF-β1 也有助于肾纤维化中的 EMT 诱导和 MF 分化。 550只血浆中 TGF-β1 水平升高的转基因小鼠出现进行性肾病,其特征是肾小球硬化和肾小管间质纤维化,伴有 TIMP 过度表达和 ECM 在内皮下和系膜位置沉积。 551、552
In fibrotic livers, TGF-β1 expression increases markedly with fibrogenic activity.553,554,555,556 Induction of TGF-β1 expression in murine livers leads to hepatic fibrosis characterized by prominent ECM deposition in peri-sinusoidal areas with activation of HSCs and apoptosis of hepatocytes.557,558 Notably, activated HSCs which play a major role in hepatic fibrosis can provide an important source of TGF-β,559 while overproduced TGF-β can in turn activate several signaling pathways such as those of SMAD, MEK, JNK, PI3K, and JAK/STAT in HSCs to contribute to their functions.236,237
在纤维化肝脏中,TGF-β1 表达随着纤维化活性显着增加。 553 , 554 , 555 , 556在小鼠肝脏中诱导 TGF-β1 表达会导致肝纤维化,其特征是在肝窦周围区域出现显着的 ECM 沉积,并伴有 HSC 激活和肝细胞凋亡。第557章、第558章HSC 中的 JAK/STAT 有助于发挥其功能。 236 , 237
As for the cardiovascular system, TGF-β is also elevated during myocardial fibrosis, valve fibrosis, and arteriosclerosis, generally attributed to the expression by SMCs, fibroblasts, endothelial cells, and inflammatory cells such as macrophages.560,561,562,563,564,565,566,567,568,569 On one hand, TGF-β can stimulate cardiovascular fibroblasts to differentiate into MFs and produce ECM components and remodelers,562,563,570,571,572,573 on the other hand, it can also stimulate endothelial cells to undergo EndMT to induce their fibrogenic phenotype.569,574,575
对于心血管系统,TGF-β在心肌纤维化、瓣膜纤维化和动脉硬化期间也会升高,通常归因于平滑肌细胞、成纤维细胞、内皮细胞和巨噬细胞等炎症细胞的表达。 560 , 561 , 562 , 563 , 564 , 565 , 566 , 567 , 568 , 569一方面,TGF-β可以刺激心血管成纤维细胞分化为MF并产生ECM成分和重塑剂, 562 , 563 , 570 , 571 , 572 , 573另一方面,它还可以刺激内皮细胞进行 EndMT,诱导其纤维化表型。 569、574、575
Furthermore, TGF-β is widely involved in the fibrosis of many other tissues and diseases as in the cases of cutaneous fibrosis,576,577 muscular fibrosis,578 pancreatic fibrosis,579,580,581,582 myelofibrosis,583,584 adenomyosis,585 autoimmune diseases,238,527,573,586,587,588,589 and infectious diseases.590,591,592,593
此外,TGF-β 广泛参与许多其他组织和疾病的纤维化,如皮肤纤维化、 576、577肌肉纤维化、 578胰腺纤维化、 579、580、581、582骨髓纤维化、 583、584子宫腺肌病、 585自身免疫性疾病、 238、527、573、586、587、588、589和传染病。 590、591、592、593
Inflammatory diseases and infectious diseases
炎症性疾病和传染病
Inflammatory diseases and infectious diseases can demonstrate aberrant immune responses and various tissue injuries which usually implicate the dysfunction of TGF-β signaling.
炎症性疾病和传染病可以表现出异常的免疫反应和各种组织损伤,这通常与 TGF-β 信号传导功能障碍有关。
Inflammatory diseases 炎症性疾病
Since TGF-β acts as a negative regulator to maintain immune homeostasis, deficient TGF-β signaling can lead to hyperactive immune responses, contributing to the pathology of numerous inflammatory diseases. TGF-β1-null mice initially appear normal after birth but soon develop a rapid wasting syndrome accompanied by a multifocal inflammatory disease which leads to organ failure and early death by 3-4 weeks of age.453,594,595,596 Many organs in these mice, including heart, lungs, stomach, liver, pancreas, and muscles, all exhibit massive infiltration of inflammatory cells such as lymphocytes, macrophages, and granulocytes. Moreover, their total numbers of blood leukocytes increase mainly due to the elevated absolute numbers of neutrophils and monocytes, while their levels of autoantibodies, MHC molecules, and inflammatory cytokines such as IFN-γ, TNF-α, and CCL3 also rise correspondingly in serum or tissues.
由于 TGF-β 作为维持免疫稳态的负调节因子,TGF-β 信号传导缺陷会导致免疫反应过度活跃,从而导致多种炎症性疾病的病理。 TGF-β1缺失的小鼠出生后最初表现正常,但很快就会出现快速消耗综合征,并伴有多灶性炎症性疾病,导致器官衰竭并在3-4周龄时过早死亡。 453 , 594 , 595 , 596这些小鼠的许多器官,包括心脏、肺、胃、肝脏、胰腺和肌肉,都表现出淋巴细胞、巨噬细胞和粒细胞等炎症细胞的大量浸润。此外,其血液白细胞总数增加主要是由于中性粒细胞和单核细胞绝对数升高,而血清中自身抗体、MHC分子以及IFN-γ、TNF-α、CCL3等炎性细胞因子的水平也相应升高或纸巾。
In the absence of any pathogens, the inflammatory diseases in TGF-β1-knockout mice actually resemble a special group of inflammatory diseases known as autoimmune diseases, which are characterized by dysregulated immune responses attacking self-tissues. In fact, even cell type-specific loss of TGF-β signaling can lead to the development of various autoimmune diseases in mice.597,598,599,600,601,602,603,604 In patients with autoimmune diseases such as systemic lupus erythematosus (SLE),605,606,607 systemic sclerosis (SSc),608,609,610,611 rheumatoid arthritis (RA),612,613,614 Sjögren’s syndrome,586,614,615,616 Crohn’s disease,587,617,618,619 ulcerative colitis (UC),617,618,619,620,621,622 autoimmune hepatitis (AIH),623,624 and Hashimoto’s thyroiditis (HT),606,625,626 the levels of TGF-β or TβR in tissues or circulation are associated with the presence, activity, and severity of the diseases. Notably, although all these diseases show correlations with dysregulated TGF-β signaling, their correlations with TGF-β levels can be either positive or negative. Some cases of the diseases are likely caused by insufficient TGF-β expression and thus exhibit decreased TGF-β production.619,622,627,628,629,630 In other cases, however, the autoimmune inflammation is likely attributed to impaired cell responsiveness to TGF-β especially due to deficient TβR functions, therefore, TGF-β production is elevated as a compensatory response.624,628,631,632,633,634,635
在没有任何病原体的情况下,TGF-β1敲除小鼠的炎症性疾病实际上类似于一组特殊的炎症性疾病,称为自身免疫性疾病,其特征是攻击自身组织的免疫反应失调。事实上,即使细胞类型特异性的 TGF-β 信号传导丧失也可能导致小鼠患上各种自身免疫性疾病。 597 , 598 , 599 , 600 , 601 , 602 , 603 , 604患有自身免疫性疾病的患者,例如系统性红斑狼疮(SLE), 605 , 606 , 607系统性硬化症(SSc), 608 , 609 , 610 , 611类风湿性关节炎( RA) 、 612、613、614干燥综合征、 586、614、615、616克罗恩病、 587、617、618、619溃疡性结肠炎( UC ) 、 617、618、619、620、621、622小鼠肝炎 (AIH) 、 623 、 624和桥本甲状腺炎(HT) 606 、 625 、 626组织或循环中TGF-β 或TβR 的水平与疾病的存在、活动性和严重程度相关。值得注意的是,尽管所有这些疾病都与 TGF-β 信号传导失调相关,但它们与 TGF-β 水平的相关性可以是正相关,也可以是负相关。 某些疾病病例可能是由 TGF-β 表达不足引起的,因此表现出 TGF-β 产生减少。 619 , 622 , 627 , 628 , 629 , 630然而,在其他情况下,自身免疫性炎症可能归因于细胞对 TGF-β 的反应性受损,特别是由于 TβR 功能缺陷,因此,作为补偿反应,TGF-β 的产生升高。624、628、631、632、633、634、635
Allergic diseases, including asthma, allergic rhinitis, food allergy, and atopic dermatitis, are another group of inflammatory diseases that are caused by aberrant immune responses to harmless environmental antigens. TGF-β production is increased in the airways and serum of asthmatic patients and is further increased after allergen exposure, disease progression, or certain treatments.636,637,638,639,640,641,642,643,644,645,646 Bronchial epithelial cells, fibroblasts, SMCs, eosinophils, neutrophils, and macrophages can all contribute to the excessive TGF-β production in asthmatic patients.641,642,643,644,645,646,647,648,649,650 However, the functions of TGF-β are seemingly contradictory in the context of allergic airway inflammation, for TGF-β can either enhance or suppress the activity of eosinophils, lymphocytes, macrophages, and mast cells in asthma.648,651,652,653,654,655,656,657,658,659,660,661 Nevertheless, it is clear that TGF-β can promote asthmatic airway remodeling by inducing airway EMT,662,663 ECM production,649,650 MF differentiation,664,665 and smooth muscle hyperplasia.647 In patients with allergic rhinitis, TGF-β levels in serum are found dependent on allergen exposure, while TGF-β and TβR expression in nasal mucosa is noticed correlated with intra-epithelial mast cell abundance.666,667,668 In fact, allergen challenge can activate TGF-β signaling in the mast cells and epithelial cells in nasal mucosa which may contribute to the mast cell accumulation and goblet cell hyperplasia in allergic rhinitis.669,670 Allergen challenge can also induce the loss of TGF-β1-expressing Bregs and Tregs which function to suppress the inflammatory Th2 responses of allergic rhinitis. However, with prolonged challenging time, the proportion of TGF-β1-expressing Bregs and Tregs can gradually recover to reconstitute the immune homeostasis in nasal mucosa.671 Similarly, TGF-β can inhibit the Th2 responses of food allergy by promoting Treg activity in the intestines.603,672,673 Therefore, reduced TGF-β1 expression in the intestinal epithelial cells and mononuclear cells of patients with food allergy can partially account for the development of the disease.603,674 Moreover, TGF-β can inhibit the pathology of atopic dermatitis by suppressing B cell maturation, mast cell activation, eosinophil infiltration, as well as the secretion of IgE, TNF-α, and histamine by those cells.675,676,677 Aberrant TGF-β expression or attenuated cell responsiveness discovered in patients with atopic dermatitis may play a key role in the disorder.678,679,680
过敏性疾病,包括哮喘、过敏性鼻炎、食物过敏和特应性皮炎,是另一类由对无害环境抗原的异常免疫反应引起的炎症性疾病。哮喘患者气道和血清中 TGF-β 的产生增加,并且在接触过敏原、疾病进展或某些治疗后进一步增加。 636 , 637 , 638 , 639 , 640 , 641 , 642 , 643 , 644 , 645 , 646支气管上皮细胞、成纤维细胞、SMC、嗜酸性粒细胞、中性粒细胞和巨噬细胞均可导致哮喘患者产生过多的 TGF-β。 641 , 642 , 643 , 644 , 645 , 646 , 647 , 648 , 649 , 650然而,TGF-β 的功能在过敏性气道炎症中似乎是矛盾的,因为 TGF-β 可以增强或抑制 TGF-β 的活性。哮喘中的嗜酸性粒细胞、淋巴细胞、巨噬细胞和肥大细胞。 648 , 651 , 652 , 653 , 654 , 655 , 656 , 657 , 658 , 659 , 660 , 661然而,很明显 TGF-β 可以通过诱导气道 EMT 来促进哮喘气道重塑, 662 , 663 ECM 产生, 649 ,第650章MF分化,第664章,第665章和平滑肌增生。647在过敏性鼻炎患者中,血清中的 TGF-β 水平取决于过敏原暴露,而鼻粘膜中的 TGF-β 和 TβR 表达与上皮内肥大细胞丰度相关。 666 , 667 , 668事实上,过敏原攻击可以激活鼻粘膜肥大细胞和上皮细胞中的 TGF-β 信号传导,这可能有助于过敏性鼻炎中肥大细胞的积累和杯状细胞增生。 669 , 670过敏原攻击还可以诱导表达 TGF-β1 的 Breg 和 Tregs 的丧失,这些 Breg 和 Tregs 的功能是抑制过敏性鼻炎的炎症 Th2 反应。然而,随着攻击时间的延长,表达TGF-β1的Bregs和Tregs的比例可以逐渐恢复,从而重建鼻粘膜的免疫稳态。 671同样,TGF-β 可以通过促进肠道中的 Treg 活性来抑制食物过敏的 Th2 反应。 603 , 672 , 673因此,食物过敏患者肠上皮细胞和单核细胞中 TGF-β1 表达的减少可以部分解释该疾病的发展。 603 , 674此外,TGF-β 可以通过抑制 B 细胞成熟、肥大细胞活化、嗜酸性粒细胞浸润以及这些细胞分泌 IgE、TNF-α 和组胺来抑制特应性皮炎的病理。 675 , 676 , 677在特应性皮炎患者中发现的异常 TGF-β 表达或减弱的细胞反应性可能在该疾病中发挥关键作用。678 , 679 , 680
Furthermore, TGF-β signaling is implicated in the pathology of other inflammatory diseases and inflammation-related diseases such as bronchitis,642 pancreatitis,681,682,683 glomerulonephritis,684,685 osteomyelitis,686 arthritis,687 diabetes,688 and Alzheimer’s disease (AD).689,690
第642章胰腺炎,第681章,第682章,第683章肾小球肾炎,第684章,第685章685骨髓炎,第686章关节炎,第687章糖尿病,第688章和阿尔茨海默病(第642章)广告)。 689 , 690
Infectious diseases 传染病
Infectious diseases caused by different kinds of pathogenic organisms can result in tissue damage due to diverse pathogen virulence and dysregulated host responses.
由不同种类的病原生物引起的传染病可由于不同的病原体毒力和宿主反应失调而导致组织损伤。
TGF-β can function to reduce pathogen burdens as well as tissue injuries in some cases of infection. In patients with H1N1 influenza A virus sepsis, blood TGF-β levels are negatively correlated with clinical severity scores on admission.691 Consistently, increased TGF-β activity in mice confers resistance against lethal influenza infection due to reductions in both viral titers and pulmonary inflammation.692,693 TGF-β expression also prevents mice from coxsackievirus-induced myocarditis and type 1 diabetes in a Treg-dependent manner.694,695 Moreover, TGF-β acts as a pro-survival factor to protect murine neurons and intestinal epithelial cells against cell death during reovirus infection.696,697 As for bacterial infection, TGF-β can attenuate sepsis-induced tissue injuries through mechanisms involving the induction of Tregs.698 It also enhances the pathogen clearance and host resistance of mice during the infection of Streptococcus pneumoniae,699 Streptococcus pyogenes,700 Listeria monocytogenes,701 and Yersinia enterocolitica,702 likely, by suppressing IFN-γ, TNF-α, and IL-6 production while promoting Th17 and Treg responses. In rats with pulmonary cryptococcosis, TGF-β reduces fungal burdens by promoting the lysozyme secretion of macrophages, meanwhile, it also limits inflammation by inhibiting macrophage phagocytosis, chemokine production, and oxidative burst.703 Moreover, TGF-β can be protective during parasitic infection. The lack of TGF-β exacerbates the severity of murine malaria infection, whereas TGF-β treatment, in contrast, suppresses plasmodium proliferation and prolongs mice survival with decreased TNF-α and increased IL-10 in serum.704 During Trypanosoma congolense infection, exogenous TGF-β1 confers early protection against parasitemia, anemia, splenomegaly, and mortality due to enhanced macrophage activity and Th1 responses which are characterized by increased NO, IFN-γ, TNF-α, IL-12, and IgG2a production.705 During Toxoplasma infection, TGF-β can prevent tissue damage by reducing inflammatory cell infiltration and cytokine production, while it can also improve the outcomes of infection-related abnormal pregnancy by promoting Treg functions and suppressing NK cytotoxicity.706,707,708,709 Furthermore, TGF-β can prevent the lung injuries during hookworm infection by inducing the immunosuppressive activity of myeloid cells to reduce Th2 responses.710
TGF-β 可以减少病原体负担以及某些感染病例中的组织损伤。在患有 H1N1 甲型流感病毒脓毒症的患者中,血液 TGF-β 水平与入院时的临床严重程度评分呈负相关。 691一致地,由于病毒滴度和肺部炎症的减少,小鼠体内 TGF-β 活性的增加赋予了对致命性流感感染的抵抗力。 692 , 693 TGF-β 表达还以 Treg 依赖性方式预防小鼠柯萨奇病毒诱导的心肌炎和 1 型糖尿病。 694 , 695此外,TGF-β 作为一种促生存因子,可保护小鼠神经元和肠上皮细胞在呼肠孤病毒感染期间免遭细胞死亡。 696 , 697对于细菌感染,TGF-β 可以通过诱导 Tregs 的机制减轻脓毒症引起的组织损伤。第698章还可以通过抑制IFN-γ、TNF-α和IL-6的产生,增强小鼠感染肺炎链球菌、化脓性链球菌、700单核细胞增生李斯特菌、 701和小肠结肠炎耶尔森菌、 702可能的病原体清除率和宿主抵抗力同时促进 Th17 和 Treg 反应。在患有肺隐球菌病的大鼠中,TGF-β通过促进巨噬细胞溶菌酶的分泌来减轻真菌负荷,同时还通过抑制巨噬细胞的吞噬作用、趋化因子的产生和氧化爆发来限制炎症。703此外,TGF-β 在寄生虫感染期间可以发挥保护作用。 TGF-β 的缺乏会加剧小鼠疟疾感染的严重性,而 TGF-β 治疗则相反,可抑制疟原虫增殖并延长小鼠的存活时间,同时血清中的 TNF-α 减少,IL-10 增加。 704在刚果锥虫感染期间,外源性 TGF-β1 可提供早期保护,防止寄生虫血症、贫血、脾肿大和死亡,因为巨噬细胞活性和 Th1 反应增强,其特点是 NO、IFN-γ、TNF-α、IL-12 和IgG2a 的产生。 705在弓形虫感染期间,TGF-β 可以通过减少炎症细胞浸润和细胞因子产生来预防组织损伤,同时还可以通过促进 Treg 功能和抑制 NK 细胞毒性来改善感染相关的异常妊娠的结局。 706 , 707 , 708 , 709此外,TGF-β 可以通过诱导骨髓细胞的免疫抑制活性来减少 Th2 反应,从而预防钩虫感染期间的肺损伤。710
In other cases of infection, however, TGF-β can turn to facilitate pathogen infection and tissue injuries. In clinical patients, circulating TGF-β1 levels are positively correlated with the severity and mortality of severe community-acquired pneumonia (CAP)711 and sepsis-induced acute respiratory distress syndrome (ARDS).712 Increased TGF-β production can impair the anti-bacterial functions of neutrophils, uncouple the cytokine production and glycolysis of macrophages, and suppress the IL-2 expression and proliferation of T cells to participate in the pathology of sepsis.713,714,715 As for bacterial infection in local tissues, on one hand, TGF-β can upregulate fibronectin and integrins in hosts to promote bacterial adhesion and invasion,716,717 on the other hand, it can attenuate anti-infectious innate responses and Th1 responses while inducing immunotolerant Treg responses to facilitate the immune escape of the pathogens.718,719,720,721,722 TGF-β-mediated immunosuppression can also contribute to viral infection, as elevated TGF-β expression during viral infection not only impairs early innate immunity such as IFN responses, NK functions, and macrophage activity but also suppresses the adaptive immune responses of T cells and B cells.428,723,724,725,726,727,728,729,730,731 Notably, TGF-β can also enhance viral infection through certain pathogen-specific mechanisms as in the cases of human immunodeficiency virus type 1 (HIV-1) infection,732,733,734 human T-cell leukemia virus type I (HTLV-I) infection,735 hepatitis C virus (HCV) infection,736 Zika virus (ZIKV) infection,737 as well as rubella virus (RuV) infection.738 Furthermore, TGF-β can promote the survival and growth of parasites in hosts through downregulation of NO, IFN-γ, TNF-α, IL-6, IL-17, and Th17 cells as well as upregulation of IL-4, IL-10, and Treg cells, contributing to the infection of Fasciola hepatica,739 Echinococcus multilocularis,740 Toxoplasma gondii,741 Leishmania,742 and Plasmodium.743,744
然而,在其他感染情况下,TGF-β 可能会促进病原体感染和组织损伤。在临床患者中,循环TGF-β1水平与严重社区获得性肺炎(CAP) 711和脓毒症引起的急性呼吸窘迫综合征(ARDS)的严重程度和死亡率呈正相关。 712 TGF-β 产生增加可损害中性粒细胞的抗菌功能,解开巨噬细胞的细胞因子产生和糖酵解作用,并抑制 T 细胞的 IL-2 表达和增殖,从而参与脓毒症的病理。 713 , 714 , 715对于局部组织的细菌感染,TGF-β一方面可以上调宿主体内的纤连蛋白和整合素,促进细菌粘附和侵袭, 716 , 717另一方面,可以减弱抗感染的先天反应和 Th1 反应,同时诱导免疫耐受的 Treg 反应,以促进病原体的免疫逃逸。 718 , 719 , 720 , 721 , 722 TGF-β 介导的免疫抑制也可能导致病毒感染,因为病毒感染期间 TGF-β 表达升高不仅损害早期先天免疫,如 IFN 反应、NK 功能和巨噬细胞活性,而且抑制 T 细胞和 B 细胞的适应性免疫反应。428 , 723 , 724 , 725 , 726 , 727 , 728 , 729 , 730 , 731值得注意的是,TGF-β 还可以通过某些病原体特异性机制增强病毒感染,例如人类免疫缺陷病毒 1 型 (HIV-1)第732章733 、第734章人类T细胞白血病病毒I型(HTLV-I)感染、第735章丙型肝炎病毒(HCV)感染、第736章寨卡病毒(ZIKV)感染、第737章以及风疹病毒(RuV)感染。 738此外,TGF-β 可以通过下调 NO、IFN-γ、TNF-α、IL-6、IL-17 和 Th17 细胞以及上调 IL-4、IL,促进宿主体内寄生虫的存活和生长。第739章多房棘球绦虫,第740章弓形虫,第741章利什曼原虫,第742章和疟原虫。743 , 744
Tumors 肿瘤
It is generally accepted that TGF-β acts as a tumor suppressor during the early stages of tumorigenesis but turns into a tumor promotor at later stages of tumor development.
人们普遍认为,TGF-β在肿瘤发生的早期阶段充当肿瘤抑制因子,但在肿瘤发展的后期变成肿瘤促进因子。
Tumorigenesis 肿瘤发生
Evidence from animal models firmly establishes the suppressor role of TGF-β signaling in early tumorigenesis. TGF-β and its receptors can be strongly induced in the murine epidermis upon exposure to carcinogens that tend to disrupt tissue homeostasis and cause oncogenic transformation.745,746 Increased TGF-β expression in murine epidermis can potently attenuate cell proliferation and confer resistance to hyperproliferation induced by carcinogens.316,746,747 Similarly, in murine mammary epithelia, the overexpression of TGF-β or TβR can result in remarkable protection from carcinogen-induced tumorigenesis with reduced premalignant lesions, prolonged tumor latency, and decreased cancer incidence.25,491,748,749,750 Such tumor-inhibitory effects by TGF-β signaling are attributed to the early apoptosis of differentiating cells and, more importantly, the premature senescence of stem cells which reduces the reproductive capacity of the mammary epithelia and thus decreases the frequency with which transforming mutations may occur and be fixed in the cell population.491,748
来自动物模型的证据明确证实了 TGF-β 信号传导在早期肿瘤发生中的抑制作用。当小鼠接触致癌物质时,TGF-β及其受体会在小鼠表皮中被强烈诱导,从而破坏组织稳态并导致致癌转化。 745 , 746小鼠表皮中 TGF-β 表达的增加可以有效减弱细胞增殖并赋予对致癌物诱导的过度增殖的抵抗力。 316 , 746 , 747同样,在小鼠乳腺上皮中,TGF-β 或 TβR 的过度表达可以显着防止致癌物诱导的肿瘤发生,减少癌前病变,延长肿瘤潜伏期,并降低癌症发病率。 25 , 491 , 748 , 749 , 750 TGF-β 信号传导的这种肿瘤抑制作用归因于分化细胞的早期凋亡,更重要的是干细胞的过早衰老,从而降低了乳腺上皮的生殖能力,从而降低细胞群中可能发生并固定的转化突变的频率。 491 , 748
In contrast, loss of TGF-β signaling can be an early event that contributes to tumorigenesis. In clinical patients, heterogeneous patterns of TβRII expression in normal breast lobular units as well as loss of TβRII expression in breast epithelial hyperplastic lesions are both associated with increased risks of invasive breast cancer.751 More convincing evidence is provided by germline mutations of TGF-β signaling components which show strong correlations with increased risks of tumorigenesis. Loss-of-function TβRI mutations can result in an autosomal dominant skin cancer condition known as multiple self-healing squamous epithelioma (MSSE) or Ferguson-Smith disease (FSD) which is characterized by multiple squamous-carcinoma-like skin tumors that invade locally and then regress spontaneously after several months.752,753 Inactivating TβRII mutations are considered causative of some cases of hereditary nonpolyposis colorectal cancer (HNPCC) or Lynch syndrome, an autosomal dominant cancer predisposition syndrome, by impairing cell growth inhibition in response to TGF-β.754 Moreover, germline mutations of SMAD4 are responsible for juvenile polyposis, an autosomal dominant syndrome predisposing to gastrointestinal hamartomatous polyps and cancers.755,756 Mechanically, impaired TGF-β signaling can cause serious disturbance to tissue homeostasis, thus largely facilitating the development of pre-neoplastic lesions, as well as subsequent tumors, as shown in different murine tissues with deficiencies in the activity of TGF-β,757,758 TβR,749,759,760,761,762,763,764,765,766,767,768,769,770 or SMAD.657,771,772,773,774,775,776 Among them, TβR-deleted murine epithelia exhibit significant reductions in p15 and p21 and remarkable increases in MYC expression and RAS/ERK signaling, accompanied by elevated cell proliferation, reduced cell apoptosis, and enhanced cell malignant transformation to become tumorigenic.762,763,764,765
相反,TGF-β信号传导的丧失可能是导致肿瘤发生的早期事件。在临床患者中,正常乳腺小叶单位中 TβRII 表达的异质性以及乳腺上皮增生性病变中 TβRII 表达的缺失均与浸润性乳腺癌风险增加相关。 751 TGF-β 信号成分的种系突变提供了更有说服力的证据,这些突变与肿瘤发生风险增加密切相关。功能丧失的 TβRI 突变可导致常染色体显性皮肤癌,称为多发性自愈性鳞状上皮瘤 (MSSE) 或弗格森-史密斯病 (FSD),其特征是局部侵袭的多发性鳞状癌样皮肤肿瘤然后几个月后自然消退。 752 , 753 TβRII 失活突变被认为是某些遗传性非息肉病性结直肠癌 (HNPCC) 或 Lynch 综合征(一种常染色体显性癌症易感综合征)病例的病因,其原因是损害对 TGF-β 的细胞生长抑制。 754此外,SMAD4 的种系突变导致幼年性息肉病,这是一种易患胃肠道错构瘤性息肉和癌症的常染色体显性综合征。755 , 756从机制上讲,受损的 TGF-β 信号传导可导致组织稳态严重紊乱,从而在很大程度上促进肿瘤前病变以及后续肿瘤的发展,如不同 TGF-β 活性缺陷的小鼠组织所示、 757、758TβR 、 749、759、760、761、762、763、764、765、766、767、768、769、770或SMAD 。 657 , 771 , 772 , 773 , 774 , 775 , 776其中,TβR 缺失的小鼠上皮表现出 p15 和 p21 显着减少,MYC 表达和 RAS/ERK 信号显着增加,伴随着细胞增殖增加,细胞凋亡减少,并增强细胞恶性转化以产生致瘤性。762 , 763 , 764 , 765
Furthermore, TGF-β can provide additional protection against tumorigenesis by controlling pathogen infection,777 inhibiting excessive inflammation,778,779,780,781 reducing genomic instability,782 inducing replicative senescence,783 and regulating epithelial-mesenchymal interaction.784
此外,TGF-β 可以通过控制病原体感染、 777抑制过度炎症、 778、779、780、781减少基因组不稳定性、 782诱导复制性衰老、 783和调节上皮-间质相互作用来提供针对肿瘤发生的额外保护。第784章
Tumor growth 肿瘤生长
TGF-β can inhibit tumor growth by triggering cytostasis and apoptosis through similar mechanisms as it does in cells from normal tissues. In tumor cells, TGF-β signaling induces cell cycle arrest by targeting effectors, such as p15,354,356 p21,355,785,786 p27,361 MYC,363 ID,787 and CDC25A,374,786 while it also induces apoptotic cell death through effectors including CTGF,788 programmed cell death 4 (PDCD4),789 Fas receptor,790 death-associated protein kinase (DAPK),791 DAXX,383 IκB-α,384,386 sex-determining region Y (SRY)-box 4 (SOX4),792 ARTS,382 TIEGs,793 as well as several BCL-2 family members.794,795,796,797,798,799,800 Consistently, primary tumors induced from murine tissues with intact TGF-β signaling pathways are initially responsive to TGF-β-mediated inhibitory effects.491,749,759,764,801,802
TGF-β 可以通过与正常组织细胞中类似的机制触发细胞停滞和细胞凋亡来抑制肿瘤生长。在肿瘤细胞中,TGF-β信号传导通过靶向效应子(例如p15、354、356 p21、355、785、786 p27、361 MYC 、 363 ID、 787和 CDC25A、 374、786 )诱导细胞周期停滞,同时还诱导细胞凋亡第788章程序性细胞死亡4(PDCD4)、第789章Fas受体、第790章死亡相关蛋白激酶(DAPK)、第791章DAXX、第383章IκB-α、第384章、第386章box 4 (SOX4)、 792 ARTS、 382 TIEG、 793以及几个 BCL-2 家族成员。 794 , 795 , 796 , 797 , 798 , 799 , 800一致地,由具有完整 TGF-β 信号通路的小鼠组织诱导的原发性肿瘤最初对 TGF-β 介导的抑制作用有反应。 491、749、759、764、801、802
On the contrary, deficient TGF-β signaling can potently promote the growth of tumors. The downregulation of tumor TGF-β signaling in many cases is attributed to reduced expression or inactivating mutations of TβR or SMAD, as shown in various tumor types such as leukemia,772 lymphoma,803,804 esophageal cancer,805,806,807 gastric cancer,808 colorectal cancer,30,807,809,810,811 pancreatic cancer,32,812,813 biliary cancer,812 ampullary cancer,814 thyroid cancer,815 prostate cancer,816,817 breast cancer,818 ovarian cancer,819 endometrial cancer,808 genital squamous cell carcinomas (SCC),764 head and neck SCC,820,821,822,823 etc. These changes are able to confer resistance to the tumor-inhibitory effects of TGF-β. In mouse models, tumors developed from tissues with deletion or inactivation of TβR exhibit increased cell proliferation and decreased cell apoptosis, accompanied by reduction in p15, p21, and p27, the elevation of MYC, cyclin D1, and epidermal growth factor receptor (EGFR), as well as activation of STAT3 and PI3K/AKT pathways.761,762,763,764,765 Interestingly, reconstituted expression of TβRII in tumor cells with corresponding deficiency not only restores the inhibitory responses to TGF-β but also significantly attenuates the tumorigenicity of these cells.824
相反,TGF-β信号传导缺陷可以有效促进肿瘤的生长。在许多情况下,肿瘤 TGF-β 信号传导的下调归因于 TβR 或 SMAD 的表达减少或失活突变,如多种肿瘤类型所示,如白血病、 772淋巴瘤、 803 、 804食管癌、 805 、 806 、 807胃癌, 808结直肠癌, 30 , 807 , 809 , 810 , 811胰腺癌, 32 , 812 , 813胆道癌, 812壶腹癌, 814甲状腺癌, 815前列腺癌, 816 , 817乳腺癌, 818卵巢癌, 819子宫内膜癌, 808生殖器鳞状细胞癌 (SCC), 764头颈 SCC, 820 , 821 , 822 , 823等。这些变化能够赋予对 TGF-β 肿瘤抑制作用的抵抗力。在小鼠模型中,由 TβR 缺失或失活的组织发展而来的肿瘤表现出细胞增殖增加和细胞凋亡减少,并伴有 p15、p21 和 p27 减少,MYC、细胞周期蛋白 D1 和表皮生长因子受体 (EGFR) 升高以及 STAT3 和 PI3K/AKT 通路的激活。761 , 762 , 763 , 764 , 765有趣的是,在具有相应缺陷的肿瘤细胞中重建 TβRII 表达不仅恢复了对 TGF-β 的抑制反应,而且还显着减弱了这些细胞的致瘤性。第824章
Notably, TGF-β can fail to suppress the growth of tumors where there is likely no loss of functional TGF-β signaling components, and even formerly inhibited tumor cells can subsequently resume proliferating in vitro and develop larger tumor masses in vivo.354,825,826 On one hand, such resistance may result from the dysfunction of the downstream targets of TGF-β signaling such as CKIs.354 On the other hand, the tumor-suppressive signaling of TGF-β can be offset or interfered by enhanced I-SMAD activity827 or potent oncogenic factors such as E1A,828,829 EVI1,148,830 SKI,150,152 SNO,153 MYC,831 ID2,832 mutant p53,833 as well as RAS/RAF/ERK signaling.162,834 Moreover, TGF-β-mediated tumor-promoting effects can also account for the enhanced tumor growth in vivo, as discussed in a later section.
值得注意的是,在功能性 TGF-β 信号成分可能没有丧失的情况下,TGF-β 可能无法抑制肿瘤的生长,甚至以前被抑制的肿瘤细胞随后也可以在体外恢复增殖并在体内形成更大的肿瘤块。 354 , 825 , 826一方面,这种抵抗可能是由于 TGF-β 信号下游靶点(例如 CKI)的功能障碍造成的。 354另一方面,TGF-β 的肿瘤抑制信号传导可被增强的 I-SMAD 活性抵消或干扰827或强效致癌因子,如 E1A、 828、829 EVI1、148、830 SKI 、 150、152 SNO 、第153章MYC,第831章ID2,第832章突变体p53,第833章以及RAS/RAF/ERK信号传导。 162 , 834此外,TGF-β 介导的肿瘤促进作用也可以解释体内肿瘤生长增强的原因,如后面部分所述。
Tumor invasion and metastasis
肿瘤侵袭和转移
Contrary to its role as a suppressor of tumor growth, TGF-β generally acts as a promoter of tumor invasion and metastasis especially in advanced tumors. Upregulation of TGF-β as well as its receptors is associated with disease progression and poor prognosis in some patients with tumors such as breast cancer,835,836 pancreatic cancer,837,838 and gastric cancer.839 Consistently, TGF-β overexpression or pre-treatment enables tumor cells to form increased metastases in vivo,825,840 while loss of TGF-β responsiveness due to the introduction of dominant negative TβRII decreases the metastatic efficiency of high-grade tumor cells.841,842 Moreover, tumors derived from transgenic murine epithelia that overexpress TGF-β or TβR are significantly more malignant and more invasive.491,749,750,802,843 Notably, these TGF-β-overexpressing tumor cells are more likely to undergo the transition from epithelial cell phenotype into spindle cell phenotype which is the most malignant and invasive cell type.802,843 This indicates that TGF-β can facilitate the progression of epithelial-derived tumors through the induction of EMT which is inoperative in tumors with deficiencies in TβR or SMAD.761,770,774,842,843 Similar to the EMT of normal cells, TGF-β-induced EMT of tumor cells is characterized by changes in keratin, integrin, cadherin, catenin, claudin, vimentin, occludin, fibronectin, and MMP expression which can contribute to the invasive and metastatic capacity of tumors.197,198,200,203,230,232,750,774,802,843,844,845,846,847,848
与其作为肿瘤生长抑制剂的作用相反,TGF-β通常充当肿瘤侵袭和转移的促进者,尤其是在晚期肿瘤中。 TGF-β及其受体的上调与某些肿瘤患者的疾病进展和不良预后相关,例如乳腺癌、 835、836胰腺癌、 837、838和胃癌。 839一致地,TGF-β 过度表达或预处理使肿瘤细胞在体内形成增加的转移, 825、840而由于显性失活 TβRII 的引入而导致 TGF-β 反应性丧失,从而降低了高级肿瘤细胞的转移效率。 841 , 842此外,源自过度表达 TGF-β 或 TβR 的转基因鼠上皮的肿瘤明显更恶性且更具侵袭性。 491 , 749 , 750 , 802 , 843值得注意的是,这些TGF-β过表达的肿瘤细胞更有可能从上皮细胞表型转变为梭形细胞表型,梭形细胞表型是最具恶性和侵袭性的细胞类型。 802 , 843这表明 TGF-β 可以通过诱导 EMT 来促进上皮源性肿瘤的进展,而 EMT 在 TβR 或 SMAD 缺陷的肿瘤中不起作用。761 , 770 , 774 , 842 , 843与正常细胞的 EMT 类似,TGF-β 诱导的肿瘤细胞 EMT 的特点是角蛋白、整合素、钙粘蛋白、连环蛋白、claudin、vimentin、occludin、纤连蛋白和 MMP 表达的变化这有助于肿瘤的侵袭和转移能力。197、198、200、203、230、232、750、774、802、843、844、845、846、847、848
However, loss of functional TGF-β signaling components can occur in tumor cells during disease progression.759,809 In fact, reduced TGF-β signaling can also contribute to tumor invasion and metastasis. For some patients, decreased expression of TβR is correlated with higher tumor grades, later clinical stages, and worse clinical prognosis.805,816,818 A large number of cell models and mouse models also demonstrate that tumors lacking TGF-β signaling tend to be more malignant and more aggressive.758,760,761,762,764,801,841,843,849,850,851,852 Relevant mechanisms in these cases involve the loss of E-cadherin,761 the reduction in PAI,849 the increase in RHO/RAC signaling,843 the activation of integrin/focal adhesion kinase (FAK)/SRC/MAPK pathway,764 and more importantly, the overexpression of various pro-invasive and pro-metastatic factors. In mouse models, deficient TGF-β signaling can stimulate tumor cells and stromal cells to produce high levels of TGF-β and other tumor-promoting factors such as CTGF, VEGF, IL-1β, C-X-C motif chemokine ligand (CXCL8), CXCL12, cyclooxygenase(COX)-2, MMPs, collagen, and tenascin C (TNC) which can strongly promote tumor angiogenesis, fibroblasts activation, immune infiltration, and ECM remodeling.760,761,762,763,764,774,843,850
然而,在疾病进展过程中,肿瘤细胞中可能会出现功能性 TGF-β 信号传导成分的丧失。 759 , 809事实上,TGF-β 信号传导减少也可能导致肿瘤侵袭和转移。对于某些患者来说,TβR 表达降低与较高的肿瘤分级、较晚的临床分期和较差的临床预后相关。 805 , 816 , 818大量细胞模型和小鼠模型也证明缺乏TGF-β信号传导的肿瘤往往更恶性且更具侵袭性。 758、760、761、762、764、801、841、843、849、850、851、852这些病例的相关机制涉及E-钙粘蛋白的丢失、 761 PAI 的减少、 849 RHO/RAC 信号传导的增加第843章整合素/粘着斑激酶(FAK)/SRC/MAPK通路的激活,第764章更重要的是,各种促侵袭和促转移因子的过度表达。在小鼠模型中,TGF-β信号传导缺陷可以刺激肿瘤细胞和基质细胞产生高水平的TGF-β和其他促肿瘤因子,如CTGF、VEGF、IL-1β、CXC基序趋化因子配体(CXCL8)、CXCL12、环加氧酶(COX)-2、MMP、胶原蛋白和肌腱蛋白C(TNC)能够强烈促进肿瘤血管生成、成纤维细胞活化、免疫浸润和ECM重塑。 760、761、762、763、764、774、843、850
Tumor microenvironment (TME) remodeling
肿瘤微环境(TME)重塑
TGF-β can stimulate tumor progression even when its signaling pathways are unavailable in the tumor cells, indicating its additional tumor-promoting effects exerted on tumor stroma.760,761,762,763,764,843 Fibroblasts, endothelial cells, and immune cells are the major stromal cell types in TME and can all be manipulated by TGF-β in favor of tumor progression.
即使肿瘤细胞中不存在其信号通路,TGF-β也可以刺激肿瘤进展,这表明它对肿瘤基质具有额外的促肿瘤作用。 760 , 761 , 762 , 763 , 764 , 843成纤维细胞、内皮细胞和免疫细胞是 TME 中的主要基质细胞类型,都可以被 TGF-β 操纵,有利于肿瘤进展。
Actively produced TGF-β in the TME can stimulate the chemotactic migration of fibroblasts and convert them into MFs which are also known as cancer-associated fibroblasts (CAFs) in terms of tumors.305,853 Activated CAFs can in turn repay TME with more TGF-β as well as other tumor-promoting factors such as TGF-α, FGF, HGF, PDGF, and CTGF to exert a strong stimulation on tumor growth.324,853,854,855,856,857 Moreover, TGF-β regulates the production of various ECM components and remodelers by CAFs to facilitate the migration of tumor cells during invasion and metastasis.855,858 Interestingly, fibroblasts with the loss of TβRII can also contribute to tumor development through the production of TGF-α, HGF, and macrophage-stimulating protein (MSP).859
TME中活跃产生的TGF-β可以刺激成纤维细胞的趋化迁移,并将其转化为MF,在肿瘤方面也称为癌症相关成纤维细胞(CAF)。 305 , 853激活的CAF反过来可以回报TME更多的TGF-β以及其他促肿瘤因子如TGF-α、FGF、HGF、PDGF和CTGF,对肿瘤生长产生强烈刺激。 324 , 853 , 854 , 855 , 856 , 857此外,TGF-β 调节 CAF 产生各种 ECM 成分和重塑剂,以促进肿瘤细胞在侵袭和转移过程中的迁移。 855 , 858有趣的是,TβRII 缺失的成纤维细胞也可以通过产生 TGF-α、HGF 和巨噬细胞刺激蛋白 (MSP) 来促进肿瘤的发展。第859章
Endothelial cells can also be converted into CAFs through TGF-β-mediated EndMT.860 More importantly, TGF-β promotes the angiogenesis of endothelial cells by inducing VEGF production in tumor cells and fibroblasts.323,354,750,861,862 TGF-β also disrupts inter-endothelial junctions to increase the vascular permeability in TME through the process of EndMT and the induction of angiopoietin-like 4 (ANGPTL4).863 Therefore, TGF-β-mediated angiogenesis not only increases the blood supply to tumors to favor their growth but also provides tumors with more accessible entrances into the circulation to form metastasis.
内皮细胞也可以通过 TGF-β 介导的 EndMT 转化为 CAF。 860更重要的是,TGF-β 通过诱导肿瘤细胞和成纤维细胞产生 VEGF 来促进内皮细胞的血管生成。 323、354、750、861、862 TGF - β还通过 EndMT 过程和诱导血管生成素样 4 (ANGPTL4) 破坏内皮间连接,从而增加 TME 中的血管通透性。 863因此,TGF-β介导的血管生成不仅增加了肿瘤的血液供应以有利于其生长,而且还为肿瘤提供了更容易进入循环的入口以形成转移。
Furthermore, TGF-β can modulate immune cell activity to facilitate tumor survival and development. TGF-β inhibits the tumoricidal activity of macrophages and neutrophils and polarizes them into tumor-promoting M2 macrophages and N2 neutrophils, which are also known as tumor-associated macrophages (TAMs) and tumor-associated neutrophils (TANs) in terms of tumors.439,442,444,448,449,864 It also promotes the functions of Tregs while suppressing the cytotoxicity of CTLs and NK cells to facilitate tumor evasion from immune surveillance.865,866,867,868,869 Moreover, TGF-β can inhibit the expression of MHC antigens in tumor cells to further attenuate their recognition by adaptive anti-tumor immunity.870,871 However, TGF-β-mediated downregulation of MHC antigens and NKG2D ligands can increase tumor susceptibility to NK cytotoxicity to some extent.233,872
此外,TGF-β可以调节免疫细胞活性,促进肿瘤的存活和发展。 TGF-β抑制巨噬细胞和中性粒细胞的杀肿瘤活性,并将其极化为促癌M2巨噬细胞和N2中性粒细胞,在肿瘤方面也称为肿瘤相关巨噬细胞(TAM)和肿瘤相关中性粒细胞(TAN)。 439 , 442 , 444 , 448 , 449 , 864它还促进Tregs的功能,同时抑制CTL和NK细胞的细胞毒性,以促进肿瘤逃避免疫监视。 865 , 866 , 867 , 868 , 869此外,TGF-β可以抑制肿瘤细胞中MHC抗原的表达,通过适应性抗肿瘤免疫进一步减弱它们的识别。 870 , 871然而,TGF-β 介导的 MHC 抗原和 NKG2D 配体的下调可以在一定程度上增加肿瘤对 NK 细胞毒性的敏感性。 233 , 872
TGF-β-targeting therapies
TGF-β靶向疗法
To rectify the dysfunction of TGF-β in different kinds of diseases, several targeted therapies have been developed to regulate TGF-β activity at the levels of biosynthesis, activation, and signaling. Many completed clinical trials have preliminarily confirmed the safety and efficacy of some therapeutic strategies, while there are still numerous clinical trials ongoing at present (Table 1).
为了纠正不同类型疾病中 TGF-β 的功能障碍,已经开发了几种靶向疗法来在生物合成、激活和信号传导水平上调节 TGF-β 活性。许多已完成的临床试验已初步证实了一些治疗策略的安全性和有效性,同时仍有大量临床试验正在进行中(表1 )。
表1 正在进行的TGF-β靶向疗法的临床试验
Alteration of TGF-β biosynthesis
TGF-β生物合成的改变
Targeting TGF-β mRNAs 靶向 TGF-β mRNA
Trabedersen (AP 12009 or OT-101) is an antisense oligonucleotide complementary to human TGF-β2 mRNA and can specifically inhibit TGF-β2 biosynthesis. It is hypothesized that trabedersen mainly acts by reversing TGF-β2-mediated immunosuppression to facilitate immune responses against tumors. A phase 2b clinical trial showed no advantage in early tumor control rate but in long-term survival rate for glioma patients treated with trabedersen in comparison with standard chemotherapy. Tumor responses which continued to increase long after discontinuation in the study suggested that the clinically relevant beneficial effects of trabedersen might increase over time. Moreover, compared with the standard chemotherapy group, drug-related or possibly drug-related adverse events in the trabedersen group were less common and mostly nervous system disorders. The study also indicated that the optimal dose of trabedersen is 10 µM, as both its efficacy and safety tended to be superior to the 80 µM dose, although the mechanism for this counterintuitive result has not been fully understood.873 TGF-β1 antisense oligonucleotides or small interfering RNAs (siRNAs) were also developed and evaluated in different pre-clinical models, suggested as potential therapeutic strategies for tuberculosis,874,875 wound scarring,876,877 and several renal diseases.878,879,880,881
Trabedersen(AP 12009 或 OT-101)是一种与人 TGF-β2 mRNA 互补的反义寡核苷酸,可以特异性抑制 TGF-β2 生物合成。据推测,trabedersen 主要通过逆转 TGF-β2 介导的免疫抑制来促进针对肿瘤的免疫反应。一项 2b 期临床试验显示,与标准化疗相比,接受 Trabedersen 治疗的神经胶质瘤患者在早期肿瘤控制率方面没有优势,但在长期生存率方面没有优势。研究中止后很长时间内肿瘤反应持续增加,这表明 Trabedersen 的临床相关有益效果可能会随着时间的推移而增加。而且,与标准化疗组相比,trabedersen组中药物相关或可能与药物相关的不良事件较少见,且多为神经系统疾病。研究还表明,trabedersen 的最佳剂量是 10 µM,因为其功效和安全性往往优于 80 µM 剂量,尽管这种违反直觉的结果的机制尚未完全了解。 [第873章]第874章878、879、880、881
TGF-β antisense gene-modified tumor cell vaccines are designed to exhibit increased immunogenicity due to reduced TGF-β expression in the tumor cells that comprise the vaccines. Vaccine Lucanix (belagenpumatucel-L) made from allogeneic non-small cell lung cancer (NSCLC) cell lines was well tolerated and brought survival advantages to NSCLC patients who were randomized within 12 weeks of completion of platinum-based chemotherapy and in those who had received prior radiation, as shown in a phase 3 trial which, however, failed to demonstrate a significant increase in survival in the overall patient population.882 TGF-β antisense-modified autologous tumor cell vaccines have also been tested in advanced glioma and other solid tumors, respectively, in two phase 1 studies in which enhanced anti-tumor activity and improved survival were observed.34,883 Notably, in the study among glioma patients, the most common treatment-related adverse events were delayed-type hypersensitivity-like reactions observed at the sites of the second and subsequent vaccinations in all patients. Some of these patients also experienced transient, flu-like symptoms consisting of musculoskeletal aches and pains and fatigue during the course of treatment.34
TGF-β 反义基因修饰的肿瘤细胞疫苗被设计为由于构成疫苗的肿瘤细胞中 TGF-β 表达减少而表现出增强的免疫原性。由同种异体非小细胞肺癌 (NSCLC) 细胞系制成的疫苗 Lucanix (belagenpumatucel-L) 具有良好的耐受性,为完成铂类化疗后 12 周内随机分配的 NSCLC 患者以及接受过铂类化疗的患者带来了生存优势。先前的放射治疗,如第 3 期试验所示,但未能证明总体患者群体的生存率显着增加。 882 TGF-β 反义修饰的自体肿瘤细胞疫苗也在两项 1 期研究中分别在晚期神经胶质瘤和其他实体瘤中进行了测试,观察到抗肿瘤活性增强和生存率提高。 34 , 883值得注意的是,在神经胶质瘤患者的研究中,最常见的治疗相关不良事件是在所有患者第二次及后续疫苗接种部位观察到的迟发型超敏反应样反应。其中一些患者在治疗过程中还经历了短暂的流感样症状,包括肌肉骨骼疼痛和疲劳。 34
Targeting furin 靶向弗林蛋白酶
Convertase furin is a therapeutic target participating in the post-translational processing of TGF-β. Vigil (FANG or Gemogenovatucel-T) is an autologous tumor cell vaccine incorporating a plasmid encoding granulocyte-macrophage colony-stimulating factor (GMCSF) and a bifunctional short-hairpin RNA (shRNA) targeting the expression of furin. A phase 1 study confirmed its safety and efficacy in various advanced solid tumors, with significant survival differences noted between patients who received less than four vaccinations and those who received no less than four vaccinations.884 A later phase 2b trial also demonstrated significant clinical benefit in homologous recombination proficient ovarian cancer (NCT02346747).885 Both studies reported no treatment-related serious adverse events, while the most common grade one and two adverse events related to study medication were local reactions at the injection site.
弗林蛋白酶转化酶是参与 TGF-β 翻译后加工的治疗靶点。 Vigil(FANG 或 Gemogenovatucel-T)是一种自体肿瘤细胞疫苗,包含编码粒细胞巨噬细胞集落刺激因子 (GMCSF) 的质粒和靶向弗林蛋白酶表达的双功能短发夹 RNA (shRNA)。一项一期研究证实了其在各种晚期实体瘤中的安全性和有效性,在接受少于四次疫苗接种的患者和接受不少于四次疫苗接种的患者之间观察到显着的生存差异。 884后来的 2b 期试验也证明了同源重组熟练的卵巢癌具有显着的临床益处 (NCT02346747)。 885两项研究均未报告与治疗相关的严重不良事件,而与研究药物相关的最常见的一级和二级不良事件是注射部位的局部反应。
Alteration of TGF-β activation
TGF-β 激活的改变
Targeting latent TGF-β complex
靶向潜在的 TGF-β 复合物
SRK-181 is an antibody that selectively binds to latent TGF-β1 to inhibit its activation. Co-administration of SRK-181 and anti-PD-1 antibody induced profound anti-tumor responses and survival benefit in mice, with increased infiltrating CD8+ T cells and decreased immunosuppressive myeloid cells observed in tumors refractory to anti-PD-1 treatment.886 The selective blockade of TGF-β1 by SRK-181 neither caused cardiac valvulopathy in rats as pan-TGFβ inhibitors might do nor did it induce cytokine release in human peripheral blood. Moreover, SRK-181 showed no effect on human platelet aggregation, activation, and binding.886,887 The favorable safety profile displayed in these preclinical assessments supports the ongoing phase 1 trial of SRK-181 in patients with advanced cancers (NCT04291079).
SRK-181 是一种选择性结合潜在 TGF-β1 以抑制其激活的抗体。 SRK-181 和抗 PD-1 抗体联合给药可在小鼠中诱导显着的抗肿瘤反应和生存获益,在抗 PD-1 治疗难治的肿瘤中观察到浸润性 CD8+ T 细胞增加和免疫抑制性骨髓细胞减少。 886 SRK-181 对 TGF-β1 的选择性阻断既不会像泛 TGFβ 抑制剂那样引起大鼠心脏瓣膜病,也不会诱导人外周血中细胞因子的释放。此外,SRK-181 对人血小板聚集、激活和结合没有影响。 886 , 887这些临床前评估中显示的良好安全性支持了正在进行的 SRK-181 在晚期癌症患者中的 1 期试验 (NCT04291079)。
Targeting GARP 针对 GARP
GARP expressed by Tregs, platelets, and endothelium functions to tether latent TGF-β complex to the cell surface for activation. Anti-GARP monoclonal antibody PIIO-1 proved to be an effective and safe strategy to block TGF-β activation in preclinical models, for it specifically bound to ligand-free GARP on Tregs but lacked recognition of GARP-latent TGF-β complex on platelets, actually avoiding the risk of platelet-related toxicities such as thrombocytopenia. More importantly, PIIO-1 showed therapeutic efficacy against both GARP+ and GARP- cancers alone or in combination with anti-PD-1 antibody, by preventing T cell exhaustion and enhancing CD8+ T cell migration into the TME in a C-X-C motif chemokine receptor 3 (CXCR3)-dependent manner.888
由 Tregs、血小板和内皮细胞表达的 GARP 具有将潜在的 TGF-β 复合物束缚到细胞表面以进行激活的功能。抗 GARP 单克隆抗体 PIIO-1 被证明是临床前模型中阻断 TGF-β 激活的有效且安全的策略,因为它特异性结合 Tregs 上的无配体 GARP,但缺乏对血小板上 GARP 潜在 TGF-β 复合物的识别,实际上避免了血小板相关毒性的风险,例如血小板减少症。更重要的是,PIIO-1 通过防止 T 细胞耗竭并增强 CD8+ T 细胞迁移到 CXC 基序趋化因子受体 3 中的 TME,单独或与抗 PD-1 抗体组合对 GARP+ 和 GARP- 癌症显示出治疗功效。 CXCR3)依赖的方式。第888章
Targeting αV integrins 靶向 αV 整合素
Integrins are regarded as the most important activators of TGF-β. Abituzumab (EMD 525797 or DI17E6) is an antibody against pan-αV integrins. In a phase 1/2 trial on KRAS wild-type metastatic colorectal cancer (NCT01008475), the progression-free survival (PFS) and response rates were similar among all groups in the intent-to-treat population comprising all patients randomized, although a trend toward improved overall survival (OS) was observed in the groups that received abituzumab treatment. However, exploratory analysis suggested that in patients with high αVβ6 expression, PFS and response rates might be increased with abituzumab therapy.889 This pan-αV integrin inhibitor was also found to inhibit prostate cancer-associated bone lesion formation in a randomized phase 2 trial (NCT01360840), although PFS was not significantly extended.890 Recently, abituzumab has been investigated in SSc-associated interstitial lung disease in a phase 2 trial (NCT02745145). However, the study was terminated prematurely due to slow enrollment and no meaningful conclusions could be drawn due to a small sample size.891 The most commonly reported treatment-related adverse events of abituzumab included fatigue, headache, gastrointestinal disorders, as well as abnormal biochemistry and hematology values.889,890,892
整合素被认为是TGF-β最重要的激活剂。 Abituzumab(EMD 525797 或 DI17E6)是一种针对泛 αV 整联蛋白的抗体。在一项针对 KRAS 野生型转移性结直肠癌 (NCT01008475) 的 1/2 期试验中,由随机分组的所有患者组成的意向治疗人群中所有组的无进展生存期 (PFS) 和缓解率相似,尽管在接受阿比珠单抗治疗的组中观察到总生存期 (OS) 改善的趋势。然而,探索性分析表明,在 αVβ6 高表达的患者中,阿比珠单抗治疗可能会提高 PFS 和缓解率。 889在一项随机 2 期试验 (NCT01360840) 中,还发现这种泛 αV 整合素抑制剂可抑制前列腺癌相关骨病变的形成,但 PFS 并未显着延长。 890最近,阿比珠单抗已在 2 期试验中研究了 SSc 相关间质性肺疾病 (NCT02745145)。然而,由于入组速度缓慢,该研究提前终止,并且由于样本量较小,无法得出有意义的结论。 891最常见报告的阿比珠单抗治疗相关不良事件包括疲劳、头痛、胃肠道疾病以及生化和血液学值异常。 889、890、892
Cilengitide (EMD 121974, NSC 707544) is a selective αvβ3 and αvβ5 integrin inhibitor which has been evaluated for therapeutic efficacy in NSCLC (NCT00842712),893,894 head and neck SCC (NCT00705016),895 glioblastoma (NCT00689221, NCT00813943, and NCT01124240),896,897,898,899,900,901,902,903 melanoma,904 pancreatic cancer,905 and prostate cancer906,907 in a series of phase 2 studies and one phase 3 study. Although cilengitide failed to demonstrate significant clinical benefits in these studies on tumors, it might be a novel treatment for fibrotic diseases as relevant preclinical studies suggested.908,909 Notably, the adverse events possibly related to cilengitide treatment included fatigue, arthralgia, lymphopenia, and gastrointestinal disorders.893,897,899,900,904,906,907 Furthermore, an inhibitor of pan-integrins and TGF-β known as GLPG-0187 was proved to enhance T cell killing of colorectal cancer cells in vitro, possibly by suppressing TGF-β-mediated PD-L1 upregulation.910,911
Cilengitide (EMD 121974, NSC 707544) 是一种选择性 αvβ3 和 αvβ5 整合素抑制剂,已针对 NSCLC (NCT00842712) 、 893、894头颈 SCC (NCT00705016)、 895胶质母细胞瘤 (NCT00689221、 3、NCT01124240) 、 896 、 897 、 898 、 899 、 900 、 901 、 902 、 903黑色素瘤、 904胰腺癌、 905和前列腺癌906 、 907在一系列 2 期研究和一项 3 期研究中。尽管西仑吉肽在这些肿瘤研究中未能证明显着的临床益处,但正如相关临床前研究表明的那样,它可能是纤维化疾病的一种新疗法。 908 , 909值得注意的是,可能与西仑吉肽治疗相关的不良事件包括疲劳、关节痛、淋巴细胞减少和胃肠道疾病。 893 , 897 , 899 , 900 , 904 , 906 , 907此外,一种称为 GLPG-0187 的泛整合素和 TGF-β 抑制剂被证明可以在体外增强 T 细胞对结直肠癌细胞的杀伤作用,可能是通过抑制 TGF-β -介导的PD-L1上调。 910 , 911
Targeting TSP-1 靶向 TSP-1
TSP-1 can directly activate all three TGF-β isoforms independent of other activators or cellular activity. The conserved LSKL sequence in LAP which is recognized by TSP-1 can be synthesized as peptides to block TSP-1-mediated TGF-β activation. Pre-clinical studies suggested that treatment of LSKL or relevant tripeptide SRI31277 could be novel therapeutic strategies for various cardiovascular diseases,912 pulmonary diseases,913 renal diseases,914,915,916 nervous diseases,917,918 fibrotic diseases,919,920,921 wound healing,922,923 and tumors.924,925,926 Moreover, TSP-1 antisense oligonucleotides were successfully developed and applied to inhibit TGF-β activation in a rat model of mesangial proliferative glomerulonephritis, demonstrating a remarkable prevention against renal fibrosis.927
TSP-1 可以直接激活所有三种 TGF-β 亚型,独立于其他激活剂或细胞活性。 LAP 中被 TSP-1 识别的保守 LSKL 序列可以合成肽来阻断 TSP-1 介导的 TGF-β 激活。临床前研究表明,LSKL或相关三肽SRI31277的治疗可能成为多种心血管疾病、 912肺部疾病、 913肾脏疾病、 914、915、916神经疾病、 917、918纤维化疾病、 919、920、921的新治疗策略922 、第923章伤口愈合和肿瘤。 924 , 925 , 926此外,TSP-1 反义寡核苷酸已成功开发并应用于抑制系膜增生性肾小球肾炎大鼠模型中的 TGF-β 活化,显示出显着的预防肾纤维化的作用。 927
Alteration of TGF-β signaling
TGF-β信号传导的改变
Targeting TGF-β ligands 靶向 TGF-β 配体
A TGF-β2-enriched polymeric dietary supplement known as Modulen (CT3211) was effective in inducing earlier remission of inflammatory bowel diseases (IBDs) including both Crohn’s disease and UC with significant improvements in endoscopic and histologic appearances, mucosal cytokine parameters, C-reactive protein (CRP) values, erythrocyte sedimentation rates (ESRs), serum albumin levels, as well as weight and height scores in the patients.928,929,930,931 Notably, an exclusive Modulen diet was more efficient than steroids to induce mucosal healing in children with Crohn’s disease, possibly due to its additional advantage in regulating intestinal microbiota (NCT00265772).932,933 Moreover, a pre-operative polymeric diet enriched with TGF-β2 was able to decrease post-operative complications after surgery for complicated ileocolonic Crohn’s disease.934 The side effects of Modulen were mild, including abdominal pain, flatulence, nausea, and vomiting.928,932,934 In mouse models, oral TGF-β supplementation also showed beneficial effects on food allergy prevention.935,936,937 In fact, it is believed that the presence of TGF-β in breast milk can protect the progeny from several allergic diseases such as asthma,938 eczema,939 and food allergy.940
富含 TGF-β2 的聚合物膳食补充剂,称为 Modulen (CT3211),可有效诱导炎症性肠病 (IBD) 的早期缓解,包括克罗恩病和 UC,并显着改善内镜和组织学外观、粘膜细胞因子参数、C 反应性蛋白质(CRP)值、红细胞沉降率(ESR)、血清白蛋白水平以及患者的体重和身高评分。 928 , 929 , 930 , 931值得注意的是,独家 Modulen 饮食比类固醇更有效地诱导克罗恩病儿童的粘膜愈合,这可能是由于其在调节肠道微生物群方面的额外优势 (NCT00265772)。 932 , 933此外,富含 TGF-β2 的术前聚合饮食能够减少复杂性回结肠克罗恩病手术后的术后并发症。 934 Modulen 的副作用很轻微,包括腹痛、胀气、恶心和呕吐。 928 , 932 , 934在小鼠模型中,口服 TGF-β 补充剂也显示出对预防食物过敏的有益作用。第935、936、937事实上,人们相信母乳中存在的TGF-β可以保护后代免受多种过敏性疾病的侵害,例如哮喘、湿疹、 939和食物过敏。 940
Recombinant human TGF-β3 known as avotermin (Juvista) is a potential therapy for the improvement of cutaneous scarring. In a series of phase 1/2 studies (NCT00847925, NCT00847795, NCT00629811, NCT00432211, NCT00594581, and NCT00430326), visual assessment of scar formation revealed that, in contrast to placebo, intradermal avotermin could significantly improve total scar scores which were derived from a visual analog scale to assess how closely scars resembled normal skin. The results were further confirmed by histological assessments that scars treated with avotermin showed better organized ECM of the papillary and reticular dermis. The incidence of adverse events at wound sites, including infection, exudate, erythema, pain, burning, itching, and thickening was low and similar for avotermin and controls.941,942,943,944 Although the other two TGF-β isoforms, TGF-β1 and TGF-β2, showed no therapeutic activity of scarring, they were found to improve and accelerate the healing of cutaneous wounds in animal models as well as clinical patients.304,306,307,317,321,334,945 Moreover, TGF-β also showed therapeutic potential for tissue regeneration,329,946,947 inflammatory diseases,676,687,948 and influenza949 as shown in relevant preclinical models.
重组人 TGF-β3 称为阿沃特明 (Juvista),是一种改善皮肤疤痕的潜在疗法。在一系列 1/2 期研究(NCT00847925、NCT00847795、NCT00629811、NCT00432211、NCT00594581 和 NCT00430326)中,对疤痕形成的视觉评估显示,与安慰剂相比,皮内阿沃特明可以显着改善总疤痕评分,该评分来自于视觉模拟量表来评估疤痕与正常皮肤的相似程度。组织学评估进一步证实了阿伏特明治疗的疤痕显示乳头状和网状真皮组织更好的 ECM。阿沃特明和对照组的伤口部位不良事件发生率较低,包括感染、渗出物、红斑、疼痛、烧灼感、瘙痒和增厚,且相似。 941 , 942 , 943 , 944虽然另外两种 TGF-β 异构体 TGF-β1 和 TGF-β2 没有表现出疤痕治疗活性,但在动物模型和临床研究中发现它们可以改善和加速皮肤伤口的愈合患者。 304、306、307、317、321、334、945此外, TGF - β还显示出对组织再生、 329、946、947炎症性疾病、 676、687、948和流感949的治疗潜力,如相关临床前模型所示。
TGF-β neutralizing antibodies and ligand traps can block the binding of TGF-β to its receptors. Fresolimumab (GC1008), a monoclonal antibody that neutralizes all three TGF-β isoforms demonstrated acceptable safety and preliminary evidence of anti-tumor activity in a phase 1 study on advanced malignant melanoma and renal cell carcinoma (NCT00356460).950 In a phase 2 trial (NCT01401062), a higher dose of fresolimumab is associated with longer median OS as well as improved peripheral blood mononuclear cell counts and boosted central memory CD8+ T cell levels in metastatic breast cancer patients receiving radiotherapy.951 Fresolimumab also showed therapeutic effects on SSc with decreased biomarkers of skin fibrosis and improved clinical symptoms in the patients in a phase 1 study (NCT01284322).952 Moreover, a phase 1 study evaluated the safety of fresolimumab in patients with treatment-resistant primary focal segmental glomerulosclerosis and the good tolerability supported additional evaluation in larger randomized dose-ranging clinical trials.953 Notably, the major drug-related adverse events of fresolimumab were skin disorders, bleeding episodes, and anemia. Skin toxicity was particularly significant and tumor patients assigned to high doses of treatment even developed skin tumors, including keratoacanthoma, basal cell carcinoma, and SCC.950,951,952,953,954 Another anti-TGF-β monoclonal antibody known as NIS793 was well tolerated alone or in combination with anti-PD-1 antibody in patients with advanced solid tumors in a phase 1 study (NCT02947165). Treatment-related adverse events of all patients in the study were mostly skin toxicity and gastrointestinal events, and no dose-limiting toxicities were observed during dose escalation. Notably, biomarker analyses in the study showed evidence of systemic target engagement, local signaling inhibition, and tumor immune activation.955 Apart from tumors, a recombinant human anti-TGF-β1 antibody known as CAT-192 was evaluated in the treatment of early-stage diffuse cutaneous SSc but showed no evidence of efficacy in the pilot phase 1/2 study. The most commonly reported adverse events in the study affected the gastrointestinal, musculoskeletal, respiratory, and skin systems, but none of them were considered to be related to the treatment.956 Moreover, a phase 2 study assessing the safety and efficacy of TGF-β1 monoclonal antibody in patients with diabetic nephropathy was terminated early for futility (NCT01113801). The frequencies of the various categories of adverse effects in this study were generally similar between the treatment and placebo groups.957 Furthermore, monotherapy of a selective TGF-β1/3 trap known as AVID200 in a population of patients with an advanced stage of myelofibrosis in a phase 1b trial resulted in limited toxicity as well as improvements in spleen size, symptom benefit, and platelet counts (NCT03895112). Remarkably, platelet count increase was a therapeutic effect not observed with other myelofibrosis therapies, suggesting a potential advantage of AVID200 treatment. Adverse events that occurred during the study regardless of attribution mainly included pruritus, fatigue, abdominal pain, anemia, and thrombocytopenia.958 Additionally, other potential applications of neutralizing TGF-β antibodies suggested by pre-clinical studies include wound healing,334,959,960 prostatic hyperplasia,961 pulmonary diseases,962,963 cardiovascular diseases,564,964 musculoskeletal diseases,965,966,967,968 inflammatory diseases,969,970 and Chagas disease (Trypanosoma cruzi infection).971
TGF-β 中和抗体和配体陷阱可以阻断 TGF-β 与其受体的结合。 Fresolimumab (GC1008) 是一种中和所有三种 TGF-β 同工型的单克隆抗体,在针对晚期恶性黑色素瘤和肾细胞癌的 1 期研究 (NCT00356460) 中表现出可接受的安全性和抗肿瘤活性的初步证据。 950在一项 2 期试验 (NCT01401062) 中,较高剂量的 fresolimumab 与接受放疗的转移性乳腺癌患者的中位 OS 较长、外周血单核细胞计数改善和中央记忆 CD8+ T 细胞水平升高相关。在一项 1 期研究 (NCT01284322) 中, 951 Fresolimumab 还显示出对 SSc 的治疗效果,可减少皮肤纤维化的生物标志物并改善患者的临床症状。 952此外,一项 1 期研究评估了 fresolimumab 在难治性原发性局灶节段性肾小球硬化患者中的安全性,良好的耐受性支持在更大规模的随机剂量范围临床试验中进行额外评估。 953值得注意的是,fresolimumab 的主要药物相关不良事件是皮肤病、出血事件和贫血。皮肤毒性尤其显着,接受高剂量治疗的肿瘤患者甚至出现皮肤肿瘤,包括角化棘皮瘤、基底细胞癌和鳞状细胞癌。 950、951、952、953、954另一种称为NIS793的抗 TGF-β 单克隆抗体在 1 期研究 (NCT02947165) 中,单独使用或与抗 PD-1 抗体联合使用,在晚期实体瘤患者中具有良好的耐受性。 研究中所有患者的治疗相关不良事件主要是皮肤毒性和胃肠道事件,剂量递增期间未观察到剂量限制性毒性。值得注意的是,该研究中的生物标志物分析显示了系统靶点参与、局部信号抑制和肿瘤免疫激活的证据。 955除肿瘤外,还评估了一种重组人抗 TGF-β1 抗体(称为 CAT-192)治疗早期弥漫性皮肤 SSc 的效果,但在 1/2 期试验研究中没有显示出疗效的证据。研究中最常见的不良事件影响胃肠道、肌肉骨骼、呼吸和皮肤系统,但没有一个被认为与治疗有关。 956此外,一项评估 TGF-β1 单克隆抗体在糖尿病肾病患者中的安全性和有效性的 2 期研究因无效而提前终止 (NCT01113801)。本研究中治疗组和安慰剂组中各类不良反应的发生率总体相似。 957此外,在 1b 期试验中,选择性 TGF-β1/3 陷阱(称为 AVID200)对晚期骨髓纤维化患者进行单一疗法,产生了有限的毒性,并改善了脾脏大小、症状益处和血小板计数(NCT03895112)。值得注意的是,血小板计数增加是其他骨髓纤维化疗法未观察到的治疗效果,表明 AVID200 治疗的潜在优势。研究期间发生的不良事件(无论归因如何)主要包括瘙痒、疲劳、腹痛、贫血和血小板减少。958此外,临床前研究表明中和 TGF-β 抗体的其他潜在应用包括伤口愈合、 334、959、960前列腺增生、 961肺部疾病、 962、963心血管疾病、 564、964肌肉骨骼疾病、 965、966 、第967章、第968章炎性疾病,第969章、第970章、恰加斯病(克氏锥虫感染)。第971章
Bifunctional antibody-ligand traps containing the extracellular domain of TβRII can target both TGF-β and immune checkpoints. In preclinical studies, both the anti-CTL associated protein (CTLA)-4-TβRII chimera and the anti-PD-L1-TβRII chimera exhibited superior anti-tumor efficacy compared with their parent immune checkpoint inhibitors.972 Bintrafusp alfa (M7824), a bifunctional fusion protein targeting both TGF-β and PD-L1 was assessed in several phase 1 trials (NCT02699515, NCT02517398, NCT02699515, and NCT04247282). The results showed that bintrafusp alfa had encouraging efficacy in NSCLC,973 gastric cancer,974 biliary tract cancer,975 as well as human papillomavirus (HPV)-unrelated head and neck cancer in which enhanced tumor antigen-specific immunity has been observed.976 Similar to fresolimumab, the treatment-related adverse events of bintrafusp alfa included fatigue, colitis, bleeding, anemia, hypokalemia, lipase increase, hepatic function abnormalities, as well as several skin disorders from rash, hyperkeratosis, to keratoacanthoma and SCC.973,974,975,976,977 BR102 is another bifunctional fusion protein simultaneously targeting PD-L1 and TGF-β. The efficacy and safety of BR102 demonstrated in preclinical characterization supported its further clinical development for anti-cancer therapy.978 Notably, the bifunctional antibody-ligand traps have inspired the development of chimeric antigen receptor (CAR)-T cells secreting bispecific trap protein, which co-targets PD-1 and TGF-β to enhance anti-tumor efficacy as shown in mouse models.979
含有 TβRII 胞外域的双功能抗体-配体陷阱可以同时靶向 TGF-β 和免疫检查点。在临床前研究中,与母体免疫检查点抑制剂相比,抗CTL相关蛋白(CTLA)-4-TβRII嵌合体和抗PD-L1-TβRII嵌合体均表现出更优异的抗肿瘤功效。 972 Bintrafusp alfa (M7824) 是一种同时靶向 TGF-β 和 PD-L1 的双功能融合蛋白,在多项 1 期试验(NCT02699515、NCT02517398、NCT02699515 和 NCT04247282)中进行了评估。第973章胃癌,第974章胆道癌,第975章976与 fresolimumab 类似,bintrafusp alfa 的治疗相关不良事件包括疲劳、结肠炎、出血、贫血、低钾血症、脂肪酶增加、肝功能异常,以及皮疹、角化过度、角化棘皮瘤和鳞状细胞癌等多种皮肤疾病。 973 , 974 , 975 , 976 , 977 BR102是另一种同时靶向PD-L1和TGF-β的双功能融合蛋白。临床前表征中所证明的 BR102 的功效和安全性支持其抗癌治疗的进一步临床开发。978值得注意的是,双功能抗体-配体陷阱激发了分泌双特异性陷阱蛋白的嵌合抗原受体 (CAR)-T 细胞的开发,该蛋白共同靶向 PD-1 和 TGF-β 以增强抗肿瘤功效,如小鼠模型所示。第979章
Furthermore, LAP, TβRIII (β-glycan), and decorin can bind to TGF-β as natural inhibitors. They have shown treatment effects in preclinical models of wound healing,980,981,982,983 cardiovascular diseases,984,985,986,987,988,989 nervous diseases,990,991,992 renal diseases,993,994,995,996 fibrotic diseases,997,998,999,1000 tuberculosis,1001 and tumors1002,1003,1004,1005 and thus warrant further development.
此外,LAP、TβRIII(β-聚糖)和核心蛋白聚糖可以作为天然抑制剂与 TGF-β 结合。他们在伤口愈合、 980、981、982、983心血管疾病、 984、985、986、987、988、989神经疾病、 990、991、992肾脏疾病、 993、994、995 、 996纤维化疾病, 997、998、999、1000结核病、 1001和肿瘤1002、1003、1004、1005 ,因此需要进一步开发。
Targeting TβRs 靶向 TβR
TGF-β-insensitive CAR-T cells armored with dominant-negative TβRII showed preliminary evidence for early anti-tumor function in prostate cancer, including a biomarker decline among approximately 30% of the patients in a phase 1 trial (NCT03089203). This strategy which is considered generally feasible, despite no partial response being observed in the study, and safe, with study-related serious adverse events mostly being cytokine release syndrome, warrants further validation and investigation.1006 Dominant-negative TβRII can also enhance the anti-tumor efficacy of DC vaccines, manifested by powerful tumor-specific CTL responses, inhibited tumor development, and prolonged survival times in mouse models.1007,1008 Moreover, dominant-negative TβRII showed great potential for reducing hypertrophic scars as in rabbit ear models.1009
带有显性失活 TβRII 的 TGF-β 不敏感 CAR-T 细胞显示出前列腺癌早期抗肿瘤功能的初步证据,包括在 1 期试验 (NCT03089203) 中约 30% 的患者生物标志物下降。尽管在研究中没有观察到部分反应,但该策略被认为是普遍可行的,并且是安全的,与研究相关的严重不良事件主要是细胞因子释放综合征,值得进一步验证和研究。 1006显性阴性 TβRII 还可以增强 DC 疫苗的抗肿瘤功效,表现为强大的肿瘤特异性 CTL 反应,抑制肿瘤发展并延长小鼠模型的生存时间。 1007 , 1008此外,显性失活 TβRII 在减少兔耳模型中的肥厚性疤痕方面显示出巨大潜力。 1009
Many small-molecule inhibitors have been developed to suppress the kinase activity of TβRI. In a series of phase 2 studies, a TβRI kinase inhibitor known as galunisertib (LY2157299) showed preliminary efficacy in patients with myelodysplastic syndromes (MDS) (NCT02008318),1010 NSCLC (NCT02423343),1011 hepatocellular carcinoma (NCT01246986),1012,1013 rectal cancer (NCT02688712),1014 and pancreatic cancer,1015 but failed to demonstrate clinical benefit in patients with glioma (NCT01582269 and NCT01220271).1016,1017 The most common adverse events related to galunisertib treatment included fatigue, pyrexia, anemia, nausea, vomiting, diarrhea, and abdominal pain.1010,1013,1017 Despite comprehensive cardiovascular monitoring for galunisertib did not detect medically relevant cardiac toxicity in cancer patients,1018 galunisertib-related uncontrolled cytokine release was reported in patients with advanced solid tumors in a phase 1 trial (NCT01646203).1019 Other TβRI kinase inhibitors such as SM16, SD-208, NP-40208, SB-431542, LY3200882, LY364947, and vactosertib (EW-7197) also showed therapeutic potential in pre-clinical studies on tumors1020,1021,1022,1023,1024,1025,1026 as well as many other diseases such as cardiovascular diseases,565,1027,1028,1029,1030 renal diseases,1031 ophthalmic diseases,1032 skeletal diseases,1033 fibrotic diseases,1034,1035,1036 inflammatory diseases,1037,1038,1039 Chagas disease,1040,1041 coronavirus disease 2019 (COVID-19),1042 and wound healing.1043,1044,1045
许多小分子抑制剂已被开发来抑制 TβRI 的激酶活性。在一系列 2 期研究中,一种名为 galunisertib (LY2157299) 的 TβRI 激酶抑制剂对骨髓增生异常综合征 (MDS) (NCT02008318)、 1010 NSCLC (NCT02423343)、 1011肝细胞癌 (NCT01246986) 、 1012、1013直肠癌患者显示出初步疗效。癌症(NCT02688712), 1014和胰腺癌, 1015 ,但未能证明对神经胶质瘤患者有临床益处(NCT01582269 和 NCT01220271)。 1016 , 1017与 galunisertib 治疗相关的最常见不良事件包括疲劳、发热、贫血、恶心、呕吐、腹泻和腹痛。第1010章1013章 第1017章 尽管对galunisertib进行的全面心血管监测并未检测到癌症患者的医学相关心脏毒性,但在一项1期试验(NCT01646203)中,在晚期实体瘤患者中报告了与galunisertib相关的不受控制的细胞因子释放(NCT01646203)。1019其他 TβRI 激酶抑制剂,如 SM16、SD-208、NP-40208、SB-431542、LY3200882、LY364947 和 vactosertib (EW-7197) 也在肿瘤临床前研究中显示出治疗潜力1020 、 1021 、 1022 、 1023 , 1024 , 1025 , 1026以及许多其他疾病,如心血管疾病, 565 , 1027 , 1028 , 1029 , 1030肾脏疾病, 1031眼科疾病, 1032骨骼疾病, 1033纤维化疾病, 1034 , 1035 , 1036炎症性疾病, 037 、 1038 、 1039恰加斯病、 1040 、 1041 2019 年冠状病毒病 (COVID-19)、 1042和伤口愈合。1043、1044、1045
Targeting SMADs 针对 SMAD
An oral SMAD7 antisense oligonucleotide known as mongersen (GED-0301) showed promising results in patients with active Crohn’s disease in phase 1 and 2 phase trials, but further phase 3 study failed due to lack of clinical benefit (EudraCT 2009-012465-66, EudraCT 2011-002640-27, and NCT02596893).1046,1047,1048 Meanwhile, SMAD3 antisense oligonucleotide treatment was found to improve flexor tendon repair in mice and might have possible therapeutic applications in clinical practice.877
一种名为 mongersen 的口服 SMAD7 反义寡核苷酸 (GED-0301) 在 1 期和 2 期试验中对活动性克罗恩病患者显示出有希望的结果,但进一步的 3 期研究因缺乏临床益处而失败 (EudraCT 2009-012465-66, EudraCT 2011-002640-27 和 NCT02596893)。 1046 , 1047 , 1048同时,SMAD3 反义寡核苷酸治疗被发现可以改善小鼠的屈肌腱修复,并可能在临床实践中具有潜在的治疗应用。第877章
Moreover, a small-molecule SMAD3 inhibitor known as specific inhibitor of SMAD3 (SIS3) has shown pre-clinical therapeutic efficacy in wound healing,1049 cardiovascular diseases,569,1050,1051 nervous diseases,1052 renal diseases,1053,1054 skeletal diseases,1055 fibrotic diseases,1056,1057 inflammatory diseases,1039,1058 type 2 diabetes,1059,1060 and tumors,1061,1062 suggesting a novel approach that could be further tested to treat clinical patients.
此外,一种被称为SMAD3特异性抑制剂(SIS3)的小分子SMAD3抑制剂已在伤口愈合、 1049心血管疾病、 569、1050、1051神经疾病、 1052肾脏疾病、 1053、1054骨骼疾病、第1055章纤维化疾病, 1056、1057炎症性疾病, 1039、1058 2型糖尿病, 1059、1060和肿瘤, 1061、1062提出了一种可以进一步测试以治疗临床患者的新方法。
Furthermore, several SMAD-binding peptide aptamers have been developed to selectively inhibit the binding between SMADs and their interacting factors.1063 An aptamer containing the SMAD-binding domain of transcription factor lymphoid enhancer-binding factor 1 (LEF1) can suppress tumor cell proliferation by inhibiting the interaction between SMAD4 and LEF/T cell-specific factor (TCF) to suppress MYC expression.1064 Other aptamers that bind specifically to R-SMADs through the SMAD-binding domain from SARA can impair the formation of functional SMAD oligomers to inhibit TGF-β-induced EMT.1065,1066 Moreover, aptamers that disrupt the interaction between SMAD and transcription coactivator yes-associated protein (YAP) have been designed for bone tumor therapy.1067
此外,已经开发了几种 SMAD 结合肽适体来选择性抑制 SMAD 与其相互作用因子之间的结合。 1063含有转录因子淋巴增强子结合因子 1 (LEF1) SMAD 结合域的适体可以通过抑制 SMAD4 与 LEF/T 细胞特异性因子 (TCF) 之间的相互作用来抑制 MYC 表达,从而抑制肿瘤细胞增殖。 1064其他通过 SARA 的 SMAD 结合域与 R-SMAD 特异性结合的适体可以损害功能性 SMAD 寡聚体的形成,从而抑制 TGF-β 诱导的 EMT。 1065 , 1066此外,破坏 SMAD 和转录共激活因子 yes 相关蛋白 (YAP) 之间相互作用的适体已被设计用于骨肿瘤治疗。 1067
Conclusions and future perspectives
结论和未来展望
TGF-β signaling is so extensively and indispensably involved in a large number of biological processes that it has attracted great interest and attention over the past decades during which relevant knowledge has exploded in the fields of health, disease, and therapeutics. However, there are still some specific issues that have not been fully elucidated, while some previous knowledge is facing updates and challenges.
TGF-β信号传导如此广泛且不可或缺地参与大量生物过程,以至于在过去几十年中引起了人们的极大兴趣和关注,在此期间,相关知识在健康、疾病和治疗领域得到了爆炸式增长。但仍有一些具体问题尚未完全阐明,一些以往的认识正面临更新和挑战。
Studies on embryonic development and wound healing have revealed the isoform-specific roles of TGF-β which remain poorly aware in other fields of research, as studies on immune homeostasis, fibrotic diseases, and tumor development so far have focused on the most abundant TGF-β1 isoform in particular. Since all TGF-β isoforms are believed to signal through the same receptors and downstream pathways, the causes of the differences in biological effects between isotypes have not been fully understood. Moreover, since a natural TGF-β heterodimer containing one TGF-β1 monomer and one TGF-β2 monomer has long been discovered,12,1068 it would be very interesting to identify and characterize novel TGF-β heterodimers in the future. Furthermore, with the discovery and study of TGF-β superfamily which also includes polypeptides structurally similar to TGF-β such as nodal, myostatin, inhibins, activins, Müllerian-inhibiting substance (MIS), bone morphogenetic proteins (BMPs), and growth and differentiation factors (GDFs), researchers have realized that TGF-β can also signal through pathways ‘specific’ to other TGF-β superfamily members, for example, via receptors ALK1/2/3 and transcription factors SMAD1/5/8.1069,1070,1071,1072,1073 The significance of the signaling crosstalk within the TGF-β superfamily also warrants future exploration. Notably, Reblozyl (luspatercept or ACE-536), a ligand trap that contains the extracellular domain of human activin receptor type IIB (ActRIIB) to inhibit GDF11-mediated SMAD2/3 signaling has been approved by the US Federal Drug Agency (FDA) for the treatment of anemia in adult patients with β-thalassemia or with MDS.
对胚胎发育和伤口愈合的研究揭示了 TGF-β 的亚型特异性作用,但其他研究领域对此仍知之甚少,因为迄今为止对免疫稳态、纤维化疾病和肿瘤发展的研究都集中在最丰富的 TGF-β 上。尤其是β1亚型。由于所有 TGF-β 同种型都被认为通过相同的受体和下游途径发出信号,因此同种型之间生物学效应差异的原因尚未完全了解。此外,由于含有一种 TGF-β1 单体和一种 TGF-β2 单体的天然 TGF-β 异二聚体早已被发现, 12 , 1068未来鉴定和表征新型 TGF-β 异二聚体将非常有趣。此外,随着TGF-β超家族的发现和研究,该家族还包括与TGF-β结构相似的多肽,如结节、肌生长抑制素、抑制素、激活素、苗勒氏管抑制物质(MIS)、骨形态发生蛋白(BMP)以及生长和生长因子。除了分化因子 (GDF) 之外,研究人员还意识到 TGF-β 还可以通过其他 TGF-β 超家族成员“特异”的途径发出信号,例如通过受体 ALK1/2/3 和转录因子 SMAD1/5/8。 1069 , 1070 , 1071 , 1072 , 1073 TGF-β 超家族内信号串扰的重要性也值得未来探索。值得注意的是,Reblozyl(luspatercept 或 ACE-536)是一种配体捕获剂,含有人 IIB 型激活素受体 (ActRIIB) 的胞外结构域,可抑制 GDF11 介导的 SMAD2/3 信号传导,已获得美国联邦药物管理局 (FDA) 批准用于治疗治疗患有β-地中海贫血或MDS的成年患者的贫血。
As for TGF-β-targeting therapy, the efficacy and safety of treatment are always issues of concern. The current lack of systematic studies on the dural roles of TGF-β in wound healing, infectious diseases, and tumor development may hinder the development of related therapeutics. Given the extensive impacts of TGF-β on a lot of biological processes, the development of TGF-β isoform-specific therapies and SMAD-binding peptide aptamers is expected to cause less adverse effects through more precise targeting. Moreover, the identification of the applicable population for each therapeutic approach is also important for better efficacy and less toxicity. Serum and tissue levels of TGF-β have shown potential as predictors or indicators of the development,1074,1075,1076,1077 complication,1078,1079,1080 response,1081,1082,1083,1084 recurrence,1085,1086,1087 and outcomes1088,1089,1090 of various kinds of diseases, meanwhile, bioinformatic tools of TGF-β signaling-related gene expression signatures have also been developed for patient stratification.863,1091 But so far, TGF-β or related factors as clinical biomarkers still need further development and assessment.
对于TGF-β靶向治疗,治疗的有效性和安全性一直是人们关注的问题。目前缺乏关于 TGF-β 在伤口愈合、感染性疾病和肿瘤发展中的硬脑膜作用的系统研究,可能会阻碍相关疗法的发展。鉴于 TGF-β 对许多生物过程的广泛影响,TGF-β 异构体特异性疗法和 SMAD 结合肽适体的开发预计将通过更精确的靶向来减少不良反应。此外,确定每种治疗方法的适用人群对于提高疗效和降低毒性也很重要。 TGF-β的血清和组织水平已显示出作为发展、 1074、1075、1076、1077并发症、 1078、1079、1080反应、 1081、1082、1083、1084复发、 1085、1086、1087和各种疾病的结果1088 , 1089 , 1090 ,同时,还开发了 TGF-β 信号传导相关基因表达特征的生物信息学工具用于患者分层。 863 , 1091但迄今为止,TGF-β或相关因子作为临床生物标志物仍需要进一步的开发和评估。
To summarize, this review focuses on the multiple roles of TGF-β in health and disease while emphasizing the mechanisms of TGF-β production, activation, signaling, as well as corresponding therapeutic strategies. These understandings might be instructive for the basic and applied research of relevant topics in the future.
综上所述,本综述重点关注 TGF-β 在健康和疾病中的多重作用,同时强调 TGF-β 产生、激活、信号传导的机制以及相应的治疗策略。这些认识对于未来相关课题的基础和应用研究可能具有指导意义。
Data availability 数据可用性
Not applicable. 不适用。
References 参考
de Larco, J. E. & Todaro, G. J. Growth factors from murine sarcoma virus-transformed cells. Proc. Natl Acad. Sci. USA 75, 4001–4005 (1978).
de Larco, JE & Todaro, GJ 来自鼠肉瘤病毒转化细胞的生长因子。过程。国家科学院。科学。美国75,4001–4005 (1978)。Derynck, R. et al. Human transforming growth factor-beta complementary DNA sequence and expression in normal and transformed cells. Nature 316, 701–705 (1985).
Derynck,R.等人。人转化生长因子-β 互补 DNA 序列及其在正常细胞和转化细胞中的表达。自然316 , 701–705 (1985)。Roberts, A. B., Anzano, M. A., Lamb, L. C., Smith, J. M. & Sporn, M. B. New class of transforming growth factors potentiated by epidermal growth factor: isolation from non-neoplastic tissues. Proc. Natl Acad. Sci. USA 78, 5339–5343 (1981).
Roberts, AB、Anzano, MA、Lamb, LC、Smith, JM 和 Sporn, MB 表皮生长因子增强的新型转化生长因子:从非肿瘤组织中分离。过程。国家科学院。科学。美国78,5339–5343 (1981)。Proper, J. A., Bjornson, C. L. & Moses, H. L. Mouse embryos contain polypeptide growth factor(s) capable of inducing a reversible neoplastic phenotype in nontransformed cells in culture. J. Cell Physiol. 110, 169–174 (1982).
Proper, JA, Bjornson, CL & Moses, HL 小鼠胚胎含有能够在培养的非转化细胞中诱导可逆肿瘤表型的多肽生长因子。 J.细胞生理学。 110、169-174 (1982)。Childs, C. B., Proper, J. A., Tucker, R. F. & Moses, H. L. Serum contains a platelet-derived transforming growth factor. Proc. Natl Acad. Sci. USA 79, 5312–5316 (1982).
Childs, CB, Proper, JA, Tucker, RF 和 Moses, HL 血清含有血小板衍生的转化生长因子。过程。国家科学院。科学。美国79,5312–5316 (1982)。Moses, H. L., Branum, E. L., Proper, J. A. & Robinson, R. A. Transforming growth factor production by chemically transformed cells. Cancer Res 41, 2842–2848 (1981).
Moses, HL、Branum, EL、Proper, JA 和 Robinson, RA 通过化学转化细胞生产转化生长因子。癌症研究41 , 2842–2848 (1981)。Assoian, R. K., Komoriya, A., Meyers, C. A., Miller, D. M. & Sporn, M. B. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J. Biol. Chem. 258, 7155–7160 (1983).
Assoian, RK、Komoriya, A.、Meyers, CA、Miller, DM 和 Sporn, MB 人血小板中的转化生长因子-β。主要储存地点的识别、纯化和表征。 J.Biol。化学。 258、7155–7160 (1983)。Roberts, A. B. et al. Purification and properties of a type beta transforming growth factor from bovine kidney. Biochemistry 22, 5692–5698 (1983).
罗伯茨,AB 等人。牛肾β型转化生长因子的纯化和性质。生物化学22 , 5692–5698 (1983)。Miyazono, K., Hellman, U., Wernstedt, C. & Heldin, C. H. Latent high molecular weight complex of transforming growth factor beta 1. Purification from human platelets and structural characterization. J. Biol. Chem. 263, 6407–6415 (1988).
Miyazono, K.、Hellman, U.、Wernstedt, C. 和 Heldin, CH 转化生长因子 β 1 的潜在高分子量复合物。从人血小板中纯化并进行结构表征。 J.Biol。化学。 263、6407-6415 (1988)。Wakefield, L. M., Smith, D. M., Flanders, K. C. & Sporn, M. B. Latent transforming growth factor-beta from human platelets. A high molecular weight complex containing precursor sequences. J. Biol. Chem. 263, 7646–7654 (1988).
Wakefield, LM、Smith, DM、Flanders, KC 和 Sporn, MB 来自人类血小板的潜在转化生长因子-β。含有前体序列的高分子量复合物。 J.Biol。化学。 263、7646–7654 (1988)。Lawrence, D. A., Pircher, R. & Jullien, P. Conversion of a high molecular weight latent beta-TGF from chicken embryo fibroblasts into a low molecular weight active beta-TGF under acidic conditions. Biochem Biophys. Res Commun. 133, 1026–1034 (1985).
Lawrence, DA、Pircher, R. 和 Jullien, P. 在酸性条件下将鸡胚成纤维细胞的高分子量潜在 β-TGF 转化为低分子量活性 β-TGF。生物化学生物物理学。资源通讯。 133、1026-1034 (1985)。Cheifetz, S. et al. The transforming growth factor-beta system, a complex pattern of cross-reactive ligands and receptors. Cell 48, 409–415 (1987).
切菲茨,S.等人。转化生长因子-β系统,交叉反应配体和受体的复杂模式。细胞48 , 409–415 (1987)。ten Dijke, P., Hansen, P., Iwata, K. K., Pieler, C. & Foulkes, J. G. Identification of another member of the transforming growth factor type beta gene family. Proc. Natl Acad. Sci. USA 85, 4715–4719 (1988).
10 Dijke, P.、Hansen, P.、Iwata, KK、Pieler, C. 和 Foulkes, JG 转化生长因子型 β 基因家族的另一个成员的鉴定。过程。国家科学院。科学。美国85,4715–4719 (1988)。Derynck, R. et al. A new type of transforming growth factor-beta, TGF-beta 3. EMBO J. 7, 3737–3743 (1988).
Derynck,R.等人。一种新型转化生长因子-β,TGF-β 3。EMBO J. 7,3737-3743 (1988)。Tucker, R. F., Shipley, G. D., Moses, H. L. & Holley, R. W. Growth inhibitor from BSC-1 cells closely related to platelet type beta transforming growth factor. Science 226, 705–707 (1984).
Tucker, RF, Shipley, GD, Moses, HL & Holley, RW 来自 BSC-1 细胞的生长抑制剂与血小板型 β 转化生长因子密切相关。科学226 , 705–707 (1984)。Holley, R. W., Armour, R. & Baldwin, J. H. Density-dependent regulation of growth of BSC-1 cells in cell culture: growth inhibitors formed by the cells. Proc. Natl Acad. Sci. USA 75, 1864–1866 (1978).
Holley, RW、Armour, R. 和 Baldwin, JH 细胞培养中 BSC-1 细胞生长的密度依赖性调节:细胞形成的生长抑制剂。过程。国家科学院。科学。美国75,1864-1866 (1978)。Ignotz, R. A. & Massague, J. Type beta transforming growth factor controls the adipogenic differentiation of 3T3 fibroblasts. Proc. Natl Acad. Sci. USA 82, 8530–8534 (1985).
Ignotz, RA 和 Massague, J。β 型转化生长因子控制 3T3 成纤维细胞的脂肪形成分化。过程。国家科学院。科学。美国82 , 8530–8534 (1985)。Seyedin, S. M., Thomas, T. C., Thompson, A. Y., Rosen, D. M. & Piez, K. A. Purification and characterization of two cartilage-inducing factors from bovine demineralized bone. Proc. Natl Acad. Sci. USA 82, 2267–2271 (1985).
Seyedin, SM, Thomas, TC, Thompson, AY, Rosen, DM & Piez, KA 牛脱矿骨中两种软骨诱导因子的纯化和表征。过程。国家科学院。科学。美国82,2267–2271 (1985)。Heine, U. et al. Role of transforming growth factor-beta in the development of the mouse embryo. J. Cell Biol. 105, 2861–2876 (1987).
海涅,U.等人。转化生长因子-β 在小鼠胚胎发育中的作用。 J.细胞生物学。 105、2861-2876 (1987)。Sporn, M. B. et al. Polypeptide transforming growth factors isolated from bovine sources and used for wound healing in vivo. Science 219, 1329–1331 (1983).
Sporn,MB 等人。从牛源分离并用于体内伤口愈合的多肽转化生长因子。科学219 , 1329–1331 (1983)。Rook, A. H. et al. Effects of transforming growth factor beta on the functions of natural killer cells: depressed cytolytic activity and blunting of interferon responsiveness. J. Immunol. 136, 3916–3920 (1986).
鲁克,AH 等人。转化生长因子β对自然杀伤细胞功能的影响:细胞溶解活性降低和干扰素反应性减弱。 J.免疫学。 136、3916-3920 (1986)。Kehrl, J. H. et al. Transforming growth factor beta is an important immunomodulatory protein for human B lymphocytes. J. Immunol. 137, 3855–3860 (1986).
凯尔,JH 等人。转化生长因子β是人类B淋巴细胞的重要免疫调节蛋白。 J.免疫学。 137、3855–3860 (1986)。Border, W. A., Okuda, S., Languino, L. R., Sporn, M. B. & Ruoslahti, E. Suppression of experimental glomerulonephritis by antiserum against transforming growth factor beta 1. Nature 346, 371–374 (1990).
Border, WA, Okuda, S., Languino, LR, Sporn, MB 和 Ruoslahti, E. 通过抗转化生长因子 β 1 的抗血清抑制实验性肾小球肾炎。 Nature 346 , 371–374 (1990)。Connor, T. B. Jr. et al. Correlation of fibrosis and transforming growth factor-beta type 2 levels in the eye. J. Clin. Invest 83, 1661–1666 (1989).
康纳,TB Jr. 等人。眼睛纤维化和转化生长因子-β 2 型水平的相关性。 J.克林。投资83,1661–1666 (1989)。Pierce, D. F. Jr. et al. Mammary tumor suppression by transforming growth factor beta 1 transgene expression. Proc. Natl Acad. Sci. USA 92, 4254–4258 (1995).
皮尔斯,DF Jr. 等人。通过转化生长因子β1转基因表达来抑制乳腺肿瘤。过程。国家科学院。科学。美国92,4254–4258 (1995)。Markowitz, S. et al. Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability. Science 268, 1336–1338 (1995).
马科维茨,S.等人。具有微卫星不稳定性的结肠癌细胞中 II 型 TGF-β 受体失活。科学268 , 1336–1338 (1995)。Massague, J., Czech, M. P., Iwata, K., DeLarco, J. E. & Todaro, G. J. Affinity labeling of a transforming growth factor receptor that does not interact with epidermal growth factor. Proc. Natl Acad. Sci. USA 79, 6822–6826 (1982).
Massague, J.、Czech, MP、Iwata, K.、DeLarco, JE 和 Todaro, GJ 不与表皮生长因子相互作用的转化生长因子受体的亲和标记。过程。国家科学院。科学。美国79,6822–6826 (1982)。Frolik, C. A., Wakefield, L. M., Smith, D. M. & Sporn, M. B. Characterization of a membrane receptor for transforming growth factor-beta in normal rat kidney fibroblasts. J. Biol. Chem. 259, 10995–11000 (1984).
Frolik, CA、Wakefield, LM、Smith, DM 和 Sporn, MB 正常大鼠肾成纤维细胞中转化生长因子-β 膜受体的表征。 J.Biol。化学。 259 , 10995–11000 (1984)。Massagué, J. & Like, B. Cellular receptors for type beta transforming growth factor. Ligand binding and affinity labeling in human and rodent cell lines. J. Biol. Chem. 260, 2636–2645 (1985).
Massagué, J. & Like, B. β 型转化生长因子的细胞受体。人类和啮齿动物细胞系中的配体结合和亲和标记。 J.Biol。化学。 260 , 2636–2645 (1985)。Eppert, K. et al. MADR2 maps to 18q21 and encodes a TGFbeta-regulated MAD-related protein that is functionally mutated in colorectal carcinoma. Cell 86, 543–552 (1996).
埃珀特,K.等人。 MADR2 定位于 18q21,编码一种 TGFbeta 调节的 MAD 相关蛋白,该蛋白在结直肠癌中发生功能突变。细胞86 , 543–552 (1996)。Zhang, Y., Feng, X., We, R. & Derynck, R. Receptor-associated Mad homologues synergize as effectors of the TGF-beta response. Nature 383, 168–172 (1996).
张,Y.,冯,X.,我们,R. 和德林克,R. 受体相关的 Mad 同系物作为 TGF-β 反应的效应器协同作用。自然383 , 168–172 (1996)。Hahn, S. A. et al. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science 271, 350–353 (1996).
哈恩,SA等人。 DPC4,人类染色体18q21.1的候选抑癌基因。科学271 , 350–353 (1996)。Schlingensiepen, K. H. et al. Targeted tumor therapy with the TGF-beta 2 antisense compound AP 12009. Cytokine Growth Factor Rev. 17, 129–139 (2006).
施林根西彭,KH 等人。使用 TGF-β 2 反义化合物 AP 12009 进行靶向肿瘤治疗。细胞因子生长因子 Rev. 17 , 129–139 (2006)。Fakhrai, H. et al. Phase I clinical trial of a TGF-beta antisense-modified tumor cell vaccine in patients with advanced glioma. Cancer Gene Ther. 13, 1052–1060 (2006).
Fakhrai,H. 等人。 TGF-β反义修饰肿瘤细胞疫苗在晚期神经胶质瘤患者中进行的 I 期临床试验。癌症基因疗法。 13、1052-1060 (2006)。Nemunaitis, J. et al. Phase II study of belagenpumatucel-L, a transforming growth factor beta-2 antisense gene-modified allogeneic tumor cell vaccine in non-small-cell lung cancer. J. Clin. Oncol. 24, 4721–4730 (2006).
Nemunaitis,J.等人。 belagenpumatucel-L(一种转化生长因子β2反义基因修饰的同种异体肿瘤细胞疫苗)治疗非小细胞肺癌的 II 期研究。 J.克林。安科尔。 24 , 4721–4730 (2006)。Trachtman, H. et al. A phase 1, single-dose study of fresolimumab, an anti-TGF-β antibody, in treatment-resistant primary focal segmental glomerulosclerosis. Kidney Int 79, 1236–1243 (2011).
特拉赫特曼,H.等人。 fresolimumab(一种抗 TGF-β 抗体)治疗难治性原发性局灶节段性肾小球硬化症的 1 期单剂量研究。肾脏国际79 , 1236–1243 (2011)。Rodon, J. et al. First-in-human dose study of the novel transforming growth factor-β receptor I kinase inhibitor LY2157299 monohydrate in patients with advanced cancer and glioma. Clin. Cancer Res 21, 553–560 (2015).
罗登,J.等人。新型转化生长因子-β受体 I 激酶抑制剂 LY2157299 一水合物在晚期癌症和神经胶质瘤患者中的首次人体剂量研究。临床。癌症研究21 , 553–560 (2015)。Rodón, J. et al. Pharmacokinetic, pharmacodynamic and biomarker evaluation of transforming growth factor-β receptor I kinase inhibitor, galunisertib, in phase 1 study in patients with advanced cancer. Invest N. Drugs 33, 357–370 (2015).
罗东,J.等人。转化生长因子-β 受体 I 激酶抑制剂 galunisertib 在晚期癌症患者的 1 期研究中的药代动力学、药效学和生物标志物评估。投资 N. 药物33 , 357–370 (2015)。Strauss, J. et al. Phase I Trial of M7824 (MSB0011359C), a Bifunctional Fusion Protein Targeting PD-L1 and TGFβ, in Advanced Solid Tumors. Clin. Cancer Res 24, 1287–1295 (2018).
施特劳斯,J.等人。 M7824 (MSB0011359C) 是一种针对 PD-L1 和 TGFβ 的双功能融合蛋白,在晚期实体瘤中的 I 期试验。临床。癌症研究24 , 1287–1295 (2018)。Dubois, C. M., Laprise, M. H., Blanchette, F., Gentry, L. E. & Leduc, R. Processing of transforming growth factor beta 1 precursor by human furin convertase. J. Biol. Chem. 270, 10618–10624 (1995).
Dubois,CM,Laprise,MH,Blanchette,F.,Gentry,LE 和 Leduc,R。人弗林蛋白酶转化酶对转化生长因子 β 1 前体的加工。 J.Biol。化学。 270、10618–10624 (1995)。Shi, M. et al. Latent TGF-beta structure and activation. Nature 474, 343–349 (2011).
石,M.等人。潜在的 TGF-β 结构和激活。自然474 , 343–349 (2011)。Miyazono, K., Olofsson, A., Colosetti, P. & Heldin, C. H. A role of the latent TGF-beta 1-binding protein in the assembly and secretion of TGF-beta 1. EMBO J. 10, 1091–1101 (1991).
Miyazono, K.、Olofsson, A.、Colosetti, P. 和 Heldin, CH 潜在 TGF-β 1 结合蛋白在 TGF-β 1 组装和分泌中的作用。 EMBO J. 10 , 1091–1101 ( 1991)。Lockhart-Cairns, M. P. et al. Latent TGFbeta complexes are transglutaminase cross-linked to fibrillin to facilitate TGFbeta activation. Matrix Biol. 107, 24–39 (2022).
洛克哈特-凯恩斯,议员等。潜在的 TGFbeta 复合物通过转谷氨酰胺酶与原纤维蛋白交联,以促进 TGFbeta 激活。基质生物。 107 , 24–39 (2022)。Tran, D. Q. et al. GARP (LRRC32) is essential for the surface expression of latent TGF-beta on platelets and activated FOXP3+ regulatory T cells. Proc. Natl Acad. Sci. USA 106, 13445–13450 (2009).
特兰,DQ 等人。 GARP (LRRC32) 对于血小板和激活的 FOXP3+ 调节性 T 细胞上潜在 TGF-β 的表面表达至关重要。过程。国家科学院。科学。美国106,13445–13450 (2009)。Qin, Y. et al. A Milieu Molecule for TGF-beta Required for Microglia Function in the Nervous System. Cell 174, 156–171.e116 (2018).
秦,Y.等人。神经系统小胶质细胞功能所需的 TGF-β 环境分子。单元格174,156–171.e116 (2018)。Annes, J. P., Rifkin, D. B. & Munger, J. S. The integrin alphaVbeta6 binds and activates latent TGFbeta3. FEBS Lett. 511, 65–68 (2002).
Annes, JP、Rifkin, DB 和 Munger, JS 整合素 alphaVbeta6 结合并激活潜在的 TGFbeta3。 FEBS 快报。 511 , 65–68 (2002)。Breuss, J. M. et al. Expression of the beta 6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J. Cell Sci. 108, 2241–2251 (1995).
布劳斯,JM 等人。 β6 整合素亚基在发育、肿瘤形成和组织修复中的表达表明其在上皮重塑中发挥作用。 J.细胞科学。 108、2241-2251 (1995)。Mu, D. et al. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J. Cell Biol. 157, 493–507 (2002).
穆,D.等人。整合素 α(v)beta8 通过 MT1-MMP 依赖性激活 TGF-β1 介导上皮稳态。 J.细胞生物学。 157、493-507 (2002)。Kitamura, H. et al. Mouse and human lung fibroblasts regulate dendritic cell trafficking, airway inflammation, and fibrosis through integrin alphavbeta8-mediated activation of TGF-beta. J. Clin. Invest 121, 2863–2875 (2011).
北村,H.等人。小鼠和人肺成纤维细胞通过整合素 alphavbeta8 介导的 TGF-beta 激活来调节树突状细胞运输、气道炎症和纤维化。 J.克林。投资121 , 2863–2875 (2011)。Kelly, A. et al. Human monocytes and macrophages regulate immune tolerance via integrin alphavbeta8-mediated TGFbeta activation. J. Exp. Med 215, 2725–2736 (2018).
凯利,A.等人。人类单核细胞和巨噬细胞通过整合素 alphavbeta8 介导的 TGFbeta 激活调节免疫耐受。 J.Exp。医学215 , 2725–2736 (2018)。Worthington, J. J., Czajkowska, B. I., Melton, A. C. & Travis, M. A. Intestinal dendritic cells specialize to activate transforming growth factor-beta and induce Foxp3+ regulatory T cells via integrin alphavbeta8. Gastroenterology 141, 1802–1812 (2011).
Worthington, JJ、Czajkowska, BI、Melton, AC 和 Travis, MA 肠树突细胞专门激活转化生长因子-β,并通过整合素 alphavbeta8 诱导 Foxp3+ 调节性 T 细胞。胃肠病学141 , 1802–1812 (2011)。Laine, A. et al. Regulatory T cells promote cancer immune-escape through integrin alphavbeta8-mediated TGF-beta activation. Nat. Commun. 12, 6228 (2021).
莱恩,A.等人。调节性 T 细胞通过整合素 alphavbeta8 介导的 TGF-beta 激活促进癌症免疫逃逸。纳特。交流。 12、6228 (2021)。Takasaka, N. et al. Integrin alphavbeta8-expressing tumor cells evade host immunity by regulating TGF-beta activation in immune cells. JCI Insight 3, e122591 (2018).
高坂,N.等人。表达整合素αvβ8的肿瘤细胞通过调节免疫细胞中TGF-β的激活来逃避宿主免疫。 JCI 洞察3 ,e122591 (2018)。Yang, Z. et al. Absence of integrin-mediated TGFbeta1 activation in vivo recapitulates the phenotype of TGFbeta1-null mice. J. Cell Biol. 176, 787–793 (2007).
杨,Z.等人。体内整合素介导的 TGFbeta1 激活的缺失概括了 TGFbeta1 缺失小鼠的表型。 J.细胞生物学。 176、787-793 (2007)。Aluwihare, P. et al. Mice that lack activity of alphavbeta6- and alphavbeta8-integrins reproduce the abnormalities of Tgfb1- and Tgfb3-null mice. J. Cell Sci. 122, 227–232 (2009).
Aluwihare,P. 等人。缺乏 alphavbeta6 和 alphavbeta8 整合素活性的小鼠会重现 Tgfb1 和 Tgfb3 缺失小鼠的异常。 J.细胞科学。 122、227-232 (2009)。Dong, X. et al. Force interacts with macromolecular structure in activation of TGF-beta. Nature 542, 55–59 (2017).
董,X.等人。力与大分子结构相互作用,激活 TGF-β。自然542 , 55–59 (2017)。Wang, R. et al. GARP regulates the bioavailability and activation of TGFbeta. Mol. Biol. Cell 23, 1129–1139 (2012).
王,R.等人。 GARP 调节 TGFbeta 的生物利用度和激活。摩尔。生物。细胞23,1129–1139 (2012)。Edwards, J. P. et al. Regulation of the expression of GARP/latent TGF-beta1 complexes on mouse T cells and their role in regulatory T cell and Th17 differentiation. J. Immunol. 190, 5506–5515 (2013).
爱德华兹,JP 等人。 GARP/潜在 TGF-β1 复合物在小鼠 T 细胞上表达的调节及其在调节性 T 细胞和 Th17 分化中的作用。 J.免疫学。 190、5506–5515 (2013)。Stockis, J. et al. Blocking immunosuppression by human Tregs in vivo with antibodies targeting integrin alphaVbeta8. Proc. Natl Acad. Sci. USA 114, E10161–E10168 (2017).
斯托克斯,J.等人。使用针对整合素 alphaVbeta8 的抗体在体内阻断人类 Tregs 的免疫抑制。过程。国家科学院。科学。美国114 ,E10161–E10168 (2017)。Campbell, M. G. et al. Cryo-EM Reveals Integrin-Mediated TGF-beta Activation without Release from Latent TGF-beta. Cell 180, 490–501.e416 (2020).
坎贝尔,MG 等人。冷冻电镜揭示了整合素介导的 TGF-β 激活,而不释放潜在的 TGF-β。单元180,490–501.e416 (2020)。Lawrence, D. A., Pircher, R., Krycève-Martinerie, C. & Jullien, P. Normal embryo fibroblasts release transforming growth factors in a latent form. J. Cell Physiol. 121, 184–188 (1984).
Lawrence, DA、Pircher, R.、Krycève-Martinerie, C. 和 Jullien, P. 正常胚胎成纤维细胞以潜在形式释放转化生长因子。 J.细胞生理学。 121、184-188 (1984)。Brown, P. D., Wakefield, L. M., Levinson, A. D. & Sporn, M. B. Physicochemical activation of recombinant latent transforming growth factor-beta’s 1, 2, and 3. Growth Factors 3, 35–43 (1990).
Brown, PD、Wakefield, LM、Levinson, AD 和 Sporn, MB 重组潜在转化生长因子-β 1、2 和 3 的物理化学激活。生长因子3,35-43 (1990)。Jullien, P., Berg, T. M. & Lawrence, D. A. Acidic cellular environments: activation of latent TGF-beta and sensitization of cellular responses to TGF-beta and EGF. Int J. Cancer 43, 886–891 (1989).
Jullien, P.、Berg, TM 和 Lawrence, DA 酸性细胞环境:潜在 TGF-β 的激活以及细胞对 TGF-β 和 EGF 反应的敏感性。国际癌症杂志43,886-891 (1989)。Silver, I. A., Murrills, R. J. & Etherington, D. J. Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp. Cell Res 175, 266–276 (1988).
Silver, IA, Murrills, RJ 和 Etherington, DJ 微电极研究粘附巨噬细胞和破骨细胞下方的酸性微环境。过期。细胞研究175 , 266–276 (1988)。Kottmann, R. M. et al. Lactic acid is elevated in idiopathic pulmonary fibrosis and induces myofibroblast differentiation via pH-dependent activation of transforming growth factor-beta. Am. J. Respir. Crit. Care Med 186, 740–751 (2012).
科特曼,RM 等人。乳酸在特发性肺纤维化中升高,并通过 pH 依赖性的转化生长因子-β 激活诱导肌成纤维细胞分化。是。 J.呼吸。暴击。护理医学186 , 740–751 (2012)。Jobling, M. F. et al. Isoform-specific activation of latent transforming growth factor beta (LTGF-beta) by reactive oxygen species. Radiat. Res 166, 839–848 (2006).
乔布林,MF 等人。活性氧对潜在转化生长因子β (LTGF-β) 的亚型特异性激活。辐射。第 166 号决议, 839–848 (2006)。Hayashi, H., Sakai, K., Baba, H. & Sakai, T. Thrombospondin-1 is a novel negative regulator of liver regeneration after partial hepatectomy through transforming growth factor-beta1 activation in mice. Hepatology 55, 1562–1573 (2012).
Hayashi, H.、Sakai, K.、Baba, H. 和 Sakai, T. Thrombospondin-1 是部分肝切除术后通过转化生长因子-β1 激活小鼠肝脏再生的新型负调节因子。肝病学55 , 1562–1573 (2012)。Wang, H. & Kochevar, I. E. Involvement of UVB-induced reactive oxygen species in TGF-beta biosynthesis and activation in keratinocytes. Free Radic. Biol. Med 38, 890–897 (2005).
Wang, H. & Kochevar, IE UVB 诱导的活性氧参与角质形成细胞的 TGF-β 生物合成和激活。自由基。生物。医学38 , 890–897 (2005)。Pociask, D. A., Sime, P. J. & Brody, A. R. Asbestos-derived reactive oxygen species activate TGF-beta1. Lab Invest 84, 1013–1023 (2004).
Pociask, DA, Sime, PJ 和 Brody, AR 石棉衍生的活性氧可激活 TGF-β1。实验室投资84 , 1013–1023 (2004)。Sullivan, D. E., Ferris, M., Pociask, D. & Brody, A. R. The latent form of TGFbeta(1) is induced by TNFalpha through an ERK specific pathway and is activated by asbestos-derived reactive oxygen species in vitro and in vivo. J. Immunotoxicol. 5, 145–149 (2008).
Sullivan, DE、Ferris, M.、Pociask, D. 和 Brody, AR 潜在形式的 TGFbeta(1) 由 TNFα 通过 ERK 特异性途径诱导,并在体外和体内被石棉衍生的活性氧激活。 J.免疫毒理学。 5、145-149 (2008)。Barcellos-Hoff, M. H. & Dix, T. A. Redox-mediated activation of latent transforming growth factor-beta 1. Mol. Endocrinol. 10, 1077–1083 (1996).
Barcellos-Hoff,MH 和 Dix,TA 氧化还原介导的潜在转化生长因子-β 1 的激活。Mol 。内分泌。 10、1077-1083 (1996)。Ning, W. et al. Effect of high glucose supplementation on pulmonary fibrosis involving reactive oxygen species and TGF-beta. Front Nutr. 9, 998662 (2022).
宁,W.等人。高糖补充对涉及活性氧和TGF-β的肺纤维化的影响。前螺母。 9、998662 (2022)。Zhang, D. et al. High Glucose Intake Exacerbates Autoimmunity through Reactive-Oxygen-Species-Mediated TGF-beta Cytokine Activation. Immunity 51, 671–681.e675 (2019).
张,D.等人。高葡萄糖摄入通过活性氧介导的 TGF-β 细胞因子激活加剧自身免疫。免疫51 , 671–681.e675 (2019)。Chen, W., Frank, M. E., Jin, W. & Wahl, S. M. TGF-beta released by apoptotic T cells contributes to an immunosuppressive milieu. Immunity 14, 715–725 (2001).
Chen, W.、Frank, ME、Jin, W. 和 Wahl, SM 凋亡 T 细胞释放的 TGF-β 有助于形成免疫抑制环境。免疫14 , 715–725 (2001)。Amarnath, S., Dong, L., Li, J., Wu, Y. & Chen, W. Endogenous TGF-beta activation by reactive oxygen species is key to Foxp3 induction in TCR-stimulated and HIV-1-infected human CD4+CD25- T cells. Retrovirology 4, 57 (2007).
Amarnath, S.、Dong, L.、Li, J.、Wu, Y. 和 Chen, W. 活性氧物质的内源性 TGF-β 激活是 TCR 刺激和 HIV-1 感染的人 CD4 中 Foxp3 诱导的关键+CD25- T 细胞。逆转录病毒学4 , 57 (2007)。Sweetwyne, M. T. & Murphy-Ullrich, J. E. Thrombospondin1 in tissue repair and fibrosis: TGF-beta-dependent and independent mechanisms. Matrix Biol. 31, 178–186 (2012).
Sweetwyne, MT 和 Murphy-Ullrich, JE 组织修复和纤维化中的血小板反应蛋白 1:TGF-β 依赖性和独立机制。基质生物。 31、178-186 (2012)。Murphy-Ullrich, J. E. & Suto, M. J. Thrombospondin-1 regulation of latent TGF-beta activation: A therapeutic target for fibrotic disease. Matrix Biol. 68-69, 28–43 (2018).
Murphy-Ullrich, JE & Suto, MJ Thrombospondin-1 对潜在 TGF-β 激活的调节:纤维化疾病的治疗靶点。基质生物。 68-69、28-43 (2018)。Murphy-Ullrich, J. E., Schultz-Cherry, S. & Hook, M. Transforming growth factor-beta complexes with thrombospondin. Mol. Biol. Cell 3, 181–188 (1992).
Murphy-Ullrich,JE,Schultz-Cherry,S. 和 Hook,M。用血小板反应蛋白转化生长因子-β 复合物。摩尔。生物。细胞3,181-188 (1992)。Yung, S. et al. Elevated glucose induction of thrombospondin-1 up-regulates fibronectin synthesis in proximal renal tubular epithelial cells through TGF-beta1 dependent and TGF-beta1 independent pathways. Nephrol. Dial. Transpl. 21, 1504–1513 (2006).
荣,S.等人。血小板反应蛋白-1 的葡萄糖诱导升高可通过 TGF-β1 依赖性和 TGF-β1 独立途径上调近端肾小管上皮细胞中纤连蛋白的合成。肾病。拨号。翻译。 21、1504-1513 (2006)。Naito, T. et al. Angiotensin II induces thrombospondin-1 production in human mesangial cells via p38 MAPK and JNK: a mechanism for activation of latent TGF-beta1. Am. J. Physiol. Ren. Physiol. 286, F278–F287 (2004).
内藤,T.等人。血管紧张素 II 通过 p38 MAPK 和 JNK 诱导人系膜细胞产生血小板反应蛋白-1:一种激活潜在 TGF-β1 的机制。是。 J.生理学。任。生理学。 286 ,F278-F287(2004)。Kumar, R. et al. Interstitial macrophage-derived thrombospondin-1 contributes to hypoxia-induced pulmonary hypertension. Cardiovasc Res 116, 2021–2030 (2020).
库马尔,R.等人。间质巨噬细胞衍生的血小板反应蛋白-1 有助于缺氧诱导的肺动脉高压。心血管研究116,2021–2030 (2020)。Matsuba, M., Hutcheon, A. E. & Zieske, J. D. Localization of thrombospondin-1 and myofibroblasts during corneal wound repair. Exp. Eye Res 93, 534–540 (2011).
Matsuba, M.、Hutcheon, AE 和 Zieske, JD 角膜伤口修复过程中血小板反应蛋白-1 和肌成纤维细胞的定位。过期。眼睛研究93 , 534–540 (2011)。Doyen, V. et al. Thrombospondin 1 is an autocrine negative regulator of human dendritic cell activation. J. Exp. Med 198, 1277–1283 (2003).
Doyen,V.等人。 Thrombospondin 1 是人树突状细胞活化的自分泌负调节因子。 J.Exp。医学198 , 1277–1283 (2003)。McMaken, S. et al. Thrombospondin-1 contributes to mortality in murine sepsis through effects on innate immunity. PLoS One 6, e19654 (2011).
麦克马肯,S.等人。 Thrombospondin-1 通过影响先天免疫而导致小鼠败血症死亡。 PLoS One 6 ,e19654 (2011)。Presser, L. D., Haskett, A. & Waris, G. Hepatitis C virus-induced furin and thrombospondin-1 activate TGF-beta1: role of TGF-beta1 in HCV replication. Virology 412, 284–296 (2011).
Presser, LD, Haskett, A. & Waris, G. 丙型肝炎病毒诱导的弗林蛋白酶和血小板反应蛋白-1 激活 TGF-β1:TGF-β1 在 HCV 复制中的作用。病毒学412 , 284–296 (2011)。Kumar, R. et al. TGF-beta activation by bone marrow-derived thrombospondin-1 causes Schistosoma- and hypoxia-induced pulmonary hypertension. Nat. Commun. 8, 15494 (2017).
库马尔,R.等人。骨髓来源的血小板反应蛋白-1 激活 TGF-β 会导致血吸虫和缺氧诱导的肺动脉高压。纳特。交流。 8、15494 (2017)。Zhou, Y., Poczatek, M. H., Berecek, K. H. & Murphy-Ullrich, J. E. Thrombospondin 1 mediates angiotensin II induction of TGF-beta activation by cardiac and renal cells under both high and low glucose conditions. Biochem Biophys. Res Commun. 339, 633–641 (2006).
Zhou, Y.、Poczatek, MH、Berecek, KH 和 Murphy-Ullrich, JE 在高糖和低糖条件下,血小板反应蛋白 1 介导血管紧张素 II 诱导心脏和肾细胞的 TGF-β 激活。生物化学生物物理学。资源通讯。 339、633-641 (2006)。Atanasova, V. S. et al. Thrombospondin-1 Is a Major Activator of TGF-beta Signaling in Recessive Dystrophic Epidermolysis Bullosa Fibroblasts. J. Invest Dermatol 139, 1497–1505.e1495 (2019).
阿塔纳索瓦,VS 等人。 Thrombospondin-1 是隐性营养不良性大疱性表皮松解症成纤维细胞中 TGF-β 信号转导的主要激活剂。 J. Invest Dermatol 139 , 1497–1505.e1495 (2019)。Presser, L. D., Haskett, A. & Waris, G. Hepatitis C virus-induced furin and thrombospondin-1 activate TGF-β1: role of TGF-β1 in HCV replication. Virology 412, 284–296 (2011).
Presser, LD, Haskett, A. & Waris, G. 丙型肝炎病毒诱导的弗林蛋白酶和血小板反应蛋白-1 激活 TGF-β1:TGF-β1 在 HCV 复制中的作用。病毒学412 , 284–296 (2011)。Matsumura, K. et al. Thrombospondin-1 overexpression stimulates loss of Smad4 and accelerates malignant behavior via TGF-beta signal activation in pancreatic ductal adenocarcinoma. Transl. Oncol. 26, 101533 (2022).
松村,K.等人。 Thrombospondin-1 过表达会刺激 Smad4 缺失,并通过胰腺导管腺癌中 TGF-β 信号激活加速恶性行为。译。安科尔。 26、101533 (2022)。Jenkins, G. The role of proteases in transforming growth factor-beta activation. Int J. Biochem. Cell Biol. 40, 1068–1078 (2008).
詹金斯,G. 蛋白酶在转化生长因子-β 激活中的作用。国际生物化学杂志。细胞生物学。 40、1068-1078 (2008)。Lu, L. et al. Restoration of intrahepatic regulatory T cells through MMP-9/13-dependent activation of TGF-beta is critical for immune homeostasis following acute liver injury. J. Mol. Cell Biol. 5, 369–379 (2013).
卢,L.等人。通过 MMP-9/13 依赖性 TGF-β 激活恢复肝内调节性 T 细胞对于急性肝损伤后的免疫稳态至关重要。 J.莫尔。细胞生物学。 5、369-379 (2013)。Bai, P. et al. Macrophage-Derived Legumain Promotes Pulmonary Hypertension by Activating the MMP (Matrix Metalloproteinase)-2/TGF (Transforming Growth Factor)-β1 Signaling. Arterioscler Thromb. Vasc. Biol. 39, e130–e145 (2019).
Bai,P.等人。巨噬细胞衍生的 Legumain 通过激活 MMP(基质金属蛋白酶)-2/TGF(转化生长因子)-β1 信号传导促进肺动脉高压。动脉硬化血栓。瓦斯克。生物。 39 ,e130–e145(2019)。Feng, W. et al. Matrix metalloproteinase-9 regulates afferent arteriolar remodeling and function in hypertension-induced kidney disease. Kidney Int 104, 740–753 (2023).
冯,W.等人。基质金属蛋白酶-9 调节高血压引起的肾脏疾病中的传入小动脉重塑和功能。肾脏国际104,740–753 (2023)。Espindola, M. S. et al. Differential Responses to Targeting Matrix Metalloproteinase 9 in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med 203, 458–470 (2021).
埃斯平多拉,MS 等人。特发性肺纤维化中靶向基质金属蛋白酶 9 的不同反应。是。 J.呼吸。暴击。护理医学203 , 458–470 (2021)。Yu, Q. & Stamenkovic, I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 14, 163–176 (2000).
Yu, Q. & Stamenkovic, I. 细胞表面定位的基质金属蛋白酶-9 蛋白水解激活 TGF-β 并促进肿瘤侵袭和血管生成。基因开发。 14、163-176 (2000)。Yehualaeshet, T. et al. Activation of rat alveolar macrophage-derived latent transforming growth factor beta-1 by plasmin requires interaction with thrombospondin-1 and its cell surface receptor, CD36. Am. J. Pathol. 155, 841–851 (1999).
Yehualaeshet,T.等人。纤溶酶激活大鼠肺泡巨噬细胞衍生的潜在转化生长因子 β-1 需要与血小板反应蛋白-1 及其细胞表面受体 CD36 相互作用。是。 J.帕索尔. 155、841-851 (1999)。Nunes, I., Shapiro, R. L. & Rifkin, D. B. Characterization of latent TGF-beta activation by murine peritoneal macrophages. J. Immunol. 155, 1450–1459 (1995).
Nunes, I.、Shapiro, RL 和 Rifkin, DB 小鼠腹膜巨噬细胞潜在 TGF-β 激活的表征。 J.免疫学。 155、1450–1459 (1995)。Kojima, S. & Rifkin, D. B. Mechanism of retinoid-induced activation of latent transforming growth factor-beta in bovine endothelial cells. J. Cell Physiol. 155, 323–332 (1993).
Kojima, S. & Rifkin, DB 类视黄醇诱导牛内皮细胞中潜在转化生长因子-β 激活的机制。 J.细胞生理学。 155、323-332 (1993)。Sato, Y. & Rifkin, D. B. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-beta 1-like molecule by plasmin during co-culture. J. Cell Biol. 109, 309–315 (1989).
Sato, Y. & Rifkin, DB 周细胞和平滑肌细胞对内皮细胞运动的抑制:共培养期间纤溶酶激活潜在的转化生长因子-β 1 样分子。 J.细胞生物学。 109、309-315 (1989)。Sankar, S., Mahooti-Brooks, N., Centrella, M., McCarthy, T. L. & Madri, J. A. Expression of transforming growth factor type III receptor in vascular endothelial cells increases their responsiveness to transforming growth factor beta 2. J. Biol. Chem. 270, 13567–13572 (1995).
Sankar, S.、Mahooti-Brooks, N.、Centrella, M.、McCarthy, TL 和 Madri, JA 血管内皮细胞中转化生长因子 III 型受体的表达增加了它们对转化生长因子 β 2 的反应性。化学。 270、13567-13572 (1995)。Cheifetz, S. et al. Distinct transforming growth factor-beta (TGF-beta) receptor subsets as determinants of cellular responsiveness to three TGF-beta isoforms. J. Biol. Chem. 265, 20533–20538 (1990).
切菲茨,S.等人。不同的转化生长因子-β (TGF-β) 受体子集是细胞对三种 TGF-β 亚型反应性的决定因素。 J.Biol。化学。 265、20533–20538 (1990)。Wang, X. F. et al. Expression cloning and characterization of the TGF-beta type III receptor. Cell 67, 797–805 (1991).
王XF等人。 TGF-β III 型受体的表达克隆和表征。细胞67 , 797–805 (1991)。Lopez-Casillas, F. et al. Structure and expression of the membrane proteoglycan betaglycan, a component of the TGF-beta receptor system. Cell 67, 785–795 (1991).
洛佩兹-卡西利亚斯,F. 等人。膜蛋白聚糖β聚糖的结构和表达,TGF-β受体系统的一个组成部分。细胞67 , 785–795 (1991)。Lopez-Casillas, F., Wrana, J. L. & Massague, J. Betaglycan presents ligand to the TGF beta signaling receptor. Cell 73, 1435–1444 (1993).
Lopez-Casillas, F.、Wrana, JL 和 Massague, J. Betaglycan 提供 TGF beta 信号受体的配体。细胞73,1435–1444 (1993)。Esparza-Lopez, J. et al. Ligand binding and functional properties of betaglycan, a co-receptor of the transforming growth factor-beta superfamily. Specialized binding regions for transforming growth factor-beta and inhibin A. J. Biol. Chem. 276, 14588–14596 (2001).
埃斯帕扎-洛佩兹,J. 等人。 β聚糖(转化生长因子-β超家族的共同受体)的配体结合和功能特性。用于转化生长因子-β 和抑制素的专门结合区域 A. J. Biol。化学。 276、14588-14596 (2001)。Madamanchi, A., Ingle, M., Hinck, A. P. & Umulis, D. M. Computational modeling of TGF-β2:TβRI:TβRII receptor complex assembly as mediated by the TGF-β coreceptor betaglycan. Biophys. J. 122, 1342–1354 (2023).
Madamanchi, A.、Ingle, M.、Hinck, AP 和 Umulis, DM 由 TGF-β 辅助受体 β 聚糖介导的 TGF-β2:TβRI:TβRII 受体复合物组装的计算模型。生物物理学。 J. 122 , 1342–1354 (2023)。Zhang, W. et al. Single-molecule imaging reveals transforming growth factor-beta-induced type II receptor dimerization. Proc. Natl Acad. Sci. USA 106, 15679–15683 (2009).
张,W.等人。单分子成像揭示了转化生长因子-β 诱导的 II 型受体二聚化。过程。国家科学院。科学。美国106,15679–15683 (2009)。Gilboa, L., Wells, R. G., Lodish, H. F. & Henis, Y. I. Oligomeric structure of type I and type II transforming growth factor beta receptors: homodimers form in the ER and persist at the plasma membrane. J. Cell Biol. 140, 767–777 (1998).
Gilboa, L.、Wells, RG、Lodish, HF 和 Henis, YI I 型和 II 型转化生长因子 β 受体的寡聚结构:同二聚体在 ER 中形成并持续存在于质膜上。 J.细胞生物学。 140、767–777 (1998)。Chen, R. H. & Derynck, R. Homomeric interactions between type II transforming growth factor-beta receptors. J. Biol. Chem. 269, 22868–22874 (1994).
Chen, RH 和 Derynck, R. II 型转化生长因子-β 受体之间的同源相互作用。 J.Biol。化学。 269 , 22868–22874 (1994)。Groppe, J. et al. Cooperative assembly of TGF-beta superfamily signaling complexes is mediated by two disparate mechanisms and distinct modes of receptor binding. Mol. Cell 29, 157–168 (2008).
格罗普,J.等人。 TGF-β 超家族信号复合物的协同组装是由两种不同的机制和不同的受体结合模式介导的。摩尔。细胞29 , 157–168 (2008)。Wrana, J. L. et al. TGF beta signals through a heteromeric protein kinase receptor complex. Cell 71, 1003–1014 (1992).
拉纳,JL 等人。 TGF beta 通过异聚蛋白激酶受体复合物发出信号。细胞71 , 1003–1014 (1992)。Huse, M. et al. The TGF beta receptor activation process: an inhibitor- to substrate-binding switch. Mol. Cell 8, 671–682 (2001).
胡斯,M.等人。 TGF β 受体激活过程:抑制剂与底物结合的开关。摩尔。细胞8,671–682 (2001)。Tsukazaki, T., Chiang, T. A., Davison, A. F., Attisano, L. & Wrana, J. L. SARA, a FYVE domain protein that recruits Smad2 to the TGFbeta receptor. Cell 95, 779–791 (1998).
Tsukazaki, T.、Chiang, TA、Davison, AF、Attisano, L. 和 Wrana, JL SARA,一种 FYVE 结构域蛋白,可将 Smad2 募集至 TGFbeta 受体。细胞95 , 779–791 (1998)。Wu, J. W. et al. Crystal structure of a phosphorylated Smad2. Recognition of phosphoserine by the MH2 domain and insights on Smad function in TGF-beta signaling. Mol. Cell 8, 1277–1289 (2001).
吴,JW等。磷酸化 Smad2 的晶体结构。 MH2 结构域对磷酸丝氨酸的识别以及对 TGF-β 信号传导中 Smad 功能的见解。摩尔。第 8单元,1277–1289 (2001)。Kawabata, M., Inoue, H., Hanyu, A., Imamura, T. & Miyazono, K. Smad proteins exist as monomers in vivo and undergo homo- and hetero-oligomerization upon activation by serine/threonine kinase receptors. Embo j. 17, 4056–4065 (1998).
Kawabata, M.、Inoue, H.、Hanyu, A.、Imamura, T. 和 Miyazono, K. Smad 蛋白在体内以单体形式存在,并在被丝氨酸/苏氨酸激酶受体激活后发生同源和异源寡聚化。恩博 J. 17、4056-4065 (1998)。Chacko, B. M. et al. The L3 loop and C-terminal phosphorylation jointly define Smad protein trimerization. Nat. Struct. Biol. 8, 248–253 (2001).
查科,BM 等人。 L3 环和 C 端磷酸化共同定义了 Smad 蛋白三聚化。纳特。结构。生物。 8、248-253 (2001)。Lucarelli, P. et al. Resolving the Combinatorial Complexity of Smad Protein Complex Formation and Its Link to Gene Expression. Cell Syst. 6, 75–89.e11 (2018).
卢卡雷利,P.等人。解决 Smad 蛋白复合物形成的组合复杂性及其与基因表达的联系。细胞系统。 6、75–89.e11 (2018)。Inman, G. J. & Hill, C. S. Stoichiometry of active smad-transcription factor complexes on DNA. J. Biol. Chem. 277, 51008–51016 (2002).
Inman、GJ 和 Hill、CS DNA 上活性 smad 转录因子复合物的化学计量。 J.Biol。化学。 277、51008-51016 (2002)。Shi, Y. et al. Crystal structure of a Smad MH1 domain bound to DNA: insights on DNA binding in TGF-beta signaling. Cell 94, 585–594 (1998).
石,Y.等人。与 DNA 结合的 Smad MH1 结构域的晶体结构:对 TGF-β 信号传导中 DNA 结合的见解。细胞94 , 585–594 (1998)。Martin-Malpartida, P. et al. Structural basis for genome wide recognition of 5-bp GC motifs by SMAD transcription factors. Nat. Commun. 8, 2070 (2017).
马丁-马尔帕蒂达,P. 等人。 SMAD 转录因子全基因组识别 5-bp GC 基序的结构基础。纳特。交流。 8,2070 (2017)。Zawel, L. et al. Human Smad3 and Smad4 are sequence-specific transcription activators. Mol. Cell 1, 611–617 (1998).
扎维尔,L.等人。人类 Smad3 和 Smad4 是序列特异性转录激活剂。摩尔。细胞1,611-617 (1998)。Liu, Z. et al. Global identification of SMAD2 target genes reveals a role for multiple co-regulatory factors in zebrafish early gastrulas. J. Biol. Chem. 286, 28520–28532 (2011).
刘,Z.等人。 SMAD2 靶基因的整体鉴定揭示了斑马鱼早期原肠胚中多个共同调控因子的作用。 J.Biol。化学。 286、28520–28532 (2011)。Koinuma, D. et al. Promoter-wide analysis of Smad4 binding sites in human epithelial cells. Cancer Sci. 100, 2133–2142 (2009).
Koinuma,D.等人。人上皮细胞中 Smad4 结合位点的启动子范围分析。癌症科学。 100,2133-2142 (2009)。Mullen, A. C. et al. Master transcription factors determine cell-type-specific responses to TGF-β signaling. Cell 147, 565–576 (2011).
马伦,AC 等。主转录因子决定细胞类型对 TGF-β 信号传导的特异性反应。细胞147 , 565–576 (2011)。Afrakhte, M. et al. Induction of inhibitory Smad6 and Smad7 mRNA by TGF-beta family members. Biochem Biophys. Res Commun. 249, 505–511 (1998).
Afrakhte,M.等人。 TGF-β 家族成员诱导抑制性 Smad6 和 Smad7 mRNA。生物化学生物物理学。资源通讯。 249、505-511 (1998)。Denissova, N. G., Pouponnot, C., Long, J., He, D. & Liu, F. Transforming growth factor beta -inducible independent binding of SMAD to the Smad7 promoter. Proc. Natl Acad. Sci. USA 97, 6397–6402 (2000).
Denissova,NG,Pouponnot,C.,Long,J.,He,D.和Liu,F。转化生长因子β-SMAD与Smad7启动子的诱导独立结合。过程。国家科学院。科学。美国97 , 6397–6402 (2000)。Imamura, T. et al. Smad6 inhibits signalling by the TGF-beta superfamily. Nature 389, 622–626 (1997).
今村,T.等人。 Smad6 抑制 TGF-β 超家族的信号传导。自然389 , 622–626 (1997)。Kamiya, Y., Miyazono, K. & Miyazawa, K. Smad7 inhibits transforming growth factor-beta family type i receptors through two distinct modes of interaction. J. Biol. Chem. 285, 30804–30813 (2010).
Kamiya, Y.、Miyazono, K. 和 Miyazawa, K. Smad7 通过两种不同的相互作用模式抑制转化生长因子-β 家族 i 型受体。 J.Biol。化学。 285、30804–30813 (2010)。Hanyu, A. et al. The N domain of Smad7 is essential for specific inhibition of transforming growth factor-beta signaling. J. Cell Biol. 155, 1017–1027 (2001).
羽生,A.等人。 Smad7 的 N 结构域对于特异性抑制转化生长因子-β 信号传导至关重要。 J.细胞生物学。 155、1017-1027 (2001)。Suzuki, C. et al. Smurf1 regulates the inhibitory activity of Smad7 by targeting Smad7 to the plasma membrane. J. Biol. Chem. 277, 39919–39925 (2002).
铃木,C.等人。 Smurf1 通过将 Smad7 靶向质膜来调节 Smad7 的抑制活性。 J.Biol。化学。 277 , 39919–39925 (2002)。Kavsak, P. et al. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGF beta receptor for degradation. Mol. Cell 6, 1365–1375 (2000).
Kavsak,P.等人。 Smad7 与 Smurf2 结合形成 E3 泛素连接酶,靶向 TGF β 受体进行降解。摩尔。单元6,1365–1375 (2000)。Yan, X. et al. Smad7 Protein Interacts with Receptor-regulated Smads (R-Smads) to Inhibit Transforming Growth Factor-β (TGF-β)/Smad Signaling. J. Biol. Chem. 291, 382–392 (2016).
严,X.等人。 Smad7 蛋白与受体调节的 Smad (R-Smad) 相互作用,抑制转化生长因子-β (TGF-β)/Smad 信号传导。 J.Biol。化学。 291 , 382–392 (2016)。Kuratomi, G. et al. NEDD4-2 (neural precursor cell expressed, developmentally down-regulated 4-2) negatively regulates TGF-beta (transforming growth factor-beta) signalling by inducing ubiquitin-mediated degradation of Smad2 and TGF-beta type I receptor. Biochem J. 386, 461–470 (2005).
仓富,G.等人。 NEDD4-2(神经前体细胞表达,发育下调 4-2)通过诱导泛素介导的 Smad2 和 TGF-β I 型受体降解来负向调节 TGF-β(转化生长因子-β)信号传导。生物化学杂志386 , 461–470 (2005)。Morén, A., Imamura, T., Miyazono, K., Heldin, C. H. & Moustakas, A. Degradation of the tumor suppressor Smad4 by WW and HECT domain ubiquitin ligases. J. Biol. Chem. 280, 22115–22123 (2005).
Morén, A.、Imamura, T.、Miyazono, K.、Heldin, CH 和 Moustakas, A. WW 和 HECT 域泛素连接酶对肿瘤抑制因子 Smad4 的降解。 J.Biol。化学。 280、22115–22123 (2005)。Shi, W. et al. GADD34-PP1c recruited by Smad7 dephosphorylates TGFbeta type I receptor. J. Cell Biol. 164, 291–300 (2004).
石,W.等人。 Smad7 招募的 GADD34-PP1c 使 TGFbeta I 型受体去磷酸化。 J.细胞生物学。 164、291-300 (2004)。Zhang, S. et al. Smad7 antagonizes transforming growth factor beta signaling in the nucleus by interfering with functional Smad-DNA complex formation. Mol. Cell Biol. 27, 4488–4499 (2007).
张,S.等人。 Smad7 通过干扰功能性 Smad-DNA 复合物的形成来拮抗细胞核中的转化生长因子 β 信号传导。摩尔。细胞生物学。 27、4488-4499 (2007)。Ross, S. et al. Smads orchestrate specific histone modifications and chromatin remodeling to activate transcription. Embo j. 25, 4490–4502 (2006).
罗斯,S.等人。 Smads 协调特定的组蛋白修饰和染色质重塑以激活转录。恩博 J. 25、4490-4502 (2006)。Feng, X. H., Zhang, Y., Wu, R. Y. & Derynck, R. The tumor suppressor Smad4/DPC4 and transcriptional adaptor CBP/p300 are coactivators for smad3 in TGF-beta-induced transcriptional activation. Genes Dev. 12, 2153–2163 (1998).
Feng, XH, Zhuang, Y., Wu, RY & Derynck, R. 肿瘤抑制因子 Smad4/DPC4 和转录适配器 CBP/p300 是 TGF-β 诱导的转录激活中 smad3 的共激活因子。基因开发。 12、2153-2163 (1998)。Itoh, S., Ericsson, J., Nishikawa, J., Heldin, C. H. & ten Dijke, P. The transcriptional co-activator P/CAF potentiates TGF-beta/Smad signaling. Nucleic Acids Res 28, 4291–4298 (2000).
Itoh, S.、Ericsson, J.、Nishikawa, J.、Heldin, CH 和 ten Dijke, P. 转录共激活因子 P/CAF 增强 TGF-β/Smad 信号传导。核酸研究28 , 4291–4298 (2000)。Kahata, K. et al. Regulation of transforming growth factor-beta and bone morphogenetic protein signalling by transcriptional coactivator GCN5. Genes Cells 9, 143–151 (2004).
卡哈塔,K.等人。转录共激活因子 GCN5 对转化生长因子-β 和骨形态发生蛋白信号的调节。基因细胞9 , 143–151 (2004)。Yahata, T. et al. The MSG1 non-DNA-binding transactivator binds to the p300/CBP coactivators, enhancing their functional link to the Smad transcription factors. J. Biol. Chem. 275, 8825–8834 (2000).
八幡,T.等人。 MSG1 非 DNA 结合反式激活因子与 p300/CBP 共激活因子结合,增强它们与 Smad 转录因子的功能联系。 J.Biol。化学。 275 , 8825–8834 (2000)。Postigo, A. A. Opposing functions of ZEB proteins in the regulation of the TGFbeta/BMP signaling pathway. Embo j. 22, 2443–2452 (2003).
Postigo, AA ZEB 蛋白在 TGFbeta/BMP 信号通路调节中的相反功能。恩博 J. 22、2443-2452 (2003)。Postigo, A. A., Depp, J. L., Taylor, J. J. & Kroll, K. L. Regulation of Smad signaling through a differential recruitment of coactivators and corepressors by ZEB proteins. Embo j. 22, 2453–2462 (2003).
Postigo, AA, Depp, JL, Taylor, JJ & Kroll, KL 通过 ZEB 蛋白差异性招募共激活子和辅阻遏物来调节 Smad 信号传导。恩博 J. 22、2453-2462 (2003)。Shuttleworth, V. G. et al. The methyltransferase SET9 regulates TGFB1 activation of renal fibroblasts via interaction with SMAD3. J. Cell Sci. 131, jcs207761 (2018).
沙特尔沃斯,VG 等人。甲基转移酶 SET9 通过与 SMAD3 相互作用调节肾成纤维细胞的 TGFB1 激活。 J.细胞科学。 131 、jcs207761(2018)。Kang, J. S., Alliston, T., Delston, R. & Derynck, R. Repression of Runx2 function by TGF-beta through recruitment of class II histone deacetylases by Smad3. Embo j. 24, 2543–2555 (2005).
Kang,JS,Alliston,T.,Delston,R. 和 Derynck,R。TGF-β 通过 Smad3 招募 II 类组蛋白脱乙酰酶来抑制 Runx2 功能。恩博 J. 24、2543-2555 (2005)。Wotton, D., Lo, R. S., Lee, S. & Massagué, J. A Smad transcriptional corepressor. Cell 97, 29–39 (1999).
Wotton, D.、Lo, RS、Lee, S. 和 Massagué, J. A Smad 转录辅阻遏物。细胞97 , 29–39 (1999)。Alliston, T. et al. Repression of bone morphogenetic protein and activin-inducible transcription by Evi-1. J. Biol. Chem. 280, 24227–24237 (2005).
艾利斯顿,T.等人。 Evi-1 对骨形态发生蛋白和激活素诱导转录的抑制。 J.Biol。化学。 280 , 24227–24237 (2005)。Izutsu, K. et al. The corepressor CtBP interacts with Evi-1 to repress transforming growth factor beta signaling. Blood 97, 2815–2822 (2001).
井津津,K.等人。辅阻遏物 CtBP 与 Evi-1 相互作用,抑制转化生长因子 β 信号传导。血97 , 2815–2822 (2001)。Luo, K. et al. The Ski oncoprotein interacts with the Smad proteins to repress TGFbeta signaling. Genes Dev. 13, 2196–2206 (1999).
罗,K.等人。 Ski 癌蛋白与 Smad 蛋白相互作用,抑制 TGFbeta 信号传导。基因开发。 13、2196-2206 (1999)。Akiyoshi, S. et al. c-Ski acts as a transcriptional co-repressor in transforming growth factor-beta signaling through interaction with smads. J. Biol. Chem. 274, 35269–35277 (1999).
秋吉,S.等人。 c-Ski 通过与 smad 相互作用,充当转录共阻遏物,转化生长因子-β 信号传导。 J.Biol。化学。 274 , 35269–35277 (1999)。Sun, Y. et al. Interaction of the Ski oncoprotein with Smad3 regulates TGF-beta signaling. Mol. Cell 4, 499–509 (1999).
孙,Y.等人。 Ski 癌蛋白与 Smad3 的相互作用调节 TGF-β 信号传导。摩尔。细胞4,499-509 (1999)。Stroschein, S. L., Wang, W., Zhou, S., Zhou, Q. & Luo, K. Negative feedback regulation of TGF-beta signaling by the SnoN oncoprotein. Science 286, 771–774 (1999).
Stroschein, SL, Wang, W., Zhou, S., Zhou, Q. & Luo, K. SnoN 癌蛋白对 TGF-β 信号传导的负反馈调节。科学286 , 771–774 (1999)。Feng, X. H., Liang, Y. Y., Liang, M., Zhai, W. & Lin, X. Direct Interaction of c-Myc with Smad2 and Smad3 to Inhibit TGF-β-Mediated Induction of the CDK Inhibitor p15(Ink4B). Mol. Cell 63, 1089 (2016).
Feng,XH,Liang,YY,Liang,M.,Zhai,W.和Lin,X。c-Myc与Smad2和Smad3的直接相互作用抑制TGF-β介导的CDK抑制剂p15(Ink4B)的诱导。摩尔。细胞63 , 1089 (2016)。Kim, R. H. et al. A novel smad nuclear interacting protein, SNIP1, suppresses p300-dependent TGF-beta signal transduction. Genes Dev. 14, 1605–1616 (2000).
金,RH 等人。一种新型 smad 核相互作用蛋白 SNIP1 可抑制 p300 依赖性 TGF-β 信号转导。基因开发。 14、1605-1616 (2000)。Verschueren, K. et al. SIP1, a novel zinc finger/homeodomain repressor, interacts with Smad proteins and binds to 5’-CACCT sequences in candidate target genes. J. Biol. Chem. 274, 20489–20498 (1999).
Verschueren,K.等人。 SIP1 是一种新型锌指/同源域阻遏蛋白,与 Smad 蛋白相互作用并与候选靶基因中的 5'-CACCT 序列结合。 J.Biol。化学。 274、20489–20498 (1999)。Wakabayashi, Y. et al. Histone 3 lysine 9 (H3K9) methyltransferase recruitment to the interleukin-2 (IL-2) promoter is a mechanism of suppression of IL-2 transcription by the transforming growth factor-β-Smad pathway. J. Biol. Chem. 286, 35456–35465 (2011).
若林,Y.等人。组蛋白 3 赖氨酸 9 (H3K9) 甲基转移酶招募至白细胞介素 2 (IL-2) 启动子是通过转化生长因子-β-Smad 途径抑制 IL-2 转录的机制。 J.Biol。化学。 286、35456–35465 (2011)。Du, D. et al. Smad3-mediated recruitment of the methyltransferase SETDB1/ESET controls Snail1 expression and epithelial-mesenchymal transition. EMBO Rep. 19, 135–155 (2018).
杜,D.等人。 Smad3 介导的甲基转移酶 SETDB1/ESET 的募集控制 Snail1 表达和上皮间质转化。 EMBO 报告19 , 135–155 (2018)。Brown, J. D., DiChiara, M. R., Anderson, K. R., Gimbrone, M. A. Jr. & Topper, J. N. MEKK-1, a component of the stress (stress-activated protein kinase/c-Jun N-terminal kinase) pathway, can selectively activate Smad2-mediated transcriptional activation in endothelial cells. J. Biol. Chem. 274, 8797–8805 (1999).
Brown, JD, DiChiara, MR, Anderson, KR, Gimbrone, MA Jr. & Topper, JN MEKK-1 是应激(应激激活蛋白激酶/c-Jun N 端激酶)通路的一个组成部分,可以选择性激活内皮细胞中 Smad2 介导的转录激活。 J.Biol。化学。 274、8797-8805 (1999)。Kamaraju, A. K. & Roberts, A. B. Role of Rho/ROCK and p38 MAP kinase pathways in transforming growth factor-beta-mediated Smad-dependent growth inhibition of human breast carcinoma cells in vivo. J. Biol. Chem. 280, 1024–1036 (2005).
Kamaraju, AK 和 Roberts, AB Rho/ROCK 和 p38 MAP 激酶途径在转化生长因子-β 介导的人乳腺癌细胞体内 Smad 依赖性生长抑制中的作用。 J.Biol。化学。 280、1024-1036 (2005)。Mori, S. et al. TGF-beta and HGF transmit the signals through JNK-dependent Smad2/3 phosphorylation at the linker regions. Oncogene 23, 7416–7429 (2004).
森,S.等人。 TGF-β 和 HGF 通过接头区域的 JNK 依赖性 Smad2/3 磷酸化来传递信号。癌基因23 , 7416–7429 (2004)。Lehmann, K. et al. Raf induces TGFbeta production while blocking its apoptotic but not invasive responses: a mechanism leading to increased malignancy in epithelial cells. Genes Dev. 14, 2610–2622 (2000).
莱曼,K.等人。 Raf 诱导 TGFbeta 产生,同时阻断其凋亡反应,但不阻断侵袭反应:这是一种导致上皮细胞恶性肿瘤增加的机制。基因开发。 14、2610-2622 (2000)。Funaba, M., Zimmerman, C. M. & Mathews, L. S. Modulation of Smad2-mediated signaling by extracellular signal-regulated kinase. J. Biol. Chem. 277, 41361–41368 (2002).
Funaba, M.、Zimmerman, CM 和 Mathews, LS 通过细胞外信号调节激酶调节 Smad2 介导的信号传导。 J.Biol。化学。 277 , 41361–41368 (2002)。Roelen, B. A. et al. Phosphorylation of threonine 276 in Smad4 is involved in transforming growth factor-beta-induced nuclear accumulation. Am. J. Physiol. Cell Physiol. 285, C823–C830 (2003).
罗伦,BA 等人。 Smad4 中苏氨酸 276 的磷酸化参与转化生长因子-β 诱导的核积累。是。 J.生理学。细胞生理学。 285 ,C823–C830(2003)。Guo, X. et al. Axin and GSK3- control Smad3 protein stability and modulate TGF- signaling. Genes Dev. 22, 106–120 (2008).
郭,X.等。 Axin 和 GSK3- 控制 Smad3 蛋白稳定性并调节 TGF- 信号传导。基因开发。 22、106-120 (2008)。Millet, C. et al. A negative feedback control of transforming growth factor-beta signaling by glycogen synthase kinase 3-mediated Smad3 linker phosphorylation at Ser-204. J. Biol. Chem. 284, 19808–19816 (2009).
米勒,C.等人。糖原合成酶激酶 3 介导的 Smad3 连接子 Ser-204 磷酸化对转化生长因子-β 信号传导的负反馈控制。 J.Biol。化学。 284,19808-19816 (2009)。Wang, G., Matsuura, I., He, D. & Liu, F. Transforming growth factor-{beta}-inducible phosphorylation of Smad3. J. Biol. Chem. 284, 9663–9673 (2009).
Wang, G.、Matsuura, I.、He, D. 和 Liu, F. 转化生长因子-{β}-诱导的 Smad3 磷酸化。 J.Biol。化学。 284 , 9663–9673 (2009)。Wicks, S. J., Lui, S., Abdel-Wahab, N., Mason, R. M. & Chantry, A. Inactivation of smad-transforming growth factor beta signaling by Ca(2+)-calmodulin-dependent protein kinase II. Mol. Cell Biol. 20, 8103–8111 (2000).
Wicks, SJ、Lui, S.、Abdel-Wahab, N.、Mason, RM 和 Chantry, A. Ca(2+)-钙调蛋白依赖性蛋白激酶 II 使 smad 转化生长因子 β 信号失活。摩尔。细胞生物学。 20、8103-8111 (2000)。Yakymovych, I., Ten Dijke, P., Heldin, C. H. & Souchelnytskyi, S. Regulation of Smad signaling by protein kinase C. Faseb j. 15, 553–555 (2001).
Yakymovych,I.,Ten Dijke,P.,Heldin,CH 和 Souchelnytskyi,S. 蛋白激酶 C 对 Smad 信号传导的调节。 Faseb j。 15、553-555 (2001)。Saura, M. et al. Nitric oxide regulates transforming growth factor-beta signaling in endothelial cells. Circ. Res 97, 1115–1123 (2005).
绍拉,M.等人。一氧化氮调节内皮细胞中的转化生长因子-β 信号传导。循环。第 97 号决议,1115–1123 (2005)。Alarcón, C. et al. Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF-beta pathways. Cell 139, 757–769 (2009).
阿拉尔孔,C.等人。核 CDK 驱动 BMP 和 TGF-β 途径中的 Smad 转录激活和周转。细胞139 , 757–769 (2009)。Matsuura, I. et al. Cyclin-dependent kinases regulate the antiproliferative function of Smads. Nature 430, 226–231 (2004).
松浦,I.等人。细胞周期蛋白依赖性激酶调节 Smad 的抗增殖功能。自然430 , 226–231 (2004)。Sapkota, G. et al. Dephosphorylation of the linker regions of Smad1 and Smad2/3 by small C-terminal domain phosphatases has distinct outcomes for bone morphogenetic protein and transforming growth factor-beta pathways. J. Biol. Chem. 281, 40412–40419 (2006).
萨普科塔,G.等人。小 C 端结构域磷酸酶对 Smad1 和 Smad2/3 连接区进行去磷酸化,对骨形态发生蛋白和转化生长因子-β 途径具有不同的结果。 J.Biol。化学。 281、40412–40419 (2006)。Wrighton, K. H. et al. Small C-terminal domain phosphatases dephosphorylate the regulatory linker regions of Smad2 and Smad3 to enhance transforming growth factor-beta signaling. J. Biol. Chem. 281, 38365–38375 (2006).
赖顿,KH 等人。小 C 端结构域磷酸酶使 Smad2 和 Smad3 的调节接头区域去磷酸化,以增强转化生长因子-β 信号传导。 J.Biol。化学。 281 , 38365–38375 (2006)。Lin, X. et al. PPM1A functions as a Smad phosphatase to terminate TGFbeta signaling. Cell 125, 915–928 (2006).
林,X.等人。 PPM1A 作为 Smad 磷酸酶发挥作用,终止 TGFbeta 信号传导。细胞125 , 915–928 (2006)。Yu, J. et al. MTMR4 attenuates transforming growth factor beta (TGFbeta) signaling by dephosphorylating R-Smads in endosomes. J. Biol. Chem. 285, 8454–8462 (2010).
于,J.等人。 MTMR4 通过内体中 R-Smad 的去磷酸化来减弱转化生长因子 β (TGFbeta) 信号传导。 J.Biol。化学。 285、8454–8462 (2010)。Heikkinen, P. T. et al. Hypoxia-activated Smad3-specific dephosphorylation by PP2A. J. Biol. Chem. 285, 3740–3749 (2010).
海基宁,PT 等人。 PP2A 缺氧激活 Smad3 特异性去磷酸化。 J.Biol。化学。 285、3740–3749 (2010)。Lin, X., Liang, M. & Feng, X. H. Smurf2 is a ubiquitin E3 ligase mediating proteasome-dependent degradation of Smad2 in transforming growth factor-beta signaling. J. Biol. Chem. 275, 36818–36822 (2000).
Lin, X.、Liang, M. 和 Feng, XH Smurf2 是一种泛素 E3 连接酶,在转化生长因子-β 信号传导过程中介导 Smad2 的蛋白酶体依赖性降解。 J.Biol。化学。 275 , 36818–36822 (2000)。Zhang, Y., Chang, C., Gehling, D. J., Hemmati-Brivanlou, A. & Derynck, R. Regulation of Smad degradation and activity by Smurf2, an E3 ubiquitin ligase. Proc. Natl Acad. Sci. USA 98, 974–979 (2001).
张,Y.,张,C.,Gehling,DJ,Hemmati-Brivanlou,A.&Derynck,R。Smurf2(一种 E3 泛素连接酶)对 Smad 降解和活性的调节。过程。国家科学院。科学。美国98 , 974–979 (2001)。Tang, L. Y. et al. Ablation of Smurf2 reveals an inhibition in TGF-β signalling through multiple mono-ubiquitination of Smad3. Embo j. 30, 4777–4789 (2011).
唐,LY 等人。 Smurf2 的消融揭示了通过 Smad3 的多重单泛素化对 TGF-β 信号传导的抑制。恩博 J. 30、4777-4789 (2011)。Gao, S. et al. Ubiquitin ligase Nedd4L targets activated Smad2/3 to limit TGF-beta signaling. Mol. Cell 36, 457–468 (2009).
高,S.等人。泛素连接酶 Nedd4L 靶向激活的 Smad2/3 以限制 TGF-β 信号传导。摩尔。细胞36 , 457–468 (2009)。Komuro, A. et al. Negative regulation of transforming growth factor-beta (TGF-beta) signaling by WW domain-containing protein 1 (WWP1). Oncogene 23, 6914–6923 (2004).
小室,A.等人。含 WW 结构域的蛋白 1 (WWP1) 对转化生长因子-β (TGF-β) 信号传导的负调控。癌基因23 , 6914–6923 (2004)。Seo, S. R. et al. The novel E3 ubiquitin ligase Tiul1 associates with TGIF to target Smad2 for degradation. Embo j. 23, 3780–3792 (2004).
Seo,SR 等人。新型 E3 泛素连接酶 Tiul1 与 TGIF 结合以靶向 Smad2 进行降解。恩博 J. 23、3780-3792 (2004)。Soond, S. M. & Chantry, A. Selective targeting of activating and inhibitory Smads by distinct WWP2 ubiquitin ligase isoforms differentially modulates TGFβ signalling and EMT. Oncogene 30, 2451–2462 (2011).
Soond, SM 和 Chantry, A. 通过不同的 WWP2 泛素连接酶异构体选择性靶向激活和抑制 Smad,差异调节 TGFβ 信号传导和 EMT。癌基因30 , 2451–2462 (2011)。Mavrakis, K. J. et al. Arkadia enhances Nodal/TGF-beta signaling by coupling phospho-Smad2/3 activity and turnover. PLoS Biol. 5, e67 (2007).
马夫拉基斯,KJ 等人。 Arkadia 通过耦合磷酸化 Smad2/3 活性和周转来增强 Nodal/TGF-β 信号传导。公共科学图书馆生物学。 5 、e67(2007)。Xin, H. et al. CHIP controls the sensitivity of transforming growth factor-beta signaling by modulating the basal level of Smad3 through ubiquitin-mediated degradation. J. Biol. Chem. 280, 20842–20850 (2005).
辛,H.等人。 CHIP 通过泛素介导的降解调节 Smad3 的基础水平,从而控制转化生长因子-β 信号传导的敏感性。 J.Biol。化学。 280、20842–20850 (2005)。Bai, Y., Yang, C., Hu, K., Elly, C. & Liu, Y. C. Itch E3 ligase-mediated regulation of TGF-beta signaling by modulating smad2 phosphorylation. Mol. Cell 15, 825–831 (2004).
Bai, Y.、Yang, C.、Hu, K.、Elly, C. 和 Liu, YC Itch E3 连接酶通过调节 smad2 磷酸化介导的 TGF-β 信号转导调节。摩尔。细胞15,825–831 (2004)。Fukuchi, M. et al. Ligand-dependent degradation of Smad3 by a ubiquitin ligase complex of ROC1 and associated proteins. Mol. Biol. Cell 12, 1431–1443 (2001).
福池,M.等人。 ROC1 和相关蛋白的泛素连接酶复合物对 Smad3 进行配体依赖性降解。摩尔。生物。细胞12,1431–1443 (2001)。Wan, M. et al. Smad4 protein stability is regulated by ubiquitin ligase SCF beta-TrCP1. J. Biol. Chem. 279, 14484–14487 (2004).
万,M.等人。 Smad4 蛋白稳定性受泛素连接酶 SCF beta-TrCP1 调节。 J.Biol。化学。 279、14484–14487 (2004)。Tang, L. Y. & Zhang, Y. E. Non-degradative ubiquitination in Smad-dependent TGF-β signaling. Cell Biosci. 1, 43 (2011).
Tang, LY & 张, YE Smad 依赖性 TGF-β 信号转导中的非降解泛素化。细胞生物科学。 1 , 43 (2011)。Aragón, E. et al. A Smad action turnover switch operated by WW domain readers of a phosphoserine code. Genes Dev. 25, 1275–1288 (2011).
阿拉贡,E.等人。由磷酸丝氨酸代码的 WW 域阅读器操作的 Smad 动作翻转开关。基因开发。 25、1275-1288 (2011)。Lee, M. K. et al. TGF-beta activates Erk MAP kinase signalling through direct phosphorylation of ShcA. Embo j. 26, 3957–3967 (2007).
李,MK 等人。 TGF-β 通过 ShcA 的直接磷酸化激活 Erk MAP 激酶信号传导。恩博 J. 26、3957-3967 (2007)。Lavoie, H., Gagnon, J. & Therrien, M. ERK signalling: a master regulator of cell behaviour, life and fate. Nat. Rev. Mol. Cell Biol. 21, 607–632 (2020).
Lavoie, H.、Gagnon, J. 和 Therrien, M. ERK 信号传导:细胞行为、生命和命运的主要调节因子。纳特。莫尔牧师。细胞生物学。 21、607-632 (2020)。Lu, N. & Malemud, C. J. Extracellular Signal-Regulated Kinase: A Regulator of Cell Growth, Inflammation, Chondrocyte and Bone Cell Receptor-Mediated Gene Expression. Int J. Mol. Sci. 20, 3792 (2019).
Lu, N. 和 Malemud, CJ 细胞外信号调节激酶:细胞生长、炎症、软骨细胞和骨细胞受体介导的基因表达的调节剂。国际 J. 摩尔。科学。 20、3792 (2019)。Schmidt, A. & Hall, A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 16, 1587–1609 (2002).
Schmidt, A. & Hall, A. Rho GTPases 的鸟嘌呤核苷酸交换因子:打开开关。基因开发。 16,1587-1609 (2002)。Papadimitriou, E., Kardassis, D., Moustakas, A. & Stournaras, C. TGFβ-induced early activation of the small GTPase RhoA is Smad2/3-independent and involves Src and the guanine nucleotide exchange factor Vav2. Cell Physiol. Biochem 28, 229–238 (2011).
Papadimitriou, E.、Kardassis, D.、Moustakas, A. 和 Stournaras, C. TGFβ 诱导的小 GTPase RhoA 的早期激活不依赖于 Smad2/3,并且涉及 Src 和鸟嘌呤核苷酸交换因子 Vav2。细胞生理学。生物化学28 , 229–238 (2011)。Lu, X. et al. Effect of RhoC on the epithelial-mesenchymal transition process induced by TGF-β1 in lung adenocarcinoma cells. Oncol. Rep. 36, 3105–3112 (2016).
卢,X.等人。 RhoC对TGF-β1诱导的肺腺癌细胞上皮-间质转化过程的影响。安科尔。报告36,3105–3112 (2016)。Bhowmick, N. A. et al. Transforming growth factor-beta1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol. Biol. Cell 12, 27–36 (2001).
博米克,NA 等人。转化生长因子-β1 通过 RhoA 依赖性机制介导上皮细胞向间质细胞的转分化。摩尔。生物。细胞12 , 27–36 (2001)。Shen, X. et al. The activity of guanine exchange factor NET1 is essential for transforming growth factor-beta-mediated stress fiber formation. J. Biol. Chem. 276, 15362–15368 (2001).
沉X.等人。鸟嘌呤交换因子 NET1 的活性对于转化生长因子-β 介导的应力纤维形成至关重要。 J.Biol。化学。 276、15362–15368 (2001)。Papadimitriou, E. et al. Differential regulation of the two RhoA-specific GEF isoforms Net1/Net1A by TGF-β and miR-24: role in epithelial-to-mesenchymal transition. Oncogene 31, 2862–2875 (2012).
帕帕迪米特里乌,E.等人。 TGF-β 和 miR-24 对两种 RhoA 特异性 GEF 亚型 Net1/Net1A 的差异调节:在上皮间质转化中的作用。癌基因31 , 2862–2875 (2012)。Vardouli, L., Moustakas, A. & Stournaras, C. LIM-kinase 2 and cofilin phosphorylation mediate actin cytoskeleton reorganization induced by transforming growth factor-beta. J. Biol. Chem. 280, 11448–11457 (2005).
Vardouli, L.、Moustakas, A. 和 Stournaras, C. LIM 激酶 2 和丝切蛋白磷酸化介导转化生长因子-β 诱导的肌动蛋白细胞骨架重组。 J.Biol。化学。 280、11448–11457 (2005)。Lee, J., Ko, M. & Joo, C. K. Rho plays a key role in TGF-beta1-induced cytoskeletal rearrangement in human retinal pigment epithelium. J. Cell Physiol. 216, 520–526 (2008).
Lee, J.、Ko, M. 和 Joo, CK Rho 在 TGF-β1 诱导的人视网膜色素上皮细胞骨架重排中发挥关键作用。 J.细胞生理学。 216、520–526 (2008)。Sousa-Squiavinato, A. C. M., Rocha, M. R., Barcellos-de-Souza, P., de Souza, W. F. & Morgado-Diaz, J. A. Cofilin-1 signaling mediates epithelial-mesenchymal transition by promoting actin cytoskeleton reorganization and cell-cell adhesion regulation in colorectal cancer cells. Biochim Biophys. Acta Mol. Cell Res 1866, 418–429 (2019).
Sousa-Squiavinato,ACM,Rocha,MR,Barcellos-de-Souza,P.,de Souza,WF 和 Morgado-Diaz,JA Cofilin-1 信号通过促进肌动蛋白细胞骨架重组和细胞间粘附调节介导上皮间质转化结直肠癌细胞。生物化学生物物理学。行为分子。细胞研究1866 , 418–429 (2019)。Wei, Y. H., Liao, S. L., Wang, S. H., Wang, C. C. & Yang, C. H. Simvastatin and ROCK Inhibitor Y-27632 Inhibit Myofibroblast Differentiation of Graves’ Ophthalmopathy-Derived Orbital Fibroblasts via RhoA-Mediated ERK and p38 Signaling Pathways. Front Endocrinol. (Lausanne) 11, 607968 (2020).
Wei, YH, Liao, SL, Wang, SH, Wang, CC & Yang, CH 辛伐他汀和 ROCK 抑制剂 Y-27632 通过 RhoA 介导的 ERK 和 p38 信号通路抑制 Graves 眼病源性眼眶成纤维细胞的肌成纤维细胞分化。前内分泌。 (洛桑) 11,607968 (2020)。Matoba, K. et al. Rho-Kinase Blockade Attenuates Podocyte Apoptosis by Inhibiting the Notch Signaling Pathway in Diabetic Nephropathy. Int J. Mol. Sci. 18, 1795 (2017).
马托巴,K.等人。 Rho 激酶阻断通过抑制糖尿病肾病的 Notch 信号通路来减弱足细胞凋亡。国际 J. 摩尔。科学。 18、1795 (2017)。Edlund, S., Landström, M., Heldin, C. H. & Aspenström, P. Transforming growth factor-beta-induced mobilization of actin cytoskeleton requires signaling by small GTPases Cdc42 and RhoA. Mol. Biol. Cell 13, 902–914 (2002).
Edlund, S.、Landström, M.、Heldin, CH 和 Aspenström, P. 转化生长因子-β 诱导的肌动蛋白细胞骨架动员需要小 GTPase Cdc42 和 RhoA 的信号传导。摩尔。生物。细胞13,902–914 (2002)。Jaffe, A. B. & Hall, A. Rho GTPases: biochemistry and biology. Annu Rev. Cell Dev. Biol. 21, 247–269 (2005).
Jaffe, AB 和 Hall, A. Rho GTPases:生物化学和生物学。细胞开发年鉴生物。 21、247-269 (2005)。Nomikou, E., Livitsanou, M., Stournaras, C. & Kardassis, D. Transcriptional and post-transcriptional regulation of the genes encoding the small GTPases RhoA, RhoB, and RhoC: implications for the pathogenesis of human diseases. Cell Mol. Life Sci. 75, 2111–2124 (2018).
Nomikou,E.,Livitsanou,M.,Stournaras,C. 和 Kardassis,D. 编码小 GTPases RhoA、RhoB 和 RhoC 的基因的转录和转录后调控:对人类疾病发病机制的影响。细胞分子。生命科学。 75、2111-2124 (2018)。Zhang, L. et al. TRAF4 promotes TGF-β receptor signaling and drives breast cancer metastasis. Mol. Cell 51, 559–572 (2013).
张,L.等人。 TRAF4 促进 TGF-β 受体信号传导并驱动乳腺癌转移。摩尔。细胞51 , 559–572 (2013)。Sorrentino, A. et al. The type I TGF-beta receptor engages TRAF6 to activate TAK1 in a receptor kinase-independent manner. Nat. Cell Biol. 10, 1199–1207 (2008).
索伦蒂诺,A.等人。 I 型 TGF-β 受体与 TRAF6 结合,以不依赖受体激酶的方式激活 TAK1。纳特。细胞生物学。 10、1199-1207 (2008)。Yamashita, M. et al. TRAF6 mediates Smad-independent activation of JNK and p38 by TGF-beta. Mol. Cell 31, 918–924 (2008).
山下,M.等人。 TRAF6 通过 TGF-β 介导不依赖于 Smad 的 JNK 和 p38 激活。摩尔。细胞31 , 918–924 (2008)。Engel, M. E., McDonnell, M. A., Law, B. K. & Moses, H. L. Interdependent SMAD and JNK signaling in transforming growth factor-beta-mediated transcription. J. Biol. Chem. 274, 37413–37420 (1999).
Engel, ME、McDonnell, MA、Law、BK 和 Moses,HL 转化生长因子 β 介导的转录中相互依赖的 SMAD 和 JNK 信号传导。 J.Biol。化学。 274 , 37413–37420 (1999)。Atfi, A., Djelloul, S., Chastre, E., Davis, R. & Gespach, C. Evidence for a role of Rho-like GTPases and stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) in transforming growth factor beta-mediated signaling. J. Biol. Chem. 272, 1429–1432 (1997).
Atfi, A.、Djelloul, S.、Chastre, E.、Davis, R. 和 Gespach, C. Rho 样 GTPases 和应激激活蛋白激酶/c-Jun N 末端激酶 (SAPK/ JNK)在转化生长因子β介导的信号传导中的作用。 J.Biol。化学。 272、1429-1432 (1997)。Minden, A., Lin, A., Claret, F. X., Abo, A. & Karin, M. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell 81, 1147–1157 (1995).
Minden, A.、Lin, A.、Claret, FX、Abo, A. 和 Karin, M. 小 GTPase Rac 和 Cdc42Hs 对 JNK 信号级联和 c-Jun 转录活性的选择性激活。细胞81 , 1147–1157 (1995)。Coso, O. A. et al. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell 81, 1137–1146 (1995).
科索,OA 等人。小 GTP 结合蛋白 Rac1 和 Cdc42 调节 JNK/SAPK 信号通路的活性。细胞81 , 1137–1146 (1995)。Mazars, A. et al. Differential roles of JNK and Smad2 signaling pathways in the inhibition of c-Myc-induced cell death by TGF-beta. Oncogene 19, 1277–1287 (2000).
玛泽,A.等人。 JNK 和 Smad2 信号通路在 TGF-β 抑制 c-Myc 诱导的细胞死亡中的不同作用。癌基因19 , 1277–1287 (2000)。Hocevar, B. A., Brown, T. L. & Howe, P. H. TGF-beta induces fibronectin synthesis through a c-Jun N-terminal kinase-dependent, Smad4-independent pathway. Embo j. 18, 1345–1356 (1999).
Hocevar, BA、Brown, TL 和 Howe, PH TGF-β 通过 c-Jun N 末端激酶依赖性、Smad4 独立途径诱导纤连蛋白合成。恩博 J. 18、1345-1356 (1999)。Zeke, A., Misheva, M., Reményi, A. & Bogoyevitch, M. A. JNK Signaling: Regulation and Functions Based on Complex Protein-Protein Partnerships. Microbiol Mol. Biol. Rev. 80, 793–835 (2016).
Zeke, A.、Misheva, M.、Reményi, A. 和 Bogoyevitch, MA JNK 信号传导:基于复杂蛋白质-蛋白质伙伴关系的调节和功能。微生物分子。生物。修订版80,793–835 (2016)。Yu, L., Hébert, M. C. & Zhang, Y. E. TGF-beta receptor-activated p38 MAP kinase mediates Smad-independent TGF-beta responses. Embo j. 21, 3749–3759 (2002).
Yu, L., Hébert, MC & 张,YE TGF-β 受体激活的 p38 MAP 激酶介导不依赖于 Smad 的 TGF-β 反应。恩博 J. 21、3749-3759 (2002)。Canovas, B. & Nebreda, A. R. Diversity and versatility of p38 kinase signalling in health and disease. Nat. Rev. Mol. Cell Biol. 22, 346–366 (2021).
Canovas, B. 和 Nebreda, AR p38 激酶信号在健康和疾病中的多样性和多功能性。纳特。莫尔牧师。细胞生物学。 22 , 346–366 (2021)。Hamidi, A. et al. Polyubiquitination of transforming growth factor β (TGFβ)-associated kinase 1 mediates nuclear factor-κB activation in response to different inflammatory stimuli. J. Biol. Chem. 287, 123–133 (2012).
哈米迪,A.等人。转化生长因子 β (TGFβ) 相关激酶 1 的多泛素化介导核因子 -κB 激活以响应不同的炎症刺激。 J.Biol。化学。 287 , 123–133 (2012)。Kim, H. J., Kim, J. G., Moon, M. Y., Park, S. H. & Park, J. B. IκB kinase γ/nuclear factor-κB-essential modulator (IKKγ/NEMO) facilitates RhoA GTPase activation, which, in turn, activates Rho-associated KINASE (ROCK) to phosphorylate IKKβ in response to transforming growth factor (TGF)-β1. J. Biol. Chem. 289, 1429–1440 (2014).
Kim, HJ, Kim, JG, Moon, MY, Park, SH & Park, JB IκB 激酶 γ/核因子-κB 必需调节剂 (IKKγ/NEMO) 促进 RhoA GTPase 激活,进而激活 Rho 相关激酶(ROCK) 响应转化生长因子 (TGF)-β1 磷酸化 IKKβ。 J.Biol。化学。 289、1429–1440 (2014)。Rodriguez, P. L., Sahay, S., Olabisi, O. O. & Whitehead, I. P. ROCK I-mediated activation of NF-kappaB by RhoB. Cell Signal 19, 2361–2369 (2007).
Rodriguez, PL、Sahay, S.、Olabisi, OO 和 Whitehead,IP ROCK I 介导的 RhoB 激活 NF-kappaB。细胞信号19 , 2361–2369 (2007)。Zhu, X. et al. TGF-beta1-induced PI3K/Akt/NF-kappaB/MMP9 signalling pathway is activated in Philadelphia chromosome-positive chronic myeloid leukaemia hemangioblasts. J. Biochem 149, 405–414 (2011).
朱X.等人。 TGF-β1 诱导的 PI3K/Akt/NF-kappaB/MMP9 信号通路在费城染色体阳性慢性粒细胞白血病成血管细胞中被激活。生物化学杂志149 , 405–414 (2011)。Capece, D. et al. NF-κB: blending metabolism, immunity, and inflammation. Trends Immunol. 43, 757–775 (2022).
卡佩斯,D.等人。 NF-κB:混合新陈代谢、免疫和炎症。趋势免疫学。 43 , 757–775 (2022)。Zinatizadeh, M. R. et al. The Nuclear Factor Kappa B (NF-kB) signaling in cancer development and immune diseases. Genes Dis. 8, 287–297 (2021).
Zinatizadeh,MR 等人。癌症发展和免疫疾病中的核因子 Kappa B (NF-kB) 信号传导。基因迪斯。 8,287-297 (2021)。Liu, D., Zhong, Z. & Karin, M. NF-κB: A Double-Edged Sword Controlling Inflammation. Biomedicines 10, 1250 (2022).
Liu, D.、Zhong, Z. 和 Karin, M. NF-κB:控制炎症的双刃剑。生物医学10 , 1250 (2022)。Yi, J. Y., Shin, I. & Arteaga, C. L. Type I transforming growth factor beta receptor binds to and activates phosphatidylinositol 3-kinase. J. Biol. Chem. 280, 10870–10876 (2005).
Yi, JY, Shin, I. 和 Arteaga, CL I 型转化生长因子 β 受体结合并激活磷脂酰肌醇 3-激酶。 J.Biol。化学。 280、10870–10876 (2005)。Hamidi, A. et al. TGF-β promotes PI3K-AKT signaling and prostate cancer cell migration through the TRAF6-mediated ubiquitylation of p85α. Sci. Signal 10, eaal4186 (2017).
哈米迪,A.等人。 TGF-β 通过 TRAF6 介导的 p85α 泛素化促进 PI3K-AKT 信号传导和前列腺癌细胞迁移。科学。信号10 ,eaal4186 (2017)。Bakin, A. V., Tomlinson, A. K., Bhowmick, N. A., Moses, H. L. & Arteaga, C. L. Phosphatidylinositol 3-kinase function is required for transforming growth factor beta-mediated epithelial to mesenchymal transition and cell migration. J. Biol. Chem. 275, 36803–36810 (2000).
Bakin, AV, Tomlinson, AK, Bhowmick, NA, Moses, HL 和 Arteaga, CL 磷脂酰肌醇 3-激酶功能是转化生长因子 β 介导的上皮间质转化和细胞迁移所必需的。 J.Biol。化学。 275 , 36803–36810 (2000)。Lamouille, S. & Derynck, R. Cell size and invasion in TGF-beta-induced epithelial to mesenchymal transition is regulated by activation of the mTOR pathway. J. Cell Biol. 178, 437–451 (2007).
Lamouille, S. & Derynck, R. TGF-β 诱导的上皮间质转化中的细胞大小和侵袭受 mTOR 通路激活的调节。 J.细胞生物学。 178、437-451 (2007)。Lamouille, S., Connolly, E., Smyth, J. W., Akhurst, R. J. & Derynck, R. TGF-β-induced activation of mTOR complex 2 drives epithelial-mesenchymal transition and cell invasion. J. Cell Sci. 125, 1259–1273 (2012).
Lamouille, S.、Connolly, E.、Smyth, JW、Akhurst, RJ 和 Derynck, R. TGF-β 诱导的 mTOR 复合物 2 激活可驱动上皮间质转化和细胞侵袭。 J.细胞科学。 125、1259–1273 (2012)。Chen, X. H. et al. The TGF-β-induced up-regulation of NKG2DLs requires AKT/GSK-3β-mediated stabilization of SP1. J. Cell Mol. Med 21, 860–870 (2017).
陈XH等人。 TGF-β 诱导的 NKG2DL 上调需要 AKT/GSK-3β 介导的 SP1 稳定。 J.细胞分子。医学21 , 860–870 (2017)。Kato, M. et al. Role of the Akt/FoxO3a pathway in TGF-beta1-mediated mesangial cell dysfunction: a novel mechanism related to diabetic kidney disease. J. Am. Soc. Nephrol. 17, 3325–3335 (2006).
加藤,M.等人。 Akt/FoxO3a 通路在 TGF-β1 介导的系膜细胞功能障碍中的作用:与糖尿病肾病相关的新机制。 J. Am.苏克。肾病。 17、3325-3335 (2006)。Franke, T. F. PI3K/Akt: getting it right matters. Oncogene 27, 6473–6488 (2008).
Franke,TF PI3K/Akt:正确处理很重要。癌基因27 , 6473–6488 (2008)。Liu, Y. et al. Transforming growth factor-β (TGF-β)-mediated connective tissue growth factor (CTGF) expression in hepatic stellate cells requires Stat3 signaling activation. J. Biol. Chem. 288, 30708–30719 (2013).
刘,Y.等人。肝星状细胞中转化生长因子-β (TGF-β) 介导的结缔组织生长因子 (CTGF) 表达需要 Stat3 信号激活。 J.Biol。化学。 288、30708–30719 (2013)。Tang, L. Y. et al. Transforming Growth Factor-β (TGF-β) Directly Activates the JAK1-STAT3 Axis to Induce Hepatic Fibrosis in Coordination with the SMAD Pathway. J. Biol. Chem. 292, 4302–4312 (2017).
唐,LY 等人。转化生长因子-β (TGF-β) 直接激活 JAK1-STAT3 轴,与 SMAD 通路协调诱导肝纤维化。 J.Biol。化学。 292、4302–4312 (2017)。Dees, C. et al. JAK-2 as a novel mediator of the profibrotic effects of transforming growth factor β in systemic sclerosis. Arthritis Rheum. 64, 3006–3015 (2012).
迪斯,C.等人。 JAK-2 作为系统性硬化症中转化生长因子 β 促纤维化作用的新型介质。关节炎大黄。 64、3006-3015 (2012)。Philips, R. L. et al. The JAK-STAT pathway at 30: Much learned, much more to do. Cell 185, 3857–3876 (2022).
飞利浦,RL 等。 30 岁时的 JAK-STAT 通路:学到了很多东西,还有很多事情要做。单元185,3857–3876 (2022)。Lehnert, S. A. & Akhurst, R. J. Embryonic expression pattern of TGF beta type-1 RNA suggests both paracrine and autocrine mechanisms of action. Development 104, 263–273 (1988).
Lehnert, SA 和 Akhurst, RJ TGF beta 1 型 RNA 的胚胎表达模式表明旁分泌和自分泌的作用机制。发展104 , 263–273 (1988)。Pelton, R. W., Nomura, S., Moses, H. L. & Hogan, B. L. Expression of transforming growth factor beta 2 RNA during murine embryogenesis. Development 106, 759–767 (1989).
Pelton, RW、Nomura, S.、Moses, HL 和 Hogan, BL 小鼠胚胎发生过程中转化生长因子 β 2 RNA 的表达。发展106 , 759–767 (1989)。Pelton, R. W., Dickinson, M. E., Moses, H. L. & Hogan, B. L. In situ hybridization analysis of TGF beta 3 RNA expression during mouse development: comparative studies with TGF beta 1 and beta 2. Development 110, 609–620 (1990).
Pelton, RW、Dickinson, ME、Moses, HL 和 Hogan, BL 小鼠发育过程中 TGF β 3 RNA 表达的原位杂交分析:与 TGF β 1 和 β 2 的比较研究。Development 110 , 609–620 (1990)。Millan, F. A., Denhez, F., Kondaiah, P. & Akhurst, R. J. Embryonic gene expression patterns of TGF beta 1, beta 2 and beta 3 suggest different developmental functions in vivo. Development 111, 131–143 (1991).
Millan, FA、Denhez, F.、Kondaiah, P. 和 Akhurst, RJ TGF beta 1、beta 2 和 beta 3 的胚胎基因表达模式表明体内不同的发育功能。发展111 , 131–143 (1991)。Pelton, R. W., Saxena, B., Jones, M., Moses, H. L. & Gold, L. I. Immunohistochemical localization of TGF beta 1, TGF beta 2, and TGF beta 3 in the mouse embryo: expression patterns suggest multiple roles during embryonic development. J. Cell Biol. 115, 1091–1105 (1991).
Pelton, RW、Saxena, B.、Jones, M.、Moses, HL 和 Gold, LI 小鼠胚胎中 TGF beta 1、TGF beta 2 和 TGF beta 3 的免疫组织化学定位:表达模式表明胚胎发育过程中的多种作用。 J.细胞生物学。 115、1091-1105 (1991)。Rosa, F. et al. Mesoderm induction in amphibians: the role of TGF-beta 2-like factors. Science 239, 783–785 (1988).
罗莎,F.等人。两栖动物中胚层诱导:TGF-β 2 样因子的作用。科学239 , 783–785 (1988)。Kimelman, D. & Kirschner, M. Synergistic induction of mesoderm by FGF and TGF-beta and the identification of an mRNA coding for FGF in the early Xenopus embryo. Cell 51, 869–877 (1987).
Kimelman, D. & Kirschner, M. FGF 和 TGF-β 对中胚层的协同诱导以及早期非洲爪蟾胚胎中 FGF 编码 mRNA 的鉴定。细胞51 , 869–877 (1987)。Bai, H., Xie, Y. L., Gao, Y. X., Cheng, T. & Wang, Z. Z. The balance of positive and negative effects of TGF-β signaling regulates the development of hematopoietic and endothelial progenitors in human pluripotent stem cells. Stem Cells Dev. 22, 2765–2776 (2013).
Bai, H., Xie, YL, Gau, YX, Cheng, T. & Wang, ZZ TGF-β信号传导正负效应的平衡调节人多能干细胞中造血和内皮祖细胞的发育。干细胞开发。 22、2765-2776 (2013)。Zhang, C. Y. et al. Transforming growth factor-β1 regulates the nascent hematopoietic stem cell niche by promoting gluconeogenesis. Leukemia 32, 479–491 (2018).
张,CY 等。转化生长因子-β1 通过促进糖异生来调节新生造血干细胞生态位。白血病32 , 479–491 (2018)。Challen, G. A., Boles, N. C., Chambers, S. M. & Goodell, M. A. Distinct hematopoietic stem cell subtypes are differentially regulated by TGF-beta1. Cell Stem Cell 6, 265–278 (2010).
Challen, GA、Boles, NC、Chambers, SM 和 Goodell, MA 不同的造血干细胞亚型受 TGF-β1 的差异调节。细胞干细胞6,265–278 (2010)。Xie, Y. et al. Cooperative Effect of Erythropoietin and TGF-β Inhibition on Erythroid Development in Human Pluripotent Stem Cells. J. Cell Biochem 116, 2735–2743 (2015).
谢,Y.等人。促红细胞生成素和 TGF-β 抑制对人多能干细胞红系发育的协同作用。细胞生物化学杂志116 , 2735–2743 (2015)。Ng, F. et al. PDGF, TGF-beta, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood 112, 295–307 (2008).
吴,F.等人。 PDGF、TGF-β 和 FGF 信号传导对于间充质干细胞 (MSC) 的分化和生长非常重要:转录谱可以识别在 MSC 分化为脂肪形成、软骨形成和成骨谱系中重要的标记物和信号传导途径。血液112 , 295–307 (2008)。Jian, H. et al. Smad3-dependent nuclear translocation of beta-catenin is required for TGF-beta1-induced proliferation of bone marrow-derived adult human mesenchymal stem cells. Genes Dev. 20, 666–674 (2006).
简,H.等人。 β-连环蛋白的 Smad3 依赖性核转位是 TGF-β1 诱导的骨髓来源的成人间充质干细胞增殖所必需的。基因开发。 20、666-674 (2006)。Alliston, T., Choy, L., Ducy, P., Karsenty, G. & Derynck, R. TGF-beta-induced repression of CBFA1 by Smad3 decreases cbfa1 and osteocalcin expression and inhibits osteoblast differentiation. Embo j. 20, 2254–2272 (2001).
Alliston, T.、Choy, L.、Ducy, P.、Karsenty, G. 和 Derynck, R. TGF-β 诱导的 Smad3 对 CBFA1 的抑制会降低 cbfa1 和骨钙素的表达并抑制成骨细胞分化。恩博 J. 20、2254-2272 (2001)。Liu, D., Black, B. L. & Derynck, R. TGF-beta inhibits muscle differentiation through functional repression of myogenic transcription factors by Smad3. Genes Dev. 15, 2950–2966 (2001).
Liu, D., Black, BL & Derynck, R. TGF-β 通过 Smad3 对生肌转录因子的功能性抑制来抑制肌肉分化。基因开发。 15、2950-2966 (2001)。Massagué, J., Cheifetz, S., Endo, T. & Nadal-Ginard, B. Type beta transforming growth factor is an inhibitor of myogenic differentiation. Proc. Natl Acad. Sci. USA 83, 8206–8210 (1986).
Massagué, J.、Cheifetz, S.、Endo, T. 和 Nadal-Ginard, B. β 型转化生长因子是生肌分化的抑制剂。过程。国家科学院。科学。美国83 , 8206–8210 (1986)。Florini, J. R. et al. Transforming growth factor-beta. A very potent inhibitor of myoblast differentiation, identical to the differentiation inhibitor secreted by Buffalo rat liver cells. J. Biol. Chem. 261, 16509–16513 (1986).
弗洛里尼,JR 等人。转化生长因子-β。一种非常有效的成肌细胞分化抑制剂,与水牛大鼠肝细胞分泌的分化抑制剂相同。 J.Biol。化学。 261,16509–16513 (1986)。Zhu, S., Goldschmidt-Clermont, P. J. & Dong, C. Transforming growth factor-beta-induced inhibition of myogenesis is mediated through Smad pathway and is modulated by microtubule dynamic stability. Circ. Res 94, 617–625 (2004).
Zhu, S., Goldschmidt-Clermont, PJ & Dong, C. 转化生长因子-β 诱导的肌生成抑制通过 Smad 途径介导,并受微管动态稳定性调节。循环。第 94 号决议,617-625(2004 年)。Choy, L. & Derynck, R. Transforming growth factor-beta inhibits adipocyte differentiation by Smad3 interacting with CCAAT/enhancer-binding protein (C/EBP) and repressing C/EBP transactivation function. J. Biol. Chem. 278, 9609–9619 (2003).
Choy, L. & Derynck, R. 转化生长因子-β 通过 Smad3 与 CCAAT/增强子结合蛋白 (C/EBP) 相互作用并抑制 C/EBP 反式激活功能来抑制脂肪细胞分化。 J.Biol。化学。 278 , 9609–9619 (2003)。Choy, L., Skillington, J. & Derynck, R. Roles of autocrine TGF-beta receptor and Smad signaling in adipocyte differentiation. J. Cell Biol. 149, 667–682 (2000).
Choy, L.、Skillington, J. 和 Derynck, R. 自分泌 TGF-β 受体和 Smad 信号在脂肪细胞分化中的作用。 J.细胞生物学。 149 , 667–682 (2000)。Kurpinski, K. et al. Transforming growth factor-beta and notch signaling mediate stem cell differentiation into smooth muscle cells. Stem Cells 28, 734–742 (2010).
库平斯基,K.等人。转化生长因子-β 和 notch 信号传导介导干细胞分化为平滑肌细胞。干细胞28 , 734–742 (2010)。Seyedin, S. M. et al. Cartilage-inducing factor-A. Apparent identity to transforming growth factor-beta. J. Biol. Chem. 261, 5693–5695 (1986).
Seiedin,SM 等人。软骨诱导因子-A。与转化生长因子-β 明显相同。 J.Biol。化学。 261 , 5693–5695 (1986)。Leonard, C. M. et al. Role of transforming growth factor-beta in chondrogenic pattern formation in the embryonic limb: stimulation of mesenchymal condensation and fibronectin gene expression by exogenenous TGF-beta and evidence for endogenous TGF-beta-like activity. Dev. Biol. 145, 99–109 (1991).
伦纳德,CM 等。转化生长因子-β 在胚胎肢体软骨形成模式形成中的作用:外源性 TGF-β 刺激间充质凝结和纤连蛋白基因表达以及内源性 TGF-β 样活性的证据。开发。生物。 145、99-109 (1991)。Reiss, M. & Sartorelli, A. C. Regulation of growth and differentiation of human keratinocytes by type beta transforming growth factor and epidermal growth factor. Cancer Res 47, 6705–6709 (1987).
Reiss, M. & Sartorelli, AC 通过 β 型转化生长因子和表皮生长因子调节人角质形成细胞的生长和分化。癌症研究47 , 6705–6709 (1987)。Masui, T. et al. Type beta transforming growth factor is the primary differentiation-inducing serum factor for normal human bronchial epithelial cells. Proc. Natl Acad. Sci. USA 83, 2438–2442 (1986).
Masui,T.等人。 β型转化生长因子是正常人支气管上皮细胞的主要分化诱导血清因子。过程。国家科学院。科学。美国83,2438–2442 (1986)。Yu, X. et al. The Cytokine TGF-β Promotes the Development and Homeostasis of Alveolar Macrophages. Immunity 47, 903–912.e904 (2017).
于X.等人。细胞因子 TGF-β 促进肺泡巨噬细胞的发育和稳态。免疫47 , 903–912.e904 (2017)。Clark, A. T., Young, R. J. & Bertram, J. F. In vitro studies on the roles of transforming growth factor-beta 1 in rat metanephric development. Kidney Int 59, 1641–1653 (2001).
Clark, AT、Young, RJ 和 Bertram, JF 关于转化生长因子-β 1 在大鼠后肾发育中作用的体外研究。肾脏国际59,1641–1653 (2001)。Sanvito, F. et al. TGF-beta 1 influences the relative development of the exocrine and endocrine pancreas in vitro. Development 120, 3451–3462 (1994).
桑维托,F.等人。 TGF-β1 在体外影响胰腺外分泌和内分泌的相对发育。发展120 , 3451–3462 (1994)。Böttinger, E. P. et al. Expression of a dominant-negative mutant TGF-beta type II receptor in transgenic mice reveals essential roles for TGF-beta in regulation of growth and differentiation in the exocrine pancreas. Embo j. 16, 2621–2633 (1997).
Böttinger,EP 等人。转基因小鼠中显性失活突变型 TGF-β II 型受体的表达揭示了 TGF-β 在调节外分泌胰腺生长和分化中的重要作用。恩博 J. 16、2621-2633 (1997)。Huojia, M. et al. TGF-beta3 induces ectopic mineralization in fetal mouse dental pulp during tooth germ development. Dev. Growth Differ. 47, 141–152 (2005).
霍嘉,M.等人。 TGF-β3 在牙胚发育过程中诱导胎鼠牙髓异位矿化。开发。增长不同。 47、141-152 (2005)。Yi, J. J., Barnes, A. P., Hand, R., Polleux, F. & Ehlers, M. D. TGF-beta signaling specifies axons during brain development. Cell 142, 144–157 (2010).
Yi, JJ, Barnes, AP, Hand, R., Polleux, F. & Ehlers, MD TGF-β 信号传导指定大脑发育过程中的轴突。细胞142 , 144–157 (2010)。Stipursky, J. & Gomes, F. C. TGF-beta1/SMAD signaling induces astrocyte fate commitment in vitro: implications for radial glia development. Glia 55, 1023–1033 (2007).
Stipursky, J. 和 Gomes, FC TGF-β1/SMAD 信号在体外诱导星形胶质细胞命运承诺:对放射状胶质细胞发育的影响。神经胶质细胞55 , 1023–1033 (2007)。Farkas, L. M., Dünker, N., Roussa, E., Unsicker, K. & Krieglstein, K. Transforming growth factor-beta(s) are essential for the development of midbrain dopaminergic neurons in vitro and in vivo. J. Neurosci. 23, 5178–5186 (2003).
Farkas, LM、Dünker, N.、Roussa, E.、Unsicker, K. 和 Krieglstein, K. 转化生长因子-β 对于中脑多巴胺能神经元的体外和体内发育至关重要。 J.神经科学。 23、5178-5186 (2003)。Chleilat, E. et al. TGF-β Signaling Regulates Development of Midbrain Dopaminergic and Hindbrain Serotonergic Neuron Subgroups. Neuroscience 381, 124–137 (2018).
Chleilat,E.等人。 TGF-β 信号传导调节中脑多巴胺能和后脑血清素能神经元亚群的发育。神经科学381 , 124–137 (2018)。Araujo, A. P. et al. Effects of Transforming Growth Factor Beta 1 in Cerebellar Development: Role in Synapse Formation. Front Cell Neurosci. 10, 104 (2016).
阿劳霍,美联社等人。转化生长因子 Beta 1 对小脑发育的影响:在突触形成中的作用。前细胞神经科学。 10、104 (2016)。Morris, A. D., Lewis, G. M. & Kucenas, S. Perineurial Glial Plasticity and the Role of TGF-β in the Development of the Blood-Nerve Barrier. J. Neurosci. 37, 4790–4807 (2017).
Morris, AD、Lewis, GM 和 Kucenas, S. 神经周围胶质可塑性和 TGF-β 在血神经屏障发育中的作用。 J.神经科学。 37、4790–4807 (2017)。de Sampaio e Spohr, T. C., Martinez, R., da Silva, E. F., Neto, V. M. & Gomes, F. C. Neuro-glia interaction effects on GFAP gene: a novel role for transforming growth factor-beta1. Eur. J. Neurosci. 16, 2059–2069 (2002).
de Sampaio e Spohr, TC, Martinez, R., da Silva, EF, Neto, VM & Gomes, FC 神经胶质细胞相互作用对 GFAP 基因的影响:转化生长因子-β1 的新作用。欧元。 J.神经科学。 16、2059-2069 (2002)。Miettinen, P. J., Ebner, R., Lopez, A. R. & Derynck, R. TGF-beta induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J. Cell Biol. 127, 2021–2036 (1994).
Miettinen,PJ,Ebner,R.,Lopez,AR 和 Derynck,R. TGF-β 诱导乳腺上皮细胞向间充质细胞的转分化:I 型受体的参与。 J.细胞生物学。 127,2021-2036 (1994)。Lamouille, S., Xu, J. & Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 15, 178–196 (2014).
Lamouille, S.、Xu, J. 和 Derynck, R. 上皮-间质转化的分子机制。纳特。莫尔牧师。细胞生物学。 15、178-196 (2014)。Jalali, A., Zhu, X., Liu, C. & Nawshad, A. Induction of palate epithelial mesenchymal transition by transforming growth factor β3 signaling. Dev. Growth Differ. 54, 633–648 (2012).
Jalali, A.、Zhu, X.、Liu, C. 和 Nawshad, A. 通过转化生长因子 β3 信号传导诱导腭上皮间质转化。开发。增长不同。 54、633-648 (2012)。Nawshad, A. & Hay, E. D. TGFbeta3 signaling activates transcription of the LEF1 gene to induce epithelial mesenchymal transformation during mouse palate development. J. Cell Biol. 163, 1291–1301 (2003).
Nawshad, A. 和 Hay, ED TGFbeta3 信号传导激活 LEF1 基因的转录,从而在小鼠上颚发育过程中诱导上皮间质转化。 J.细胞生物学。 163、1291-1301 (2003)。Pelton, R. W., Hogan, B. L., Miller, D. A. & Moses, H. L. Differential expression of genes encoding TGFs beta 1, beta 2, and beta 3 during murine palate formation. Dev. Biol. 141, 456–460 (1990).
Pelton, RW、Hogan, BL、Miller, DA 和 Moses, HL 在小鼠上颚形成过程中编码 TGFs beta 1、beta 2 和 beta 3 的基因的差异表达。开发。生物。 141、456-460 (1990)。Fitzpatrick, D. R., Denhez, F., Kondaiah, P. & Akhurst, R. J. Differential expression of TGF beta isoforms in murine palatogenesis. Development 109, 585–595 (1990).
Fitzpatrick, DR、Denhez, F.、Kondaiah, P. 和 Akhurst, RJ 小鼠腭发育中 TGF β 同种型的差异表达。发展109 , 585–595 (1990)。Brunet, C. L., Sharpe, P. M. & Ferguson, M. W. Inhibition of TGF-beta 3 (but not TGF-beta 1 or TGF-beta 2) activity prevents normal mouse embryonic palate fusion. Int J. Dev. Biol. 39, 345–355 (1995).
Brunet, CL, Sharpe, PM 和 Ferguson, MW 抑制 TGF-β 3(但不包括 TGF-β 1 或 TGF-β 2)活性可阻止正常的小鼠胚胎腭融合。 Int J. Dev。生物。 39、345-355 (1995)。Sabbineni, H., Verma, A. & Somanath, P. R. Isoform-specific effects of transforming growth factor beta on endothelial-to-mesenchymal transition. J. Cell Physiol. 233, 8418–8428 (2018).
Sabbineni, H.、Verma, A. 和 Somanath, PR 转化生长因子β对内皮-间质转化的异构体特异性影响。 J.细胞生理学。 233、8418–8428 (2018)。Molin, D. G. et al. Expression patterns of Tgfbeta1-3 associate with myocardialisation of the outflow tract and the development of the epicardium and the fibrous heart skeleton. Dev. Dyn. 227, 431–444 (2003).
莫林,DG 等人。 Tgfbeta1-3 的表达模式与流出道的心肌化以及心外膜和纤维心脏骨骼的发育相关。开发。动态。 227、431-444 (2003)。Dickson, M. C., Slager, H. G., Duffie, E., Mummery, C. L. & Akhurst, R. J. RNA and protein localisations of TGF beta 2 in the early mouse embryo suggest an involvement in cardiac development. Development 117, 625–639 (1993).
Dickson, MC、Slager, HG、Duffie, E.、Mummery, CL 和 Akhurst, RJ 早期小鼠胚胎中 TGF beta 2 的 RNA 和蛋白质定位表明参与心脏发育。发展117 , 625–639 (1993)。Akhurst, R. J., Lehnert, S. A., Faissner, A. & Duffie, E. TGF beta in murine morphogenetic processes: the early embryo and cardiogenesis. Development 108, 645–656 (1990).
Akhurst, RJ, Lehnert, SA, Faissner, A. & Duffie, E. 小鼠形态发生过程中的 TGF beta:早期胚胎和心脏发生。发展108 , 645–656 (1990)。Camenisch, T. D. et al. Temporal and distinct TGFbeta ligand requirements during mouse and avian endocardial cushion morphogenesis. Dev. Biol. 248, 170–181 (2002).
卡梅尼施,TD 等人。小鼠和禽类心内膜垫形态发生过程中对时间和不同 TGFbeta 配体的需求。开发。生物。 248 , 170–181 (2002)。Potts, J. D. & Runyan, R. B. Epithelial-mesenchymal cell transformation in the embryonic heart can be mediated, in part, by transforming growth factor beta. Dev. Biol. 134, 392–401 (1989).
Potts, JD 和 Runyan, RB 胚胎心脏中的上皮间质细胞转化可以部分通过转化生长因子 β 介导。开发。生物。 134、392-401 (1989)。Azhar, M. et al. Ligand-specific function of transforming growth factor beta in epithelial-mesenchymal transition in heart development. Dev. Dyn. 238, 431–442 (2009).
阿扎尔,M.等人。转化生长因子β在心脏发育中上皮间质转化中的配体特异性功能。开发。动态。 238、431-442 (2009)。Nakajima, Y., Yamagishi, T., Nakamura, H., Markwald, R. R. & Krug, E. L. An autocrine function for transforming growth factor (TGF)-beta3 in the transformation of atrioventricular canal endocardium into mesenchyme during chick heart development. Dev. Biol. 194, 99–113 (1998).
Nakajima,Y.,Yamagishi,T.,Nakamura,H.,Markwald,RR 和 Krug,EL 鸡心脏发育过程中房室管心内膜转化为间充质过程中转化生长因子 (TGF)-β3 的自分泌功能。开发。生物。 194 , 99–113 (1998)。Compton, L. A., Potash, D. A., Mundell, N. A. & Barnett, J. V. Transforming growth factor-beta induces loss of epithelial character and smooth muscle cell differentiation in epicardial cells. Dev. Dyn. 235, 82–93 (2006).
Compton, LA、Potash, DA、Mundell, NA 和 Barnett, JV 转化生长因子-β 会诱导心外膜细胞上皮特性的丧失和平滑肌细胞的分化。开发。动态。 235 , 82–93 (2006)。Austin, A. F., Compton, L. A., Love, J. D., Brown, C. B. & Barnett, J. V. Primary and immortalized mouse epicardial cells undergo differentiation in response to TGFbeta. Dev. Dyn. 237, 366–376 (2008).
Austin, AF、Compton, LA、Love, JD、Brown, CB 和 Barnett, JV 原代和永生化小鼠心外膜细胞响应 TGFbeta 进行分化。开发。动态。 237、366-376 (2008)。Liu, M. et al. Transforming Growth Factor-induced Protein Promotes NF-κB-mediated Angiogenesis during Postnatal Lung Development. Am. J. Respir. Cell Mol. Biol. 64, 318–330 (2021).
刘,M.等人。转化生长因子诱导蛋白促进出生后肺发育过程中 NF-κB 介导的血管生成。是。 J.呼吸。细胞分子。生物。 64 , 318–330 (2021)。Ahmed, S., Liu, C. C. & Nawshad, A. Mechanisms of palatal epithelial seam disintegration by transforming growth factor (TGF) beta3. Dev. Biol. 309, 193–207 (2007).
Ahmed, S.、Liu, CC 和 Nawshad, A. 转化生长因子 (TGF) beta3 导致腭上皮缝崩解的机制。开发。生物。 309、193-207 (2007)。Dunker, N., Schmitt, K. & Krieglstein, K. TGF-beta is required for programmed cell death in interdigital webs of the developing mouse limb. Mech. Dev. 113, 111–120 (2002).
Dunker, N.、Schmitt, K. 和 Krieglstein, K. TGF-β 是发育中小鼠肢体指间网中程序性细胞死亡所必需的。机甲。开发。 113、111-120 (2002)。Krieglstein, K. et al. Reduction of endogenous transforming growth factors beta prevents ontogenetic neuron death. Nat. Neurosci. 3, 1085–1090 (2000).
克里格斯坦,K.等人。内源性转化生长因子β的减少可防止个体发育神经元死亡。纳特。神经科学。 3、1085-1090 (2000)。Dunker, N., Schuster, N. & Krieglstein, K. TGF-beta modulates programmed cell death in the retina of the developing chick embryo. Development 128, 1933–1942 (2001).
Dunker, N.、Schuster, N. 和 Krieglstein, K. TGF-β 调节发育中鸡胚视网膜中的程序性细胞死亡。发展128,1933-1942 (2001)。Schuster, N., Dunker, N. & Krieglstein, K. Transforming growth factor-beta induced cell death in the developing chick retina is mediated via activation of c-jun N-terminal kinase and downregulation of the anti-apoptotic protein Bcl-X(L). Neurosci. Lett. 330, 239–242 (2002).
Schuster, N.、Dunker, N. 和 Krieglstein, K. 发育中的小鸡视网膜中转化生长因子-β 诱导的细胞死亡是通过 c-jun N 末端激酶的激活和抗凋亡蛋白 Bcl-X 的下调介导的(L)。神经科学。莱特。 330、239-242 (2002)。Braunger, B. M. et al. TGF-β signaling protects retinal neurons from programmed cell death during the development of the mammalian eye. J. Neurosci. 33, 14246–14258 (2013).
布劳格,BM 等人。 TGF-β信号传导可保护视网膜神经元在哺乳动物眼睛发育过程中免遭程序性细胞死亡。 J.神经科学。 33、14246–14258 (2013)。Ruiz-Canada, C., Bernabe-Garcia, A., Liarte, S., Rodriguez-Valiente, M. & Nicolas, F. J. Chronic Wound Healing by Amniotic Membrane: TGF-beta and EGF Signaling Modulation in Re-epithelialization. Front Bioeng. Biotechnol. 9, 689328 (2021).
Ruiz-Canada, C.、Bernabe-Garcia, A.、Liarte, S.、Rodriguez-Valiente, M. 和 Nicolas, FJ 羊膜慢性伤口愈合:上皮再形成中的 TGF-β 和 EGF 信号传导调节。前生物工程。生物技术。 9、689328 (2021)。McMullen, H. et al. Spatial and temporal expression of transforming growth factor-beta isoforms during ovine excisional and incisional wound repair. Wound Repair Regen. 3, 141–156 (1995).
麦克马伦,H.等人。绵羊切除和切口伤口修复过程中转化生长因子-β亚型的空间和时间表达。伤口修复再生。 3,141-156 (1995)。Gold, L. I., Sung, J. J., Siebert, J. W. & Longaker, M. T. Type I (RI) and type II (RII) receptors for transforming growth factor-beta isoforms are expressed subsequent to transforming growth factor-beta ligands during excisional wound repair. Am. J. Pathol. 150, 209–222 (1997).
Gold、LI、Sung、JJ、Siebert、JW 和 Longaker,MT I 型 (RI) 和 II 型 (RII) 转化生长因子-β 同种型受体在切除伤口修复过程中在转化生长因子-β 配体之后表达。是。 J.帕索尔. 150、209-222 (1997)。Mustoe, T. A. et al. Accelerated healing of incisional wounds in rats induced by transforming growth factor-beta. Science 237, 1333–1336 (1987).
穆斯托,TA 等人。转化生长因子-β 诱导大鼠切口伤口加速愈合。科学237 , 1333–1336 (1987)。Postlethwaite, A. E., Keski-Oja, J., Moses, H. L. & Kang, A. H. Stimulation of the chemotactic migration of human fibroblasts by transforming growth factor beta. J. Exp. Med 165, 251–256 (1987).
Postlethwaite, AE、Keski-Oja, J.、Moses, HL 和 Kang, AH 通过转化生长因子 β 刺激人成纤维细胞的趋化迁移。 J.Exp。医学165 , 251–256 (1987)。Pierce, G. F. et al. Platelet-derived growth factor and transforming growth factor-beta enhance tissue repair activities by unique mechanisms. J. Cell Biol. 109, 429–440 (1989).
皮尔斯,GF 等人。血小板衍生生长因子和转化生长因子-β 通过独特的机制增强组织修复活性。 J.细胞生物学。 109 , 429–440 (1989)。Puolakkainen, P. A. et al. Acceleration of wound healing in aged rats by topical application of transforming growth factor-beta(1). Wound Repair Regen. 3, 330–339 (1995).
普奥拉凯宁,PA 等人。局部应用转化生长因子-β(1) 加速老年大鼠伤口愈合。伤口修复再生。 3、330-339 (1995)。Wahl, S. M. et al. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc. Natl Acad. Sci. USA 84, 5788–5792 (1987).
瓦尔,SM 等人。 β 型转化生长因子诱导单核细胞趋化性和生长因子产生。过程。国家科学院。科学。美国84,5788–5792 (1987)。Rappolee, D. A., Mark, D., Banda, M. J. & Werb, Z. Wound macrophages express TGF-alpha and other growth factors in vivo: analysis by mRNA phenotyping. Science 241, 708–712 (1988).
Rappolee, DA, Mark, D., Banda, MJ & Werb, Z. 伤口巨噬细胞在体内表达 TGF-α 和其他生长因子:mRNA 表型分析。科学241 , 708–712 (1988)。Kane, C. J., Hebda, P. A., Mansbridge, J. N. & Hanawalt, P. C. Direct evidence for spatial and temporal regulation of transforming growth factor beta 1 expression during cutaneous wound healing. J. Cell Physiol. 148, 157–173 (1991).
Kane, CJ、Hebda, PA、Mansbridge, JN 和 Hanawalt, PC 皮肤伤口愈合过程中转化生长因子 β 1 表达的空间和时间调节的直接证据。 J.细胞生理学。 148、157-173 (1991)。Zambruno, G. et al. Transforming growth factor-beta 1 modulates beta 1 and beta 5 integrin receptors and induces the de novo expression of the alpha v beta 6 heterodimer in normal human keratinocytes: implications for wound healing. J. Cell Biol. 129, 853–865 (1995).
赞布鲁诺,G.等人。转化生长因子-β 1 调节 β 1 和 β 5 整合素受体并诱导正常人角质形成细胞中 α v β 6 异二聚体的从头表达:对伤口愈合的影响。 J.细胞生物学。 129、853–865 (1995)。Jeong, H. W. & Kim, I. S. TGF-beta1 enhances betaig-h3-mediated keratinocyte cell migration through the alpha3beta1 integrin and PI3K. J. Cell Biochem 92, 770–780 (2004).
Jeong, HW & Kim, IS TGF-β1 通过 alpha3beta1 整合素和 PI3K 增强 betaig-h3 介导的角质形成细胞迁移。细胞生物化学杂志92 , 770–780 (2004)。Bandyopadhyay, B. et al. A “traffic control” role for TGFbeta3: orchestrating dermal and epidermal cell motility during wound healing. J. Cell Biol. 172, 1093–1105 (2006).
Bandyopadhyay,B.等人。 TGFbeta3 的“交通控制”作用:在伤口愈合过程中协调真皮和表皮细胞的运动。 J.细胞生物学。 172、1093-1105 (2006)。Heimark, R. L., Twardzik, D. R. & Schwartz, S. M. Inhibition of endothelial regeneration by type-beta transforming growth factor from platelets. Science 233, 1078–1080 (1986).
Heimark, RL、Twardzik, DR 和 Schwartz, SM 通过血小板中的 β 型转化生长因子抑制内皮再生。科学233 , 1078–1080 (1986)。Roberts, A. B. et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc. Natl Acad. Sci. USA 83, 4167–4171 (1986).
罗伯茨,AB 等人。 β 型转化生长因子:体内快速诱导纤维化和血管生成,体外刺激胶原蛋白形成。过程。国家科学院。科学。美国83,4167–4171 (1986)。Wang, X. J., Liefer, K. M., Tsai, S., O’Malley, B. W. & Roop, D. R. Development of gene-switch transgenic mice that inducibly express transforming growth factor beta1 in the epidermis. Proc. Natl Acad. Sci. USA 96, 8483–8488 (1999).
Wang, XJ, Liefer, KM, Tsai, S., O'Malley, BW & Roop, DR 开发在表皮中诱导表达转化生长因子β1的基因开关转基因小鼠。过程。国家科学院。科学。美国96,8483–8488 (1999)。Lynch, S. E., Colvin, R. B. & Antoniades, H. N. Growth factors in wound healing. Single and synergistic effects on partial thickness porcine skin wounds. J. Clin. Invest 84, 640–646 (1989).
Lynch, SE, Colvin, RB 和 Antoniades, HN 伤口愈合中的生长因子。对部分厚猪皮肤伤口的单一和协同作用。 J.克林。投资84 , 640–646 (1989)。Madri, J. A., Pratt, B. M. & Tucker, A. M. Phenotypic modulation of endothelial cells by transforming growth factor-beta depends upon the composition and organization of the extracellular matrix. J. Cell Biol. 106, 1375–1384 (1988).
Madri,JA,Pratt,BM 和 Tucker,AM 通过转化生长因子-β 对内皮细胞的表型调节取决于细胞外基质的组成和组织。 J.细胞生物学。 106、1375-1384 (1988)。Iruela-Arispe, M. L. & Sage, E. H. Endothelial cells exhibiting angiogenesis in vitro proliferate in response to TGF-beta 1. J. Cell Biochem 52, 414–430 (1993).
Iruela-Arispe, ML & Sage, EH 表现出体外血管生成的内皮细胞响应 TGF-β 1 增殖。 J. Cell Biochem 52 , 414–430 (1993)。Lu, S. L. et al. Overexpression of transforming growth factor beta1 in head and neck epithelia results in inflammation, angiogenesis, and epithelial hyperproliferation. Cancer Res 64, 4405–4410 (2004).
卢,SL 等。头颈上皮中转化生长因子β1的过度表达会导致炎症、血管生成和上皮过度增殖。癌症研究64 , 4405–4410 (2004)。Cox, D. A., Kunz, S., Cerletti, N., McMaster, G. K. & Burk, R. R. Wound healing in aged animals-effects of locally applied transforming growth factor beta 2 in different model systems. Exs 61, 287–295 (1992).
Cox, DA、Kunz, S.、Cerletti, N.、McMaster, GK 和 Burk, RR 老年动物的伤口愈合——局部应用转化生长因子 β 2 在不同模型系统中的影响。前61 , 287–295 (1992)。Frank, S. et al. Regulation of vascular endothelial growth factor expression in cultured keratinocytes. Implications for normal and impaired wound healing. J. Biol. Chem. 270, 12607–12613 (1995).
弗兰克,S.等人。培养的角质形成细胞中血管内皮生长因子表达的调节。对正常和受损伤口愈合的影响。 J.Biol。化学。 270、12607-12613 (1995)。Pertovaara, L. et al. Vascular endothelial growth factor is induced in response to transforming growth factor-beta in fibroblastic and epithelial cells. J. Biol. Chem. 269, 6271–6274 (1994).
佩托瓦拉,L.等人。成纤维细胞和上皮细胞中的转化生长因子-β 会诱导血管内皮生长因子的产生。 J.Biol。化学。 269、6271–6274 (1994)。Strutz, F. et al. TGF-beta 1 induces proliferation in human renal fibroblasts via induction of basic fibroblast growth factor (FGF-2). Kidney Int 59, 579–592 (2001).
斯特鲁茨,F.等人。 TGF-β 1 通过诱导碱性成纤维细胞生长因子 (FGF-2) 来诱导人肾成纤维细胞增殖。肾脏国际59 , 579–592 (2001)。Battegay, E. J., Raines, E. W., Seifert, R. A., Bowen-Pope, D. F. & Ross, R. TGF-beta induces bimodal proliferation of connective tissue cells via complex control of an autocrine PDGF loop. Cell 63, 515–524 (1990).
Battegay, EJ, Raines, EW, Seifert, RA, Bowen-Pope, DF & Ross, R. TGF-β 通过自分泌 PDGF 环的复杂控制诱导结缔组织细胞的双峰增殖。细胞63 , 515–524 (1990)。Ignotz, R. A. & Massagué, J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J. Biol. Chem. 261, 4337–4345 (1986).
Ignotz, RA 和 Massagué, J. 转化生长因子-β 刺激纤连蛋白和胶原蛋白的表达及其与细胞外基质的结合。 J.Biol。化学。 261 , 4337–4345 (1986)。Clark, R. A., McCoy, G. A., Folkvord, J. M. & McPherson, J. M. TGF-beta 1 stimulates cultured human fibroblasts to proliferate and produce tissue-like fibroplasia: a fibronectin matrix-dependent event. J. Cell Physiol. 170, 69–80 (1997).
Clark, RA、McCoy, GA、Folkvord, JM 和 McPherson, JM TGF-β1 刺激培养的人成纤维细胞增殖并产生组织样纤维增生:一种纤连蛋白基质依赖性事件。 J.细胞生理学。 170、69-80 (1997)。Murata, H. et al. TGF-beta3 stimulates and regulates collagen synthesis through TGF-beta1-dependent and independent mechanisms. J. Invest Dermatol 108, 258–262 (1997).
村田,H.等人。 TGF-β3 通过 TGF-β1 依赖和独立机制刺激和调节胶原蛋白合成。 J. Invest Dermatol 108 , 258–262 (1997)。Tyrone, J. W. et al. Transforming growth factor beta3 promotes fascial wound healing in a new animal model. Arch. Surg. 135, 1154–1159 (2000).
蒂龙,JW 等人。转化生长因子 beta3 在新的动物模型中促进筋膜伤口愈合。拱。外科医生。 135、1154-1159 (2000)。Edwards, D. R. et al. Transforming growth factor beta modulates the expression of collagenase and metalloproteinase inhibitor. Embo j. 6, 1899–1904 (1987).
爱德华兹,DR 等人。转化生长因子β调节胶原酶和金属蛋白酶抑制剂的表达。恩博 J. 6、1899-1904 (1987)。Lund, L. R. et al. Transforming growth factor-beta is a strong and fast acting positive regulator of the level of type-1 plasminogen activator inhibitor mRNA in WI-38 human lung fibroblasts. Embo j. 6, 1281–1286 (1987).
隆德,LR 等人。转化生长因子-β 是 WI-38 人肺成纤维细胞中 1 型纤溶酶原激活剂抑制剂 mRNA 水平的强而快速的正调节剂。恩博 J. 6、1281-1286 (1987)。Overall, C. M., Wrana, J. L. & Sodek, J. Independent regulation of collagenase, 72-kDa progelatinase, and metalloendoproteinase inhibitor expression in human fibroblasts by transforming growth factor-beta. J. Biol. Chem. 264, 1860–1869 (1989).
总体而言,CM、Wrana、JL 和 Sodek,J。通过转化生长因子-β 独立调节人成纤维细胞中胶原酶、72-kDa 原明胶酶和金属内蛋白酶抑制剂的表达。 J.Biol。化学。 264,1860-1869 (1989)。Wright, J. K., Cawston, T. E. & Hazleman, B. L. Transforming growth factor beta stimulates the production of the tissue inhibitor of metalloproteinases (TIMP) by human synovial and skin fibroblasts. Biochim. Biophys. Acta 1094, 207–210 (1991).
Wright, JK、Cawston, TE 和 Hazleman, BL 转化生长因子 β 刺激人类滑膜和皮肤成纤维细胞产生金属蛋白酶组织抑制剂 (TIMP)。生物化学。生物物理学。 Acta 1094,207-210 (1991)。Shah, M., Foreman, D. M. & Ferguson, M. W. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J. Cell Sci. 108, 985–1002 (1995).
Shah, M., Foreman, DM & Ferguson, MW 中和 TGF-β 1 和 TGF-β 2 或向大鼠皮肤伤口外源添加 TGF-β 3 可减少疤痕形成。 J.细胞科学。 108、985-1002 (1995)。Montesano, R. & Orci, L. Transforming growth factor beta stimulates collagen-matrix contraction by fibroblasts: implications for wound healing. Proc. Natl Acad. Sci. USA 85, 4894–4897 (1988).
Montesano, R. & Orci, L. 转化生长因子β刺激成纤维细胞的胶原基质收缩:对伤口愈合的影响。过程。国家科学院。科学。美国85,4894–4897 (1988)。Meckmongkol, T. T., Harmon, R., McKeown-Longo, P. & Van De Water, L. The fibronectin synergy site modulates TGF-beta-dependent fibroblast contraction. Biochem Biophys. Res Commun. 360, 709–714 (2007).
Meckmongkol, TT、Harmon, R.、McKeown-Longo, P. 和 Van De Water, L. 纤连蛋白协同位点调节 TGF-β 依赖性成纤维细胞收缩。生物化学生物物理学。资源通讯。 360、709–714 (2007)。Desmoulière, A., Geinoz, A., Gabbiani, F. & Gabbiani, G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J. Cell Biol. 122, 103–111 (1993).
Desmoulière,A.,Geinoz,A.,Gabbiani,F. 和 Gabbiani,G。转化生长因子-β 1 诱导肉芽组织肌成纤维细胞以及静止和生长的培养成纤维细胞中的 α-平滑肌肌动蛋白表达。 J.细胞生物学。 122、103-111 (1993)。Jakowlew, S. B. et al. Transforming growth factor-beta (TGF-beta) isoforms in rat liver regeneration: messenger RNA expression and activation of latent TGF-beta. Cell Regul. 2, 535–548 (1991).
Jakowlew,SB 等人。大鼠肝脏再生中的转化生长因子-β (TGF-β) 同工型:信使 RNA 表达和潜在 TGF-β 的激活。细胞调节。 2,535-548 (1991)。Armendariz-Borunda, J. et al. Transforming growth factor beta gene expression is transiently enhanced at a critical stage during liver regeneration after CCl4 treatment. Lab Invest 69, 283–294 (1993).
Armendariz-Borunda,J. 等人。 CCl4处理后肝脏再生过程中的关键阶段转化生长因子β基因表达短暂增强。实验室投资69 , 283–294 (1993)。Braun, L. et al. Transforming growth factor beta mRNA increases during liver regeneration: a possible paracrine mechanism of growth regulation. Proc. Natl Acad. Sci. USA 85, 1539–1543 (1988).
布劳恩,L.等人。转化生长因子 β mRNA 在肝再生过程中增加:一种可能的生长调节旁分泌机制。过程。国家科学院。科学。美国85 , 1539–1543 (1988)。Nishikawa, Y., Wang, M. & Carr, B. I. Changes in TGF-beta receptors of rat hepatocytes during primary culture and liver regeneration: increased expression of TGF-beta receptors associated with increased sensitivity to TGF-beta-mediated growth inhibition. J. Cell Physiol. 176, 612–623 (1998).
Nishikawa, Y.、Wang, M. 和 Carr, BI 原代培养和肝再生过程中大鼠肝细胞 TGF-β 受体的变化:TGF-β 受体表达增加与对 TGF-β 介导的生长抑制敏感性增加相关。 J.细胞生理学。 176、612-623 (1998)。Grasl-Kraupp, B. et al. Levels of transforming growth factor beta and transforming growth factor beta receptors in rat liver during growth, regression by apoptosis and neoplasia. Hepatology 28, 717–726 (1998).
Grasl-Kraupp,B. 等人。大鼠肝脏在生长、细胞凋亡和肿瘤消退过程中转化生长因子β和转化生长因子β受体的水平。肝病学28 , 717–726 (1998)。Riesle, E. et al. Increased expression of transforming growth factor beta s after acute oedematous pancreatitis in rats suggests a role in pancreatic repair. Gut 40, 73–79 (1997).
Riesle,E.等人。大鼠急性水肿性胰腺炎后转化生长因子β表达增加表明其在胰腺修复中发挥作用。肠道40 , 73–79 (1997)。Friess, H. et al. Enhanced expression of TGF-betas and their receptors in human acute pancreatitis. Ann. Surg. 227, 95–104 (1998).
弗里斯,H.等人。人类急性胰腺炎中 TGF-β 及其受体的表达增强。安.外科医生。 227 , 95–104 (1998)。Gress, T. et al. Enhancement of transforming growth factor beta 1 expression in the rat pancreas during regeneration from caerulein-induced pancreatitis. Eur. J. Clin. Invest 24, 679–685 (1994).
格雷斯,T.等人。雨蛙素诱导的胰腺炎再生过程中大鼠胰腺中转化生长因子β1表达的增强。欧元。 J.克林。投资24 , 679–685 (1994)。Wan, M. et al. Injury-activated transforming growth factor β controls mobilization of mesenchymal stem cells for tissue remodeling. Stem Cells 30, 2498–2511 (2012).
万,M.等人。损伤激活的转化生长因子β控制间充质干细胞的动员以进行组织重塑。干细胞30 , 2498–2511 (2012)。Bax, N. A. et al. In vitro epithelial-to-mesenchymal transformation in human adult epicardial cells is regulated by TGFβ-signaling and WT1. Basic Res Cardiol. 106, 829–847 (2011).
巴克斯,NA 等人。成人心外膜细胞的体外上皮-间质转化受 TGFβ 信号传导和 WT1 调节。心脏基础研究。 106、829–847 (2011)。Redini, F., Galera, P., Mauviel, A., Loyau, G. & Pujol, J. P. Transforming growth factor beta stimulates collagen and glycosaminoglycan biosynthesis in cultured rabbit articular chondrocytes. FEBS Lett. 234, 172–176 (1988).
Redini, F.、Galera, P.、Mauviel, A.、Loyau, G. 和 Pujol, JP 转化生长因子 β 可刺激培养的兔关节软骨细胞中的胶原蛋白和糖胺聚糖生物合成。 FEBS 快报。 234、172-176 (1988)。Malemud, C. J., Killeen, W., Hering, T. M. & Purchio, A. F. Enhanced sulfated-proteoglycan core protein synthesis by incubation of rabbit chondrocytes with recombinant transforming growth factor-beta 1. J. Cell Physiol. 149, 152–159 (1991).
Malemud, CJ、Killeen, W.、Hering, TM 和 Purchio, AF 通过将兔软骨细胞与重组转化生长因子-β 1 一起孵育来增强硫酸化蛋白聚糖核心蛋白的合成。J . Cell Physiol。 149、152-159 (1991)。Buss, A. et al. TGF-beta1 and TGF-beta2 expression after traumatic human spinal cord injury. Spinal Cord. 46, 364–371 (2008).
巴斯,A.等人。人类脊髓损伤后 TGF-β1 和 TGF-β2 的表达。脊髓。 46、364-371 (2008)。Lehrmann, E. et al. Microglia and macrophages are major sources of locally produced transforming growth factor-beta1 after transient middle cerebral artery occlusion in rats. Glia 24, 437–448 (1998).
莱尔曼,E.等人。小胶质细胞和巨噬细胞是大鼠大脑中动脉短暂闭塞后局部产生的转化生长因子-β1 的主要来源。神经胶质细胞24 , 437–448 (1998)。Hannon, G. J. & Beach, D. p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature 371, 257–261 (1994).
Hannon, GJ 和 Beach, D. p15INK4B 是 TGF-β 诱导的细胞周期停滞的潜在效应子。自然371 , 257–261 (1994)。Datto, M. B. et al. Transforming growth factor beta induces the cyclin-dependent kinase inhibitor p21 through a p53-independent mechanism. Proc. Natl Acad. Sci. USA 92, 5545–5549 (1995).
达托,MB 等人。转化生长因子β通过p53独立机制诱导细胞周期蛋白依赖性激酶抑制剂p21。过程。国家科学院。科学。美国92,5545–5549 (1995)。Rich, J. N., Zhang, M., Datto, M. B., Bigner, D. D. & Wang, X. F. Transforming growth factor-beta-mediated p15(INK4B) induction and growth inhibition in astrocytes is SMAD3-dependent and a pathway prominently altered in human glioma cell lines. J. Biol. Chem. 274, 35053–35058 (1999).
Rich,JN,Zhang,M.,Datto,MB,Bigner,DD 和 Wang,XF 星形胶质细胞中转化生长因子-β 介导的 p15 (INK4B) 诱导和生长抑制是 SMAD3 依赖性的,并且是人胶质瘤细胞中显着改变的途径线。 J.Biol。化学。 274、35053–35058 (1999)。Seoane, J., Le, H. V., Shen, L., Anderson, S. A. & Massagué, J. Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell 117, 211–223 (2004).
Seoane, J.、Le, HV、Shen, L.、Anderson, SA 和 Massagué, J. Smad 和叉头通路在神经上皮和胶质母细胞瘤细胞增殖控制中的整合。单元格117 , 211–223 (2004)。Gomis, R. R., Alarcón, C., Nadal, C., Van Poznak, C. & Massagué, J. C/EBPbeta at the core of the TGFbeta cytostatic response and its evasion in metastatic breast cancer cells. Cancer Cell 10, 203–214 (2006).
Gomis, RR、Alarcón, C.、Nadal, C.、Van Poznak, C. 和 Massagué, J. C/EBPbeta 是转移性乳腺癌细胞中 TGFbeta 细胞抑制反应及其逃避的核心。癌细胞10 , 203–214 (2006)。Feng, X. H., Lin, X. & Derynck, R. Smad2, Smad3 and Smad4 cooperate with Sp1 to induce p15(Ink4B) transcription in response to TGF-beta. Embo j. 19, 5178–5193 (2000).
Feng, XH, Lin, X. & Derynck, R. Smad2、Smad3 和 Smad4 与 Sp1 配合诱导 p15(Ink4B) 转录以响应 TGF-β。恩博 J. 19、5178-5193 (2000)。Pardali, K. et al. Role of Smad proteins and transcription factor Sp1 in p21(Waf1/Cip1) regulation by transforming growth factor-beta. J. Biol. Chem. 275, 29244–29256 (2000).
帕达利,K.等人。 Smad 蛋白和转录因子 Sp1 在转化生长因子-β 调节 p21(Waf1/Cip1)中的作用。 J.Biol。化学。 275 , 29244–29256 (2000)。Reynisdóttir, I., Polyak, K., Iavarone, A. & Massagué, J. Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-beta. Genes Dev. 9, 1831–1845 (1995).
Reynisdóttir, I.、Polyak, K.、Iavarone, A. 和 Massagué, J. Kip/Cip 和 Ink4 Cdk 抑制剂协同诱导响应 TGF-β 的细胞周期停滞。基因开发。 9、1831-1845 (1995)。Reynisdóttir, I. & Massagué, J. The subcellular locations of p15(Ink4b) and p27(Kip1) coordinate their inhibitory interactions with cdk4 and cdk2. Genes Dev. 11, 492–503 (1997).
Reynisdóttir, I. & Massagué, J. p15(Ink4b) 和 p27(Kip1) 的亚细胞位置协调它们与 cdk4 和 cdk2 的抑制相互作用。基因开发。 11,492-503 (1997)。Kamesaki, H., Nishizawa, K., Michaud, G. Y., Cossman, J. & Kiyono, T. TGF-beta 1 induces the cyclin-dependent kinase inhibitor p27Kip1 mRNA and protein in murine B cells. J. Immunol. 160, 770–777 (1998).
Kamesaki, H.、Nishizawa, K.、Michaud, GY、Cossman, J. 和 Kiyono, T. TGF-β 1 诱导小鼠 B 细胞中的细胞周期蛋白依赖性激酶抑制剂 p27Kip1 mRNA 和蛋白质。 J.免疫学。 160、770–777 (1998)。Scandura, J. M., Boccuni, P., Massagué, J. & Nimer, S. D. Transforming growth factor beta-induced cell cycle arrest of human hematopoietic cells requires p57KIP2 up-regulation. Proc. Natl Acad. Sci. USA 101, 15231–15236 (2004).
Scandura, JM、Baccuni, P.、Massagué, J. 和 Nimer, SD 转化生长因子 β 诱导的人类造血细胞细胞周期停滞需要 p57KIP2 上调。过程。国家科学院。科学。美国101,15231–15236 (2004)。Chen, C. R., Kang, Y., Siegel, P. M. & Massagué, J. E2F4/5 and p107 as Smad cofactors linking the TGFbeta receptor to c-myc repression. Cell 110, 19–32 (2002).
Chen, CR, Kang, Y., Siegel, PM & Massagué, J. E2F4/5 和 p107 作为 Smad 辅助因子,将 TGFbeta 受体与 c-myc 抑制联系起来。细胞110,19-32 (2002)。Yagi, K. et al. c-myc is a downstream target of the Smad pathway. J. Biol. Chem. 277, 854–861 (2002).
八木,K.等人。 c-myc 是 Smad 通路的下游靶标。 J.Biol。化学。 277、854-861 (2002)。Kang, Y., Chen, C. R. & Massagué, J. A self-enabling TGFbeta response coupled to stress signaling: Smad engages stress response factor ATF3 for Id1 repression in epithelial cells. Mol. Cell 11, 915–926 (2003).
Kang, Y., Chen, CR & Massagué, J. 与应激信号耦合的自我激活 TGFbeta 反应:Smad 参与应激反应因子 ATF3 来抑制上皮细胞中的 Id1。摩尔。细胞11,915-926 (2003)。Lasorella, A., Noseda, M., Beyna, M., Yokota, Y. & Iavarone, A. Id2 is a retinoblastoma protein target and mediates signalling by Myc oncoproteins. Nature 407, 592–598 (2000).
Lasorella, A.、Noseda, M.、Beyna, M.、Yokota, Y. 和 Iavarone, A. Id2 是视网膜母细胞瘤蛋白靶标,通过 Myc 癌蛋白介导信号传导。自然407 , 592–598 (2000)。Siegel, P. M., Shu, W. & Massagué, J. Mad upregulation and Id2 repression accompany transforming growth factor (TGF)-beta-mediated epithelial cell growth suppression. J. Biol. Chem. 278, 35444–35450 (2003).
Siegel, PM, Shu, W. 和 Massagué, J. Mad 上调和 Id2 抑制伴随着转化生长因子 (TGF)-β 介导的上皮细胞生长抑制。 J.Biol。化学。 278 , 35444–35450 (2003)。Seoane, J. et al. TGFbeta influences Myc, Miz-1 and Smad to control the CDK inhibitor p15INK4b. Nat. Cell Biol. 3, 400–408 (2001).
Seoane,J.等人。 TGFbeta 影响 Myc、Miz-1 和 Smad 以控制 CDK 抑制剂 p15INK4b。纳特。细胞生物学。 3、400-408 (2001)。Seoane, J., Le, H. V. & Massagué, J. Myc suppression of the p21(Cip1) Cdk inhibitor influences the outcome of the p53 response to DNA damage. Nature 419, 729–734 (2002).
Seoane, J.、Le, HV 和 Massagué, J. p21(Cip1) Cdk 抑制剂的 Myc 抑制会影响 p53 对 DNA 损伤的反应结果。自然419 , 729–734 (2002)。Prabhu, S., Ignatova, A., Park, S. T. & Sun, X. H. Regulation of the expression of cyclin-dependent kinase inhibitor p21 by E2A and Id proteins. Mol. Cell Biol. 17, 5888–5896 (1997).
Prabhu, S.、Ignatova, A.、Park, ST 和 Sun, XH E2A 和 Id 蛋白对细胞周期蛋白依赖性激酶抑制剂 p21 表达的调节。摩尔。细胞生物学。 17、5888-5896 (1997)。Asirvatham, A. J., Carey, J. P. & Chaudhary, J. ID1-, ID2-, and ID3-regulated gene expression in E2A positive or negative prostate cancer cells. Prostate 67, 1411–1420 (2007).
Asirvatham,AJ,Carey,JP 和 Chaudhary,J。E2A 阳性或阴性前列腺癌细胞中 ID1、ID2 和 ID3 调节的基因表达。前列腺67 , 1411–1420 (2007)。Iavarone, A. & Massagué, J. E2F and histone deacetylase mediate transforming growth factor beta repression of cdc25A during keratinocyte cell cycle arrest. Mol. Cell Biol. 19, 916–922 (1999).
Iavarone, A. & Massagué, J. E2F 和组蛋白脱乙酰酶介导角质形成细胞周期停滞期间 cdc25A 的转化生长因子 β 抑制。摩尔。细胞生物学。 19、916-922 (1999)。Bhowmick, N. A. et al. TGF-beta-induced RhoA and p160ROCK activation is involved in the inhibition of Cdc25A with resultant cell-cycle arrest. Proc. Natl Acad. Sci. USA 100, 15548–15553 (2003).
博米克,NA 等人。 TGF-β 诱导的 RhoA 和 p160ROCK 激活参与 Cdc25A 的抑制,从而导致细胞周期停滞。过程。国家科学院。科学。美国100 强,15548–15553 (2003)。Ray, D. et al. Transforming growth factor beta facilitates beta-TrCP-mediated degradation of Cdc25A in a Smad3-dependent manner. Mol. Cell Biol. 25, 3338–3347 (2005).
雷,D.等人。转化生长因子β以Smad3依赖性方式促进β-TrCP介导的Cdc25A降解。摩尔。细胞生物学。 25、3338-3347 (2005)。Leof, E. B. et al. Induction of c-sis mRNA and activity similar to platelet-derived growth factor by transforming growth factor beta: a proposed model for indirect mitogenesis involving autocrine activity. Proc. Natl Acad. Sci. USA 83, 2453–2457 (1986).
莱夫,EB 等人。通过转化生长因子β诱导c-sis mRNA和与血小板衍生生长因子相似的活性:一种涉及自分泌活性的间接有丝分裂发生的拟议模型。过程。国家科学院。科学。美国83,2453–2457 (1986)。Wildey, G. M., Patil, S. & Howe, P. H. Smad3 potentiates transforming growth factor beta (TGFbeta)-induced apoptosis and expression of the BH3-only protein Bim in WEHI 231 B lymphocytes. J. Biol. Chem. 278, 18069–18077 (2003).
Wildey, GM、Patil, S. 和 Howe, PH Smad3 增强转化生长因子 β (TGFbeta) 诱导的细胞凋亡以及 WEHI 231 B 淋巴细胞中仅 BH3 蛋白 Bim 的表达。 J.Biol。化学。 278、18069-18077 (2003)。Ramjaun, A. R., Tomlinson, S. & Eddaoudi, A. & Downward, J. Upregulation of two BH3-only proteins, Bmf and Bim, during TGF beta-induced apoptosis. Oncogene 26, 970–981 (2007).
Ramjaun, AR、Tomlinson, S. & Eddaoudi, A. & Downward, J. 在 TGF beta 诱导的细胞凋亡过程中两种仅 BH3 蛋白 Bmf 和 Bim 的上调。癌基因26 , 970–981 (2007)。Yoshimoto, T. et al. Involvement of smad2 and Erk/Akt cascade in TGF-β1-induced apoptosis in human gingival epithelial cells. Cytokine 75, 165–173 (2015).
吉本,T.等人。 smad2 和 Erk/Akt 级联参与 TGF-β1 诱导的人牙龈上皮细胞凋亡。细胞因子75 , 165–173 (2015)。Schiffer, M. et al. Apoptosis in podocytes induced by TGF-beta and Smad7. J. Clin. Invest 108, 807–816 (2001).
希弗,M.等人。 TGF-β 和 Smad7 诱导足细胞凋亡。 J.克林。投资108 , 807–816 (2001)。Francis, J. M. et al. Transforming growth factor-beta 1 induces apoptosis independently of p53 and selectively reduces expression of Bcl-2 in multipotent hematopoietic cells. J. Biol. Chem. 275, 39137–39145 (2000).
弗朗西斯,JM 等人。转化生长因子-β 1 独立于 p53 诱导细胞凋亡,并选择性降低多能造血细胞中 Bcl-2 的表达。 J.Biol。化学。 275 , 39137–39145 (2000)。Schulz, R., Vogel, T., Dressel, R. & Krieglstein, K. TGF-beta superfamily members, ActivinA and TGF-beta1, induce apoptosis in oligodendrocytes by different pathways. Cell Tissue Res 334, 327–338 (2008).
Schulz, R.、Vogel, T.、Dressel, R. 和 Krieglstein, K. TGF-β 超家族成员 ActivinA 和 TGF-β1 通过不同途径诱导少突胶质细胞凋亡。细胞组织研究334 , 327–338 (2008)。Larisch, S. et al. A novel mitochondrial septin-like protein, ARTS, mediates apoptosis dependent on its P-loop motif. Nat. Cell Biol. 2, 915–921 (2000).
拉里什,S.等人。一种新型线粒体脓毒蛋白样蛋白 ARTS 依赖于其 P 环基序介导细胞凋亡。纳特。细胞生物学。 2,915-921 (2000)。Perlman, R., Schiemann, W. P., Brooks, M. W., Lodish, H. F. & Weinberg, R. A. TGF-beta-induced apoptosis is mediated by the adapter protein Daxx that facilitates JNK activation. Nat. Cell Biol. 3, 708–714 (2001).
Perlman, R.、Schiemann, WP、Brooks, MW、Lodish, HF 和 Weinberg, RA TGF-β 诱导的细胞凋亡是由促进 JNK 激活的接头蛋白 Daxx 介导的。纳特。细胞生物学。 3、708-714 (2001)。Arsura, M., Wu, M. & Sonenshein, G. E. TGF beta 1 inhibits NF-kappa B/Rel activity inducing apoptosis of B cells: transcriptional activation of I kappa B alpha. Immunity 5, 31–40 (1996).
Arsura, M.、Wu, M. 和 Sonenshein, GE TGF beta 1 抑制 NF-kappa B/Rel 活性,诱导 B 细胞凋亡:I kappa B α 的转录激活。免疫5 , 31–40 (1996)。Arsura, M., FitzGerald, M. J., Fausto, N. & Sonenshein, G. E. Nuclear factor-kappaB/Rel blocks transforming growth factor beta1-induced apoptosis of murine hepatocyte cell lines. Cell Growth Differ. 8, 1049–1059 (1997).
Arsura, M.、FitzGerald, MJ、Fausto, N. 和 Sonenshein, GE 核因子-kappaB/Rel 可阻断转化生长因子 beta1 诱导的小鼠肝细胞系细胞凋亡。细胞生长不同。 8、1049-1059 (1997)。Arsura, M. et al. Transient activation of NF-kappaB through a TAK1/IKK kinase pathway by TGF-beta1 inhibits AP-1/SMAD signaling and apoptosis: implications in liver tumor formation. Oncogene 22, 412–425 (2003).
阿苏拉,M.等人。 TGF-β1 通过 TAK1/IKK 激酶途径瞬时激活 NF-kappaB 可抑制 AP-1/SMAD 信号传导和细胞凋亡:对肝肿瘤形成的影响。癌基因22 , 412–425 (2003)。Yoo, J. et al. Transforming growth factor-beta-induced apoptosis is mediated by Smad-dependent expression of GADD45b through p38 activation. J. Biol. Chem. 278, 43001–43007 (2003).
尤,J.等人。转化生长因子-β 诱导的细胞凋亡是由 GADD45b 的 Smad 依赖性表达通过 p38 激活介导的。 J.Biol。化学。 278 , 43001–43007 (2003)。Schiffer, M., Mundel, P., Shaw, A. S. & Böttinger, E. P. A novel role for the adaptor molecule CD2-associated protein in transforming growth factor-beta-induced apoptosis. J. Biol. Chem. 279, 37004–37012 (2004).
Schiffer, M.、Mundel, P.、Shaw, AS 和 Böttinger, EP 接头分子 CD2 相关蛋白在转化生长因子-β 诱导的细胞凋亡中的新作用。 J.Biol。化学。 279 , 37004–37012 (2004)。Conery, A. R. et al. Akt interacts directly with Smad3 to regulate the sensitivity to TGF-beta induced apoptosis. Nat. Cell Biol. 6, 366–372 (2004).
科纳里,AR 等人。 Akt 直接与 Smad3 相互作用,调节对 TGF-β 诱导的细胞凋亡的敏感性。纳特。细胞生物学。 6,366-372 (2004)。Remy, I., Montmarquette, A. & Michnick, S. W. PKB/Akt modulates TGF-beta signalling through a direct interaction with Smad3. Nat. Cell Biol. 6, 358–365 (2004).
Remy, I.、Montmarquette, A. 和 Michnick, SW PKB/Akt 通过与 Smad3 直接相互作用来调节 TGF-β 信号传导。纳特。细胞生物学。 6、358-365 (2004)。Valderrama-Carvajal, H. et al. Activin/TGF-beta induce apoptosis through Smad-dependent expression of the lipid phosphatase SHIP. Nat. Cell Biol. 4, 963–969 (2002).
Valderrama-Carvajal,H. 等人。激活素/TGF-β 通过脂质磷酸酶 SHIP 的 Smad 依赖性表达诱导细胞凋亡。纳特。细胞生物学。 4,963-969 (2002)。Bender, H., Wang, Z., Schuster, N. & Krieglstein, K. TIEG1 facilitates transforming growth factor-beta-mediated apoptosis in the oligodendroglial cell line OLI-neu. J. Neurosci. Res 75, 344–352 (2004).
Bender, H.、Wang, Z.、Schuster, N. 和 Krieglstein, K. TIEG1 促进少突胶质细胞系 OLI-neu 中转化生长因子-β 介导的细胞凋亡。 J.神经科学。第 75 号决议,344–352(2004 年)。Wang, Z., Spittau, B., Behrendt, M., Peters, B. & Krieglstein, K. Human TIEG2/KLF11 induces oligodendroglial cell death by downregulation of Bcl-XL expression. J. Neural Transm. (Vienna) 114, 867–875 (2007).
Wang, Z.、Spittau, B.、Behrendt, M.、Peters, B. 和 Krieglstein, K。人 TIEG2/KLF11 通过下调 Bcl-XL 表达诱导少突胶质细胞死亡。 J.神经传输。 (维也纳) 114 , 867–875 (2007)。Chalaux, E. et al. A zinc-finger transcription factor induced by TGF-beta promotes apoptotic cell death in epithelial Mv1Lu cells. FEBS Lett. 457, 478–482 (1999).
查劳克斯,E.等人。 TGF-β 诱导的锌指转录因子可促进上皮 Mv1Lu 细胞的凋亡细胞死亡。 FEBS 快报。 457、478-482 (1999)。Poulsen, K. T. et al. TGF beta 2 and TGF beta 3 are potent survival factors for midbrain dopaminergic neurons. Neuron 13, 1245–1252 (1994).
波尔森,KT 等人。 TGF beta 2 和 TGF beta 3 是中脑多巴胺能神经元的有效生存因子。神经元13,1245–1252 (1994)。Roussa, E., Farkas, L. M. & Krieglstein, K. TGF-beta promotes survival on mesencephalic dopaminergic neurons in cooperation with Shh and FGF-8. Neurobiol. Dis. 16, 300–310 (2004).
Roussa, E.、Farkas, LM 和 Krieglstein, K. TGF-β 与 Shh 和 FGF-8 合作促进中脑多巴胺能神经元的存活。神经生物学。迪斯。 16、300-310 (2004)。Krieglstein, K., Farkas, L. & Unsicker, K. TGF-beta regulates the survival of ciliary ganglionic neurons synergistically with ciliary neurotrophic factor and neurotrophins. J. Neurobiol. 37, 563–572 (1998).
Krieglstein, K.、Farkas, L. 和 Unsicker, K. TGF-β 与睫状神经营养因子和神经营养蛋白协同调节睫状神经节神经元的存活。 J.神经生物学。 37、563-572 (1998)。Bye, N., Zieba, M., Wreford, N. G. & Nichols, N. R. Resistance of the dentate gyrus to induced apoptosis during ageing is associated with increases in transforming growth factor-beta1 messenger RNA. Neuroscience 105, 853–862 (2001).
Bye, N.、Zieba, M.、Wreford, NG 和 Nichols, NR 衰老过程中齿状回对诱导细胞凋亡的抵抗与转化生长因子-β1 信使 RNA 的增加有关。神经科学105 , 853–862 (2001)。Shin, I., Bakin, A. V., Rodeck, U., Brunet, A. & Arteaga, C. L. Transforming growth factor beta enhances epithelial cell survival via Akt-dependent regulation of FKHRL1. Mol. Biol. Cell 12, 3328–3339 (2001).
Shin, I.、Bakin, AV、Rodeck, U.、Brunet, A. 和 Arteaga, CL 转化生长因子 β 通过 Akt 依赖性调节 FKHRL1 增强上皮细胞存活。摩尔。生物。细胞12,3328–3339 (2001)。Lanvin, O. et al. TGF-beta1 modulates Fas (APO-1/CD95)-mediated apoptosis of human pre-B cell lines. Eur. J. Immunol. 33, 1372–1381 (2003).
朗万,O.等人。 TGF-β1 调节 Fas (APO-1/CD95) 介导的人前 B 细胞系凋亡。欧元。 J.免疫学。 33、1372-1381 (2003)。Huang, Y. et al. Transforming growth factor-beta 1 suppresses serum deprivation-induced death of A549 cells through differential effects on c-Jun and JNK activities. J. Biol. Chem. 275, 18234–18242 (2000).
黄,Y.等人。转化生长因子-β 1 通过对 c-Jun 和 JNK 活性的不同作用来抑制血清剥夺诱导的 A549 细胞死亡。 J.Biol。化学。 275 , 18234–18242 (2000)。Saile, B., Matthes, N., El Armouche, H., Neubauer, K. & Ramadori, G. The bcl, NFkappaB and p53/p21WAF1 systems are involved in spontaneous apoptosis and in the anti-apoptotic effect of TGF-beta or TNF-alpha on activated hepatic stellate cells. Eur. J. Cell Biol. 80, 554–561 (2001).
Saile, B.、Matthes, N.、El Armouche, H.、Neubauer, K. 和 Ramadori, G. bcl、NFkappaB 和 p53/p21WAF1 系统参与自发凋亡和 TGF-β 的抗凋亡作用或激活的肝星状细胞上的 TNF-α。欧元。 J.细胞生物学。 80、554-561 (2001)。Letterio, J. J. & Roberts, A. B. Regulation of immune responses by TGF-beta. Annu Rev. Immunol. 16, 137–161 (1998).
Letterio, JJ 和 Roberts, AB 通过 TGF-β 调节免疫反应。免疫学年鉴。 16、137-161 (1998)。Thomas, D. A. & Massagué, J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell 8, 369–380 (2005).
Thomas, DA 和 Massagué, J. 在肿瘤逃避免疫监视过程中,TGF-β 直接靶向细胞毒性 T 细胞功能。癌细胞8,369–380 (2005)。Ahmadzadeh, M. & Rosenberg, S. A. TGF-beta 1 attenuates the acquisition and expression of effector function by tumor antigen-specific human memory CD8 T cells. J. Immunol. 174, 5215–5223 (2005).
Ahmadzadeh, M. 和 Rosenberg, SA TGF-β 1 减弱肿瘤抗原特异性人类记忆 CD8 T 细胞效应功能的获得和表达。 J.免疫学。 174、5215–5223 (2005)。Genestier, L., Kasibhatla, S., Brunner, T. & Green, D. R. Transforming growth factor beta1 inhibits Fas ligand expression and subsequent activation-induced cell death in T cells via downregulation of c-Myc. J. Exp. Med 189, 231–239 (1999).
Genestier, L.、Kasibhatla, S.、Brunner, T. 和 Green, DR 转化生长因子 beta1 通过下调 c-Myc 抑制 T 细胞中 Fas 配体表达和随后激活诱导的细胞死亡。 J.Exp。医学189 , 231–239 (1999)。Chen, C. H. et al. Transforming growth factor beta blocks Tec kinase phosphorylation, Ca2+ influx, and NFATc translocation causing inhibition of T cell differentiation. J. Exp. Med 197, 1689–1699 (2003).
陈,CH 等人。转化生长因子β可阻断 Tec 激酶磷酸化、Ca2+ 内流和 NFATc 易位,从而抑制 T 细胞分化。 J.Exp。医学197 , 1689–1699 (2003)。Gorelik, L., Constant, S. & Flavell, R. A. Mechanism of transforming growth factor beta-induced inhibition of T helper type 1 differentiation. J. Exp. Med 195, 1499–1505 (2002).
Gorelik, L.、Constant, S. 和 Flavell, RA 转化生长因子 β 诱导的 T 辅助细胞 1 型分化抑制机制。 J.Exp。医学195 , 1499–1505 (2002)。Gorelik, L., Fields, P. E. & Flavell, R. A. Cutting edge: TGF-beta inhibits Th type 2 development through inhibition of GATA-3 expression. J. Immunol. 165, 4773–4777 (2000).
Gorelik, L.、Fields, PE 和 Flavell, RA 最前沿:TGF-β 通过抑制 GATA-3 表达来抑制 Th 2 型发育。 J.免疫学。 165 , 4773–4777 (2000)。Heath, V. L., Murphy, E. E., Crain, C., Tomlinson, M. G. & O’Garra, A. TGF-beta1 down-regulates Th2 development and results in decreased IL-4-induced STAT6 activation and GATA-3 expression. Eur. J. Immunol. 30, 2639–2649 (2000).
Heath, VL, Murphy, EE, Crain, C., Tomlinson, MG & O'Garra, A. TGF-β1 下调 Th2 发育并导致 IL-4 诱导的 STAT6 激活和 GATA-3 表达减少。欧元。 J.免疫学。 30、2639-2649 (2000)。Chen, W. et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med 198, 1875–1886 (2003).
陈,W.等人。通过转录因子 Foxp3 的 TGF-β 诱导,将外周 CD4+CD25- 初始 T 细胞转化为 CD4+CD25+ 调节性 T 细胞。 J.Exp。医学198 , 1875–1886 (2003)。Fantini, M. C. et al. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25- T cells through Foxp3 induction and down-regulation of Smad7. J. Immunol. 172, 5149–5153 (2004).
范蒂尼,MC 等人。最前沿:TGF-β 通过 Foxp3 诱导和 Smad7 下调,诱导 CD4+CD25-T 细胞中的调节表型。 J.免疫学。 172、5149–5153 (2004)。Zheng, S. G., Wang, J., Wang, P., Gray, J. D. & Horwitz, D. A. IL-2 is essential for TGF-beta to convert naive CD4+CD25- cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J. Immunol. 178, 2018–2027 (2007).
Cheng, SG, Wang, J., Wang, P., Gray, JD & Horwitz, DA IL-2 对于 TGF-β 将初始 CD4+CD25- 细胞转化为 CD25+Foxp3+ 调节性 T 细胞以及这些细胞的扩增至关重要细胞。 J.免疫学。 178,2018-2027 (2007)。Davidson, T. S., DiPaolo, R. J., Andersson, J. & Shevach, E. M. Cutting Edge: IL-2 is essential for TGF-beta-mediated induction of Foxp3+ T regulatory cells. J. Immunol. 178, 4022–4026 (2007).
Davidson, TS、DiPaolo, RJ、Andersson, J. 和 Shevach, EM 前沿:IL-2 对于 TGF-β 介导的 Foxp3+ T 调节细胞的诱导至关重要。 J.免疫学。 178、4022–4026 (2007)。Sawamukai, N. et al. Cell-autonomous role of TGFβ and IL-2 receptors in CD4+ and CD8+ inducible regulatory T-cell generation during GVHD. Blood 119, 5575–5583 (2012).
泽向井,N.等人。 GVHD 期间 TGFβ 和 IL-2 受体在 CD4+ 和 CD8+ 诱导调节性 T 细胞生成中的细胞自主作用。血液119,5575–5583 (2012)。Rich, S., Seelig, M., Lee, H. M. & Lin, J. Transforming growth factor beta 1 costimulated growth and regulatory function of staphylococcal enterotoxin B-responsive CD8+ T cells. J. Immunol. 155, 609–618 (1995).
Rich, S.、Seelig, M.、Lee, HM 和 Lin, J. 转化生长因子 β1 共刺激葡萄球菌肠毒素 B 反应性 CD8+ T 细胞的生长和调节功能。 J.免疫学。 155、609-618 (1995)。Dardalhon, V. et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(-) effector T cells. Nat. Immunol. 9, 1347–1355 (2008).
Dardalhon,V.等人。 IL-4 抑制 TGF-β 诱导的 Foxp3+ T 细胞,并与 TGF-β 一起产生 IL-9+ IL-10+ Foxp3(-) 效应 T 细胞。纳特。免疫学。 9、1347-1355 (2008)。Chang, H. C. et al. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat. Immunol. 11, 527–534 (2010).
张,HC 等。转录因子 PU.1 是产生 IL-9 的 T 细胞和过敏性炎症的发育所必需的。纳特。免疫学。 11、527-534 (2010)。Goswami, R. et al. STAT6-dependent regulation of Th9 development. J. Immunol. 188, 968–975 (2012).
戈斯瓦米,R.等人。 Th9 发育的 STAT6 依赖性调节。 J.免疫学。 188 , 968–975 (2012)。Veldhoen, M. et al. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat. Immunol. 9, 1341–1346 (2008).
Veldhoen,M.等人。转化生长因子-β 可“重新编程”辅助 T 细胞 2 细胞的分化,并促进产生白细胞介素 9 的亚群。纳特。免疫学。 9、1341-1346 (2008)。Manel, N., Unutmaz, D. & Littman, D. R. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat. Immunol. 9, 641–649 (2008).
Manel, N.、Unutmaz, D. 和 Littman, DR 人类 T(H)-17 细胞的分化需要转化生长因子-β 和核受体 RORgammat 的诱导。纳特。免疫学。 9、641-649 (2008)。Volpe, E. et al. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat. Immunol. 9, 650–657 (2008).
沃尔普,E.等人。转化生长因子-β、白细胞介素 23 和促炎细胞因子在驱动和调节人类 T(H)-17 反应中的关键功能。纳特。免疫学。 9、650-657 (2008)。Kehrl, J. H., Thevenin, C., Rieckmann, P. & Fauci, A. S. Transforming growth factor-beta suppresses human B lymphocyte Ig production by inhibiting synthesis and the switch from the membrane form to the secreted form of Ig mRNA. J. Immunol. 146, 4016–4023 (1991).
Kehrl, JH、Thevenin, C.、Rieckmann, P. 和 Fauci, AS 转化生长因子-β 通过抑制 Ig mRNA 的合成和从膜形式向分泌形式的转变来抑制人 B 淋巴细胞 Ig 的产生。 J.免疫学。 146、4016-4023 (1991)。Rehmann, J. A. & LeBien, T. W. Transforming growth factor-beta regulates normal human pre-B cell differentiation. Int Immunol. 6, 315–322 (1994).
Rehmann, JA 和 LeBien, TW 转化生长因子-β 调节正常的人类前 B 细胞分化。国际免疫学。 6,315-322 (1994)。Bouchard, C., Fridman, W. H. & Sautès, C. Mechanism of inhibition of lipopolysaccharide-stimulated mouse B-cell responses by transforming growth factor-beta 1. Immunol. Lett. 40, 105–110 (1994).
Bouchard, C.、Fridman, WH 和 Sautès, C. 通过转化生长因子-β 1 抑制脂多糖刺激的小鼠 B 细胞反应的机制。免疫学。莱特。 40、105-110 (1994)。Lebman, D. A., Lee, F. D. & Coffman, R. L. Mechanism for transforming growth factor beta and IL-2 enhancement of IgA expression in lipopolysaccharide-stimulated B cell cultures. J. Immunol. 144, 952–959 (1990).
Lebman, DA、Lee, FD 和 Coffman, RL 转化生长因子 β 和 IL-2 增强脂多糖刺激的 B 细胞培养物中 IgA 表达的机制。 J.免疫学。 144、952-959 (1990)。Zhong, Z. et al. Pro- and Anti- Effects of Immunoglobulin A- Producing B Cell in Tumors and Its Triggers. Front Immunol. 12, 765044 (2021).
钟,Z.等人。肿瘤及其触发因素中产生免疫球蛋白 A 的 B 细胞的促和抗作用。前免疫学。 12、765044 (2021)。Ferreira-Gomes, M. et al. SARS-CoV-2 in severe COVID-19 induces a TGF-β-dominated chronic immune response that does not target itself. Nat. Commun. 12, 1961 (2021).
费雷拉-戈麦斯,M.等人。严重的 COVID-19 中的 SARS-CoV-2 会诱导以 TGF-β 为主的慢性免疫反应,该反应不针对自身。纳特。交流。 1961 年12 月(2021 年)。Balkwill, F., Montfort, A. & Capasso, M. B regulatory cells in cancer. Trends Immunol. 34, 169–173 (2013).
Balkwill, F.、Montfort, A. 和 Capasso, M. B 癌症中的调节细胞。趋势免疫学。 34、169-173 (2013)。Catalan, D. et al. Immunosuppressive Mechanisms of Regulatory B Cells. Front Immunol. 12, 611795 (2021).
加泰罗尼亚,D.等人。调节性 B 细胞的免疫抑制机制。前免疫学。 12、611795 (2021)。Shang, J., Zha, H. & Sun, Y. Phenotypes, Functions, and Clinical Relevance of Regulatory B Cells in Cancer. Front Immunol. 11, 582657 (2020).
Shang, J.、Zha, H. 和 Sun, Y. 癌症中调节性 B 细胞的表型、功能和临床相关性。前免疫学。 11、582657 (2020)。Wang, L., Fu, Y. & Chu, Y. Regulatory B Cells. Adv. Exp. Med. Biol. 1254, 87–103 (2020).
Wang, L.、Fu, Y. 和 Chu, Y. 调节性 B 细胞。副词。过期。医学。生物。 1254 , 87–103 (2020)。Tang, P. M. et al. Smad3 promotes cancer progression by inhibiting E4BP4-mediated NK cell development. Nat. Commun. 8, 14677 (2017).
唐,PM 等。 Smad3 通过抑制 E4BP4 介导的 NK 细胞发育来促进癌症进展。纳特。交流。 8、14677 (2017)。Trotta, R. et al. TGF-beta utilizes SMAD3 to inhibit CD16-mediated IFN-gamma production and antibody-dependent cellular cytotoxicity in human NK cells. J. Immunol. 181, 3784–3792 (2008).
特罗塔,R.等人。 TGF-β 利用 SMAD3 抑制人 NK 细胞中 CD16 介导的 IFN-γ 产生和抗体依赖性细胞毒性。 J.免疫学。 181 , 3784–3792 (2008)。Castriconi, R. et al. Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: consequences for the NK-mediated killing of dendritic cells. Proc. Natl Acad. Sci. USA 100, 4120–4125 (2003).
卡斯特里科尼,R.等人。转化生长因子 β 1 抑制 NKp30 和 NKG2D 受体的表达:NK 介导的树突状细胞杀伤的后果。过程。国家科学院。科学。美国100,4120–4125 (2003)。Fujii, R. et al. An IL-15 superagonist/IL-15Rα fusion complex protects and rescues NK cell-cytotoxic function from TGF-β1-mediated immunosuppression. Cancer Immunol. Immunother. 67, 675–689 (2018).
藤井,R.等人。 IL-15 超级激动剂/IL-15Rα 融合复合物可保护和挽救 NK 细胞的细胞毒性功能,使其免受 TGF-β1 介导的免疫抑制的影响。癌症免疫学。免疫疗法。 67、675–689 (2018)。Piskurich, J. F., Wang, Y., Linhoff, M. W., White, L. C. & Ting, J. P. Identification of distinct regions of 5’ flanking DNA that mediate constitutive, IFN-gamma, STAT1, and TGF-beta-regulated expression of the class II transactivator gene. J. Immunol. 160, 233–240 (1998).
Piskurich, JF, Wang, Y., Linhoff, MW, White, LC & Ting, JP 介导 II 类组成型、IFN-γ、STAT1 和 TGF-β 调节表达的 5' 侧翼 DNA 不同区域的鉴定反式激活基因。 J.免疫学。 160、233-240 (1998)。Nandan, D. & Reiner, N. E. TGF-beta attenuates the class II transactivator and reveals an accessory pathway of IFN-gamma action. J. Immunol. 158, 1095–1101 (1997).
Nandan, D. 和 Reiner, NE TGF-β 减弱 II 类反式激活因子并揭示 IFN-γ 作用的辅助途径。 J.免疫学。 158、1095-1101 (1997)。Shaul, M. E. et al. Tumor-associated neutrophils display a distinct N1 profile following TGFβ modulation: A transcriptomics analysis of pro- vs. antitumor TANs. Oncoimmunology 5, e1232221 (2016).
沙乌尔,ME 等人。肿瘤相关中性粒细胞在 TGFβ 调节后表现出独特的 N1 特征:亲肿瘤 TAN 与抗肿瘤 TAN 的转录组学分析。肿瘤免疫学5 ,e1232221 (2016)。Geissmann, F. et al. TGF-beta 1 prevents the noncognate maturation of human dendritic Langerhans cells. J. Immunol. 162, 4567–4575 (1999).
盖斯曼,F.等人。 TGF-β1 阻止人树突状朗格汉斯细胞的非同源成熟。 J.免疫学。 162、4567–4575 (1999)。Takeuchi, M., Alard, P. & Streilein, J. W. TGF-beta promotes immune deviation by altering accessory signals of antigen-presenting cells. J. Immunol. 160, 1589–1597 (1998).
Takeuchi, M.、Alard, P. 和 Streilein, JW TGF-β 通过改变抗原呈递细胞的辅助信号来促进免疫偏差。 J.免疫学。 160、1589-1597 (1998)。Espevik, T. et al. Inhibition of cytokine production by cyclosporin A and transforming growth factor beta. J. Exp. Med 166, 571–576 (1987).
埃斯佩维克,T.等人。环孢菌素 A 和转化生长因子 β 抑制细胞因子的产生。 J.Exp。医学166 , 571–576 (1987)。Bogdan, C., Paik, J., Vodovotz, Y. & Nathan, C. Contrasting mechanisms for suppression of macrophage cytokine release by transforming growth factor-beta and interleukin-10. J. Biol. Chem. 267, 23301–23308 (1992).
Bogdan, C.、Paik, J.、Vodovotz, Y. 和 Nathan, C. 通过转化生长因子-β 和白介素-10 抑制巨噬细胞细胞因子释放的对比机制。 J.Biol。化学。 267、23301–23308 (1992)。Nelson, B. J., Ralph, P., Green, S. J. & Nacy, C. A. Differential susceptibility of activated macrophage cytotoxic effector reactions to the suppressive effects of transforming growth factor-beta 1. J. Immunol. 146, 1849–1857 (1991).
Nelson, BJ、Ralph, P.、Green, SJ 和 Nacy, CA 激活的巨噬细胞细胞毒性效应反应对转化生长因子-β 1 抑制作用的不同敏感性。J . 免疫学。 146、1849-1857 (1991)。Vodovotz, Y., Bogdan, C., Paik, J., Xie, Q. W. & Nathan, C. Mechanisms of suppression of macrophage nitric oxide release by transforming growth factor beta. J. Exp. Med 178, 605–613 (1993).
Vodovotz,Y.,Bogdan,C.,Paik,J.,Xie,QW 和 Nathan,C。转化生长因子β抑制巨噬细胞一氧化氮释放的机制。 J.Exp。医学178 , 605–613 (1993)。Oswald, I. P., Gazzinelli, R. T., Sher, A. & James, S. L. IL-10 synergizes with IL-4 and transforming growth factor-beta to inhibit macrophage cytotoxic activity. J. Immunol. 148, 3578–3582 (1992).
Oswald, IP、Gazzinelli, RT、Sher, A. 和 James, SL IL-10 与 IL-4 和转化生长因子-β 协同作用,抑制巨噬细胞的细胞毒活性。 J.免疫学。 148、3578-3582 (1992)。Tridandapani, S. et al. TGF-beta 1 suppresses [correction of supresses] myeloid Fc gamma receptor function by regulating the expression and function of the common gamma-subunit. J. Immunol. 170, 4572–4577 (2003).
Tridandapani,S.等人。 TGF-β 1 通过调节常见 γ 亚基的表达和功能来抑制 [抑制] 骨髓 Fc γ 受体功能。 J.免疫学。 170 , 4572–4577 (2003)。Mantovani, A., Sozzani, S., Locati, M., Allavena, P. & Sica, A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 23, 549–555 (2002).
Mantovani, A.、Sozzani, S.、Locati, M.、Allavena, P. 和 Sica, A. 巨噬细胞极化:肿瘤相关巨噬细胞作为极化 M2 单核吞噬细胞的范例。趋势免疫学。 23、549-555 (2002)。Fridlender, Z. G. et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 16, 183–194 (2009).
弗里德伦德,ZG 等人。 TGF-β 引起的肿瘤相关中性粒细胞表型的极化:“N1”与“N2”TAN。癌细胞16 , 183–194 (2009)。Sagiv, J. Y. et al. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep. 10, 562–573 (2015).
萨吉夫,JY 等人。癌症中循环中性粒细胞亚群的表型多样性和可塑性。细胞报告10 , 562–573 (2015)。Larsson, J. et al. Abnormal angiogenesis but intact hematopoietic potential in TGF-beta type I receptor-deficient mice. Embo j. 20, 1663–1673 (2001).
拉尔森,J.等人。 TGF-β I 型受体缺陷小鼠的血管生成异常,但造血潜力完整。恩博 J. 20、1663-1673 (2001)。Oshima, M., Oshima, H. & Taketo, M. M. TGF-beta receptor type II deficiency results in defects of yolk sac hematopoiesis and vasculogenesis. Dev. Biol. 179, 297–302 (1996).
Oshima, M.、Oshima, H. 和 Taketo, MM II 型 TGF-β 受体缺陷会导致卵黄囊造血和血管生成缺陷。开发。生物。 179、297-302 (1996)。Shull, M. M. et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature 359, 693–699 (1992).
舒尔,MM 等人。小鼠转化生长因子-β1 基因的靶向破坏会导致多灶性炎症性疾病。自然359 , 693–699 (1992)。McLennan, I. S., Poussart, Y. & Koishi, K. Development of skeletal muscles in transforming growth factor-beta 1 (TGF-beta1) null-mutant mice. Dev. Dyn. 217, 250–256 (2000).
McLennan, IS、Poussart, Y. 和 Koishi, K. 转化生长因子-β 1 (TGF-β1) 无效突变小鼠骨骼肌的发育。开发。动态。 217、250-256 (2000)。Sanford, L. P. et al. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development 124, 2659–2670 (1997).
桑福德,LP 等。 TGFbeta2 敲除小鼠具有多种发育缺陷,这些缺陷与其他 TGFbeta 敲除表型不重叠。发展124 , 2659–2670 (1997)。Foitzik, K., Paus, R., Doetschman, T. & Dotto, G. P. The TGF-beta2 isoform is both a required and sufficient inducer of murine hair follicle morphogenesis. Dev. Biol. 212, 278–289 (1999).
Foitzik, K.、Paus, R.、Doetschman, T. 和 Dotto, GP TGF-β2 同种型是小鼠毛囊形态发生所需且充分的诱导剂。开发。生物。 212、278-289 (1999)。Bartram, U. et al. Double-outlet right ventricle and overriding tricuspid valve reflect disturbances of looping, myocardialization, endocardial cushion differentiation, and apoptosis in TGF-beta(2)-knockout mice. Circulation 103, 2745–2752 (2001).
巴特拉姆,U.等人。双出口右心室和覆盖三尖瓣反映了 TGF-β(2) 敲除小鼠中环路、心肌化、心内膜垫分化和细胞凋亡的紊乱。流通量103 , 2745–2752 (2001)。Jiao, K. et al. Tgfbeta signaling is required for atrioventricular cushion mesenchyme remodeling during in vivo cardiac development. Development 133, 4585–4593 (2006).
焦,K.等人。在体内心脏发育过程中,Tgfbeta 信号传导是房室垫间充质重塑所必需的。发展133 , 4585–4593 (2006)。Kaartinen, V. et al. Abnormal lung development and cleft palate in mice lacking TGF-beta 3 indicates defects of epithelial-mesenchymal interaction. Nat. Genet 11, 415–421 (1995).
Kaartinen,V.等人。缺乏 TGF-β 3 的小鼠肺发育异常和腭裂表明上皮间质相互作用存在缺陷。纳特。热内特11 , 415–421 (1995)。Proetzel, G. et al. Transforming growth factor-beta 3 is required for secondary palate fusion. Nat. Genet 11, 409–414 (1995).
普罗泽尔,G.等人。二次腭融合需要转化生长因子-β3。纳特。基因11 , 409–414 (1995)。Lindsay, M. E. et al. Loss-of-function mutations in TGFB2 cause a syndromic presentation of thoracic aortic aneurysm. Nat. Genet 44, 922–927 (2012).
林赛,ME 等人。 TGFB2 的功能丧失突变会导致胸主动脉瘤的综合征表现。纳特。基因44 , 922–927 (2012)。Boileau, C. et al. TGFB2 mutations cause familial thoracic aortic aneurysms and dissections associated with mild systemic features of Marfan syndrome. Nat. Genet 44, 916–921 (2012).
布瓦洛,C.等人。 TGFB2 突变导致家族性胸主动脉瘤和夹层,与马凡综合征的轻度全身特征相关。纳特。基因44 , 916–921 (2012)。Al Maskari, R. et al. A missense TGFB2 variant p.(Arg320Cys) causes a paradoxical and striking increase in aortic TGFB1/2 expression. Eur. J. Hum. Genet 25, 157–160 (2016).
Al Maskari,R. 等人。错义 TGFB2 变体 p.(Arg320Cys) 导致主动脉 TGFB1/2 表达出现矛盾且显着的增加。欧元。 J.哼。基因25 , 157–160 (2016)。Rienhoff, H. Y. Jr. et al. A mutation in TGFB3 associated with a syndrome of low muscle mass, growth retardation, distal arthrogryposis and clinical features overlapping with Marfan and Loeys-Dietz syndrome. Am. J. Med Genet A 161a, 2040–2046 (2013).
Rienhoff,HY Jr. 等人。 TGFB3 突变与低肌肉质量、生长迟缓、远端关节弯曲综合征以及与马凡综合征和 Loeys-Dietz 综合征重叠的临床特征相关。是。 J. Med Genet A 161a ,2040–2046 (2013)。Bertoli-Avella, A. M. et al. Mutations in a TGF-β ligand, TGFB3, cause syndromic aortic aneurysms and dissections. J. Am. Coll. Cardiol. 65, 1324–1336 (2015).
贝尔托利·阿维拉 (Bertoli-Avella),AM 等人。 TGF-β 配体 TGFB3 的突变会导致综合征性主动脉瘤和夹层。 J. Am.科尔。心脏。 65、1324-1336 (2015)。Kuechler, A. et al. Exome sequencing identifies a novel heterozygous TGFB3 mutation in a disorder overlapping with Marfan and Loeys-Dietz syndrome. Mol. Cell Probes 29, 330–334 (2015).
库奇勒,A.等人。外显子组测序在与马凡综合征和洛伊斯-迪茨综合征重叠的疾病中发现了一种新的杂合 TGFB3 突变。摩尔。细胞探针29 , 330–334 (2015)。Loeys, B. L. et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N. Engl. J. Med 355, 788–798 (2006).
Loeys,BL 等人。由 TGF-β 受体突变引起的动脉瘤综合征。 N. 英格兰。医学杂志355 , 788–798 (2006)。Hara, H. et al. Activation of TGF-β signaling in an aortic aneurysm in a patient with Loeys-Dietz syndrome caused by a novel loss-of-function variant of TGFBR1. Hum. Genome Var. 6, 6 (2019).
原,H.等人。由 TGFBR1 的新型功能丧失变体引起的 Loeys-Dietz 综合征患者主动脉瘤中 TGF-β 信号的激活。哼。基因组变异。 6 , 6 (2019)。Tran-Fadulu, V. et al. Analysis of multigenerational families with thoracic aortic aneurysms and dissections due to TGFBR1 or TGFBR2 mutations. J. Med Genet 46, 607–613 (2009).
Tran-Fadulu,V. 等人。因 TGFBR1 或 TGFBR2 突变而患有胸主动脉瘤和夹层的多代家庭分析。 J. Med Genet 46 , 607–613 (2009)。Kirmani, S. et al. Germline TGF-beta receptor mutations and skeletal fragility: a report on two patients with Loeys-Dietz syndrome. Am. J. Med Genet A 152a, 1016–1019 (2010).
Kirmani,S.等人。种系 TGF-β 受体突变和骨骼脆弱性:关于两名 Loeys-Dietz 综合征患者的报告。是。 J. Med Genet A 152a ,1016-1019(2010)。Cousin, M. A. et al. Functional validation reveals the novel missense V419L variant in TGFBR2 associated with Loeys-Dietz syndrome (LDS) impairs canonical TGF-β signaling. Cold Spring Harb. Mol. Case Stud. 3, a001727 (2017).
表弟,MA 等人。功能验证揭示了与 Loeys-Dietz 综合征 (LDS) 相关的 TGFBR2 中的新型错义 V419L 变体会损害典型的 TGF-β 信号传导。冷泉港。摩尔。案例螺柱。 3 、a001727(2017)。Luo, X. et al. Identification of a Pathogenic TGFBR2 Variant in a Patient With Loeys-Dietz Syndrome. Front Genet 11, 479 (2020).
罗,X.等人。 Loeys-Dietz 综合征患者致病性 TGFBR2 变异的鉴定。基因前沿11 , 479 (2020)。Cannaerts, E. et al. Novel pathogenic SMAD2 variants in five families with arterial aneurysm and dissection: further delineation of the phenotype. J. Med Genet 56, 220–227 (2019).
Cannaerts,E.等人。五个动脉瘤和夹层家族的新致病性 SMAD2 变异:进一步描述表型。医学遗传学杂志56 , 220–227 (2019)。Granadillo, J. L. et al. Variable cardiovascular phenotypes associated with SMAD2 pathogenic variants. Hum. Mutat. 39, 1875–1884 (2018).
格拉纳迪洛,JL 等人。与 SMAD2 致病性变异相关的可变心血管表型。哼。变异。 39,1875-1884 (2018)。Schepers, D. et al. A mutation update on the LDS-associated genes TGFB2/3 and SMAD2/3. Hum. Mutat. 39, 621–634 (2018).
谢珀斯,D.等人。 LDS 相关基因 TGFB2/3 和 SMAD2/3 的突变更新。哼。变异。 39、621-634 (2018)。van de Laar, I. M. et al. Mutations in SMAD3 cause a syndromic form of aortic aneurysms and dissections with early-onset osteoarthritis. Nat. Genet 43, 121–126 (2011).
范德拉尔,IM 等人。 SMAD3 突变会导致主动脉瘤和夹层夹层综合征,并伴有早发性骨关节炎。纳特。基因43 , 121–126 (2011)。van de Laar, I. M. et al. Phenotypic spectrum of the SMAD3-related aneurysms-osteoarthritis syndrome. J. Med Genet 49, 47–57 (2012).
范德拉尔,IM 等人。 SMAD3 相关动脉瘤-骨关节炎综合征的表型谱。 J. Med Genet 49 , 47–57 (2012)。Aubart, M. et al. Early-onset osteoarthritis, Charcot-Marie-Tooth like neuropathy, autoimmune features, multiple arterial aneurysms and dissections: an unrecognized and life threatening condition. PLoS One 9, e96387 (2014).
奥巴特,M.等人。早发性骨关节炎、腓骨肌萎缩症样神经病、自身免疫特征、多发性动脉瘤和夹层:一种未被识别且危及生命的疾病。 PLoS One 9 ,e96387 (2014)。Chung, B. H. et al. Hand and fibrillin-1 deposition abnormalities in Loeys-Dietz syndrome-expanding the clinical spectrum. Am. J. Med Genet A 164a, 461–466 (2014).
钟,BH 等人。 Loeys-Dietz 综合征中的手部和 fibrillin-1 沉积异常——扩大了临床谱。是。 J. Med Genet A 164a ,461-466(2014)。Barnett, C. P., Chitayat, D., Bradley, T. J., Wang, Y. & Hinek, A. Dexamethasone normalizes aberrant elastic fiber production and collagen 1 secretion by Loeys-Dietz syndrome fibroblasts: a possible treatment? Eur. J. Hum. Genet 19, 624–633 (2011).
Barnett, CP、Chitayat, D.、Bradley, TJ、Wang, Y. 和 Hinek, A. 地塞米松使 Loeys-Dietz 综合征成纤维细胞异常的弹性纤维生成和胶原蛋白 1 分泌正常化:一种可能的治疗方法?欧元。 J.哼。热内特19 , 624–633 (2011)。Loeys, B. L. et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat. Genet 37, 275–281 (2005).
Loeys,BL 等人。由 TGFBR1 或 TGFBR2 突变引起的心血管、颅面、神经认知和骨骼发育改变的综合征。纳特。基因37 , 275–281 (2005)。Maleszewski, J. J., Miller, D. V., Lu, J., Dietz, H. C. & Halushka, M. K. Histopathologic findings in ascending aortas from individuals with Loeys-Dietz syndrome (LDS). Am. J. Surg. Pathol. 33, 194–201 (2009).
Maleszewski, JJ、Miller, DV、Lu, J.、Dietz, HC 和 Halushka, MK Loeys-Dietz 综合征 (LDS) 患者升主动脉的组织病理学发现。是。 J.外科医生。病理学。 33、194-201 (2009)。Sellheyer, K. et al. Inhibition of skin development by overexpression of transforming growth factor beta 1 in the epidermis of transgenic mice. Proc. Natl Acad. Sci. USA 90, 5237–5241 (1993).
Sellheyer,K.等人。通过转基因小鼠表皮中转化生长因子β1的过度表达来抑制皮肤发育。过程。国家科学院。科学。美国90,5237–5241 (1993)。Ito, Y. et al. Overexpression of Smad2 reveals its concerted action with Smad4 in regulating TGF-beta-mediated epidermal homeostasis. Dev. Biol. 236, 181–194 (2001).
伊藤,Y.等人。 Smad2 的过度表达揭示了其与 Smad4 在调节 TGF-β 介导的表皮稳态中的协同作用。开发。生物。 236、181-194 (2001)。Erlebacher, A. & Derynck, R. Increased expression of TGF-beta 2 in osteoblasts results in an osteoporosis-like phenotype. J. Cell Biol. 132, 195–210 (1996).
Erlebacher, A. & Derynck, R. 成骨细胞中 TGF-β 2 表达增加导致骨质疏松症样表型。 J.细胞生物学。 132、195-210 (1996)。Flügel-Koch, C., Ohlmann, A., Piatigorsky, J. & Tamm, E. R. Disruption of anterior segment development by TGF-beta1 overexpression in the eyes of transgenic mice. Dev. Dyn. 225, 111–125 (2002).
Flügel-Koch, C.、Ohlmann, A.、Piatigorsky, J. 和 Tamm, ER 转基因小鼠眼中 TGF-β1 过度表达导致眼前段发育中断。开发。动态。 225、111-125 (2002)。Vicencio, A. G. et al. Conditional overexpression of bioactive transforming growth factor-beta1 in neonatal mouse lung: a new model for bronchopulmonary dysplasia? Am. J. Respir. Cell Mol. Biol. 31, 650–656 (2004).
维森西奥,AG 等人。新生小鼠肺中生物活性转化生长因子-β1的条件性过度表达:支气管肺发育不良的新模型?是。 J.呼吸。细胞分子。生物。 31、650-656 (2004)。Zeng, X., Gray, M., Stahlman, M. T. & Whitsett, J. A. TGF-beta1 perturbs vascular development and inhibits epithelial differentiation in fetal lung in vivo. Dev. Dyn. 221, 289–301 (2001).
Zeng, X.、Gray, M.、Stahlman, MT 和 Whitsett, JA TGF-β1 会扰乱胎肺体内血管发育并抑制上皮分化。开发。动态。 221、289-301 (2001)。Jhappan, C. et al. Targeting expression of a transforming growth factor beta 1 transgene to the pregnant mammary gland inhibits alveolar development and lactation. Embo j. 12, 1835–1845 (1993).
Jhappan,C.等人。将转化生长因子 β1 转基因靶向表达到怀孕乳腺可抑制肺泡发育和泌乳。恩博 J. 12、1835-1845 (1993)。Pierce, D. F. Jr. et al. Inhibition of mammary duct development but not alveolar outgrowth during pregnancy in transgenic mice expressing active TGF-beta 1. Genes Dev. 7, 2308–2317 (1993).
皮尔斯,DF Jr. 等人。表达活性 TGF-β 1 的转基因小鼠在怀孕期间抑制乳腺导管发育,但不抑制肺泡生长。Genes Dev。 7、2308-2317 (1993)。Buggiano, V. et al. Impairment of mammary lobular development induced by expression of TGFbeta1 under the control of WAP promoter does not suppress tumorigenesis in MMTV-infected transgenic mice. Int J. Cancer 92, 568–576 (2001).
布贾诺,V.等人。在 WAP 启动子控制下表达 TGFbeta1 诱导的乳腺小叶发育受损并不能抑制 MMTV 感染的转基因小鼠的肿瘤发生。国际癌症杂志92 , 568–576 (2001)。Hall, B. E. et al. Conditional overexpression of TGF-beta1 disrupts mouse salivary gland development and function. Lab Invest 90, 543–555 (2010).
霍尔,BE 等人。 TGF-β1 的条件性过度表达会破坏小鼠唾液腺的发育和功能。实验室投资90 , 543–555 (2010)。Wyss-Coray, T. et al. Increased central nervous system production of extracellular matrix components and development of hydrocephalus in transgenic mice overexpressing transforming growth factor-beta 1. Am. J. Pathol. 147, 53–67 (1995).
Wyss-Coray,T. 等人。过度表达转化生长因子-β 1 的转基因小鼠中枢神经系统细胞外基质成分的产生增加和脑积水的发生。Am。 J.帕索尔. 147、53-67 (1995)。Saito, T. et al. Domain-specific mutations of a transforming growth factor (TGF)-beta 1 latency-associated peptide cause Camurati-Engelmann disease because of the formation of a constitutively active form of TGF-beta 1. J. Biol. Chem. 276, 11469–11472 (2001).
斋藤,T.等人。转化生长因子 (TGF)-β 1 潜伏相关肽的域特异性突变会导致 Camurati-Engelmann 病,因为形成了 TGF-β 1 的组成型活性形式。化学。 276、11469–11472 (2001)。Janssens, K., ten Dijke, P., Ralston, S. H., Bergmann, C. & Van Hul, W. Transforming growth factor-beta 1 mutations in Camurati-Engelmann disease lead to increased signaling by altering either activation or secretion of the mutant protein. J. Biol. Chem. 278, 7718–7724 (2003).
Janssens, K.、10 Dijke, P.、Ralston, SH、Bergmann, C. 和 Van Hul, W. Camurati-Engelmann 病中的转化生长因子-β 1 突变通过改变突变体的激活或分泌而导致信号传导增强蛋白质。 J.Biol。化学。 278、7718–7724 (2003)。Wallace, S. E. & Wilcox, W. R. In GeneReviews(®) (eds. M. P. Adam et al.) (University of Washington, Seattle Copyright © 1993-2023, University of Washington, Seattle. GeneReviews is a registered trademark of the University of Washington, Seattle. All rights reserved., 1993).
Wallace, SE 和 Wilcox, WR In GeneReviews(®)(编辑 MP Adam 等人)(西雅图华盛顿大学版权所有 © 1993-2023,西雅图华盛顿大学。GeneReviews 是华盛顿大学的注册商标,西雅图,保留所有权利。,1993)。Janssens, K. et al. Mutations in the gene encoding the latency-associated peptide of TGF-beta 1 cause Camurati-Engelmann disease. Nat. Genet 26, 273–275 (2000).
詹森斯,K.等人。编码 TGF-β1 潜伏相关肽的基因突变会导致卡穆拉蒂-恩格尔曼病。纳特。基因26 , 273–275 (2000)。McGowan, N. W. et al. A mutation affecting the latency-associated peptide of TGFbeta1 in Camurati-Engelmann disease enhances osteoclast formation in vitro. J. Clin. Endocrinol. Metab. 88, 3321–3326 (2003).
麦高恩,NW 等人。影响 Camurati-Engelmann 病中 TGFbeta1 潜伏相关肽的突变可增强体外破骨细胞的形成。 J.克林。内分泌。元数据。 88、3321-3326 (2003)。Tang, Y. et al. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat. Med 15, 757–765 (2009).
唐,Y.等人。 TGF-β1 诱导的骨间充质干细胞迁移将骨吸收与形成耦合起来。纳特。医学15 , 757–765 (2009)。Schmid, P. et al. TGF-beta s and TGF-beta type II receptor in human epidermis: differential expression in acute and chronic skin wounds. J. Pathol. 171, 191–197 (1993).
施密德,P.等人。人表皮中的 TGF-β 和 TGF-β II 型受体:急性和慢性皮肤伤口中的差异表达。 J.帕索尔. 171、191-197 (1993)。Pastar, I. et al. Attenuation of the transforming growth factor beta-signaling pathway in chronic venous ulcers. Mol. Med 16, 92–101 (2010).
帕斯塔尔,I.等人。慢性静脉溃疡中转化生长因子β信号通路的减弱。摩尔。医学16 , 92–101 (2010)。Kim, B. C. et al. Fibroblasts from chronic wounds show altered TGF-beta-signaling and decreased TGF-beta Type II receptor expression. J. Cell Physiol. 195, 331–336 (2003).
金,BC 等人。慢性伤口的成纤维细胞表现出 TGF-β 信号传导的改变和 TGF-β II 型受体表达的降低。 J.细胞生理学。 195、331-336 (2003)。Cowin, A. J. et al. Effect of healing on the expression of transforming growth factor beta(s) and their receptors in chronic venous leg ulcers. J. Invest Dermatol 117, 1282–1289 (2001).
Cowin,AJ 等人。愈合对慢性静脉性腿部溃疡中转化生长因子β及其受体表达的影响。 J. Invest Dermatol 117 , 1282–1289 (2001)。Bitar, M. S. & Labbad, Z. N. Transforming growth factor-beta and insulin-like growth factor-I in relation to diabetes-induced impairment of wound healing. J. Surg. Res 61, 113–119 (1996).
Bitar,MS 和 Labbad,ZN 转化生长因子-β 和胰岛素样生长因子-I 与糖尿病引起的伤口愈合受损有关。 J.外科医生。第 61 号决议, 113–119 (1996)。Jude, E. B., Blakytny, R., Bulmer, J., Boulton, A. J. & Ferguson, M. W. Transforming growth factor-beta 1, 2, 3 and receptor type I and II in diabetic foot ulcers. Diabet. Med 19, 440–447 (2002).
Jude, EB、Blakytny, R.、Bulmer, J.、Boulton, AJ 和 Ferguson, MW 糖尿病足溃疡中的转化生长因子-β 1、2、3 以及 I 型和 II 型受体。糖尿病。医学19 , 440–447 (2002)。Liu, J. et al. Regenerative phenotype in mice with a point mutation in transforming growth factor beta type I receptor (TGFBR1). Proc. Natl Acad. Sci. USA 108, 14560–14565 (2011).
刘,J.等人。转化生长因子 β I 型受体 (TGFBR1) 点突变小鼠的再生表型。过程。国家科学院。科学。美国108,14560–14565 (2011)。Tredget, E. B. et al. Transforming growth factor-beta and its effect on reepithelialization of partial-thickness ear wounds in transgenic mice. Wound Repair Regen. 13, 61–67 (2005).
特雷吉特,EB 等人。转化生长因子-β及其对转基因小鼠部分厚度耳伤口上皮再生的影响。伤口修复再生。 13、61-67 (2005)。Chan, T. et al. Development, characterization, and wound healing of the keratin 14 promoted transforming growth factor-beta1 transgenic mouse. Wound Repair Regen. 10, 177–187 (2002).
陈,T.等人。角蛋白 14 的发育、表征和伤口愈合促进转化生长因子-β1 转基因小鼠。伤口修复再生。 10、177-187 (2002)。Brown, R. L., Ormsby, I., Doetschman, T. C. & Greenhalgh, D. G. Wound healing in the transforming growth factor-beta-deficient mouse. Wound Repair Regen. 3, 25–36 (1995).
Brown, RL、Ormsby, I.、Doetschman, TC 和 Greenhalgh, DG 转化生长因子-β 缺陷小鼠的伤口愈合。伤口修复再生。 3、25-36 (1995)。Crowe, M. J., Doetschman, T. & Greenhalgh, D. G. Delayed wound healing in immunodeficient TGF-beta 1 knockout mice. J. Invest Dermatol 115, 3–11 (2000).
Crowe, MJ、Doetschman, T. 和 Greenhalgh, DG 免疫缺陷 TGF-β 1 敲除小鼠的伤口愈合延迟。 J. Invest Dermatol 115 , 3-11 (2000)。Amendt, C., Mann, A., Schirmacher, P. & Blessing, M. Resistance of keratinocytes to TGFbeta-mediated growth restriction and apoptosis induction accelerates re-epithelialization in skin wounds. J. Cell Sci. 115, 2189–2198 (2002).
Amendt, C.、Mann, A.、Schirmacher, P. 和 Blessing, M. 角质形成细胞对 TGFbeta 介导的生长限制和细胞凋亡诱导的抵抗加速了皮肤伤口的上皮化。 J.细胞科学。 115、2189-2198 (2002)。Owens, P., Engelking, E., Han, G., Haeger, S. M. & Wang, X. J. Epidermal Smad4 deletion results in aberrant wound healing. Am. J. Pathol. 176, 122–133 (2010).
Owens, P.、Engelking, E.、Han, G.、Haeger, SM 和 Wang, XJ 表皮 Smad4 缺失会导致伤口愈合异常。是。 J.帕索尔. 176、122-133 (2010)。Ashcroft, G. S. et al. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat. Cell Biol. 1, 260–266 (1999).
阿什克罗夫特,GS 等人。缺乏 Smad3 的小鼠表现出伤口愈合加速和局部炎症反应受损。纳特。细胞生物学。 1,260-266 (1999)。Ghahary, A., Shen, Y. J., Scott, P. G. & Tredget, E. E. Immunolocalization of TGF-beta 1 in human hypertrophic scar and normal dermal tissues. Cytokine 7, 184–190 (1995).
Ghahary, A.、Shen, YJ、Scott, PG 和 Tredget, EE TGF-β1 在人类肥厚性疤痕和正常真皮组织中的免疫定位。细胞因子7,184–190 (1995)。Schmid, P., Itin, P., Cherry, G., Bi, C. & Cox, D. A. Enhanced expression of transforming growth factor-beta type I and type II receptors in wound granulation tissue and hypertrophic scar. Am. J. Pathol. 152, 485–493 (1998).
Schmid, P.、Itin, P.、Cherry, G.、Bi, C. 和 Cox, DA 伤口肉芽组织和肥厚性疤痕中 I 型和 II 型转化生长因子-β 受体的表达增强。是。 J.帕索尔. 152、485-493 (1998)。Wang, R. et al. Hypertrophic scar tissues and fibroblasts produce more transforming growth factor-beta1 mRNA and protein than normal skin and cells. Wound Repair Regen. 8, 128–137 (2000).
王,R.等人。肥厚性疤痕组织和成纤维细胞比正常皮肤和细胞产生更多的转化生长因子-β1 mRNA 和蛋白质。伤口修复再生。 8、128-137 (2000)。Chin, G. S. et al. Differential expression of transforming growth factor-beta receptors I and II and activation of Smad 3 in keloid fibroblasts. Plast. Reconstr. Surg. 108, 423–429 (2001).
Chin,GS 等。瘢痕疙瘩成纤维细胞中转化生长因子-β受体 I 和 II 的差异表达以及 Smad 3 的激活。塑料。重建。外科医生。 108、423-429 (2001)。Xia, W., Phan, T. T., Lim, I. J., Longaker, M. T. & Yang, G. P. Complex epithelial-mesenchymal interactions modulate transforming growth factor-beta expression in keloid-derived cells. Wound Repair Regen. 12, 546–556 (2004).
Xia, W., Phan, TT, Lim, IJ, Longaker, MT & Yang, GP 复杂的上皮-间质相互作用调节瘢痕疙瘩衍生细胞中转化生长因子-β的表达。伤口修复再生。 12、546-556 (2004)。Younai, S. et al. Modulation of collagen synthesis by transforming growth factor-beta in keloid and hypertrophic scar fibroblasts. Ann. Plast. Surg. 33, 148–151 (1994).
尤奈,S.等人。通过转化生长因子-β来调节疤痕疙瘩和肥厚性疤痕成纤维细胞中的胶原蛋白合成。安.塑料。外科医生。 33、148-151 (1994)。Chodon, T., Sugihara, T., Igawa, H. H., Funayama, E. & Furukawa, H. Keloid-derived fibroblasts are refractory to Fas-mediated apoptosis and neutralization of autocrine transforming growth factor-beta1 can abrogate this resistance. Am. J. Pathol. 157, 1661–1669 (2000).
Chodon, T., Sugihara, T., Ikawa, HH, Funayama, E. & Furukawa, H. 瘢痕疙瘩衍生的成纤维细胞对 Fas 介导的细胞凋亡具有抵抗力,中和自分泌转化生长因子-β1 可以消除这种抵抗力。是。 J.帕索尔. 157、1661-1669 (2000)。Colwell, A. S., Phan, T. T., Kong, W., Longaker, M. T. & Lorenz, P. H. Hypertrophic scar fibroblasts have increased connective tissue growth factor expression after transforming growth factor-beta stimulation. Plast. Reconstr. Surg. 116, 1387–1390 (2005). discussion 1391-1382.
Colwell, AS、Phan, TT、Kong, W.、Longaker, MT 和 Lorenz, PH 肥厚性疤痕成纤维细胞在转化生长因子-β 刺激后结缔组织生长因子表达增加。塑料。重建。外科医生。 116、1387-1390 (2005)。讨论1391-1382。Bettinger, D. A., Yager, D. R., Diegelmann, R. F. & Cohen, I. K. The effect of TGF-beta on keloid fibroblast proliferation and collagen synthesis. Plast. Reconstr. Surg. 98, 827–833 (1996).
Bettinger, DA、Yager, DR、Diegelmann, RF 和 Cohen, IK TGF-β 对疤痕疙瘩成纤维细胞增殖和胶原蛋白合成的影响。塑料。重建。外科医生。 98、827-833 (1996)。Fujiwara, M., Muragaki, Y. & Ooshima, A. Upregulation of transforming growth factor-beta1 and vascular endothelial growth factor in cultured keloid fibroblasts: relevance to angiogenic activity. Arch. Dermatol Res 297, 161–169 (2005).
Fujiwara, M., Muragaki, Y. & Ooshima, A. 培养的瘢痕疙瘩成纤维细胞中转化生长因子-β1 和血管内皮生长因子的上调:与血管生成活性的相关性。拱。皮肤科研究297 , 161–169 (2005)。Hoyt, D. G. & Lazo, J. S. Alterations in pulmonary mRNA encoding procollagens, fibronectin and transforming growth factor-beta precede bleomycin-induced pulmonary fibrosis in mice. J. Pharm. Exp. Ther. 246, 765–771 (1988).
Hoyt, DG & Lazo, JS 编码前胶原、纤连蛋白和转化生长因子-β 的肺部 mRNA 的改变先于博莱霉素诱导的小鼠肺纤维化。 J.Pharm。过期。瑟尔。 246、765–771 (1988)。Westergren-Thorsson, G. et al. Altered expression of small proteoglycans, collagen, and transforming growth factor-beta 1 in developing bleomycin-induced pulmonary fibrosis in rats. J. Clin. Invest 92, 632–637 (1993).
Westergren-Thorsson,G. 等人。在博来霉素诱导的大鼠肺纤维化过程中,小蛋白多糖、胶原蛋白和转化生长因子-β1 的表达发生改变。 J.克林。投资92 , 632–637 (1993)。Broekelmann, T. J., Limper, A. H., Colby, T. V. & McDonald, J. A. Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc. Natl Acad. Sci. USA 88, 6642–6646 (1991).
Broekelmann, TJ, Limper, AH, Colby, TV 和 McDonald, JA 转化生长因子 β1 存在于人肺纤维化的细胞外基质基因表达位点。过程。国家科学院。科学。美国88,6642–6646 (1991)。Coker, R. K. et al. Localisation of transforming growth factor beta1 and beta3 mRNA transcripts in normal and fibrotic human lung. Thorax 56, 549–556 (2001).
科克,RK 等人。正常和纤维化人肺中转化生长因子 β1 和 β3 mRNA 转录本的定位。胸部56 , 549–556 (2001)。Corrin, B. et al. Immunohistochemical localization of transforming growth factor-beta 1 in the lungs of patients with systemic sclerosis, cryptogenic fibrosing alveolitis and other lung disorders. Histopathology 24, 145–150 (1994).
科林,B.等人。转化生长因子-β1 在系统性硬化症、隐源性纤维化肺泡炎和其他肺部疾病患者肺部的免疫组织化学定位。组织病理学24 , 145–150 (1994)。Utsugi, M. et al. C-Jun-NH2-terminal kinase mediates expression of connective tissue growth factor induced by transforming growth factor-beta1 in human lung fibroblasts. Am. J. Respir. Cell Mol. Biol. 28, 754–761 (2003).
Utsugi,M.等人。 C-Jun-NH2-末端激酶介导人肺成纤维细胞中转化生长因子-β1 诱导的结缔组织生长因子的表达。是。 J.呼吸。细胞分子。生物。 28、754-761 (2003)。Togami, K., Yamaguchi, K., Chono, S. & Tada, H. Evaluation of permeability alteration and epithelial-mesenchymal transition induced by transforming growth factor-β(1) in A549, NCI-H441, and Calu-3 cells: Development of an in vitro model of respiratory epithelial cells in idiopathic pulmonary fibrosis. J. Pharm. Toxicol. Methods 86, 19–27 (2017).
Togami, K.、Yamaguchi, K.、Chono, S. 和 Tada, H. A549、NCI-H441 和 Calu-3 细胞中转化生长因子-β(1) 诱导的通透性改变和上皮间质转化的评估:特发性肺纤维化呼吸道上皮细胞体外模型的开发。 J.Pharm。毒性。方法86、19-27 (2017)。Roy, S. G., Nozaki, Y. & Phan, S. H. Regulation of alpha-smooth muscle actin gene expression in myofibroblast differentiation from rat lung fibroblasts. Int J. Biochem Cell Biol. 33, 723–734 (2001).
Roy, SG, Nozaki, Y. & Phan, SH 大鼠肺成纤维细胞分化为肌成纤维细胞时α-平滑肌肌动蛋白基因表达的调节。 Int J. Biochem 细胞生物学。 33、723-734 (2001)。Kim, K. K. et al. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc. Natl Acad. Sci. USA 103, 13180–13185 (2006).
金,KK 等人。肺泡上皮细胞间质转化在肺纤维化过程中在体内发生,并受到细胞外基质的调节。过程。国家科学院。科学。美国103,13180–13185 (2006)。Lee, C. G. et al. Early growth response gene 1-mediated apoptosis is essential for transforming growth factor beta1-induced pulmonary fibrosis. J. Exp. Med 200, 377–389 (2004).
李,CG 等人。早期生长反应基因 1 介导的细胞凋亡对于转化生长因子 β1 诱导的肺纤维化至关重要。 J.Exp。医学200 , 377–389 (2004)。Sime, P. J., Xing, Z., Graham, F. L., Csaky, K. G. & Gauldie, J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J. Clin. Invest 100, 768–776 (1997).
Sime, PJ, Xing, Z., Graham, FL, Csaky, KG & Gauldie, J. 活性转化生长因子-β1 的腺载体介导的基因转移诱导大鼠肺长期严重纤维化。 J.克林。投资100 , 768–776 (1997)。D’Alessandro-Gabazza, C. N. et al. Development and preclinical efficacy of novel transforming growth factor-β1 short interfering RNAs for pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 46, 397–406 (2012).
D'Alessandro-Gabazza,CN 等人。新型转化生长因子-β1短干扰RNA治疗肺纤维化的开发和临床前疗效。是。 J.呼吸。细胞分子。生物。 46、397-406 (2012)。Li, M. et al. Epithelium-specific deletion of TGF-β receptor type II protects mice from bleomycin-induced pulmonary fibrosis. J. Clin. Invest 121, 277–287 (2011).
李,M.等人。上皮特异性删除 II 型 TGF-β 受体可保护小鼠免受博莱霉素诱导的肺纤维化。 J.克林。投资121 , 277–287 (2011)。Xu, L. et al. Transforming growth factor β3 attenuates the development of radiation-induced pulmonary fibrosis in mice by decreasing fibrocyte recruitment and regulating IFN-γ/IL-4 balance. Immunol. Lett. 162, 27–33 (2014).
徐,L.等人。转化生长因子 β3 通过减少纤维细胞募集和调节 IFN-γ/IL-4 平衡来减轻小鼠辐射诱导的肺纤维化的发展。免疫学。莱特。 162、27-33 (2014)。Zhao, J. et al. Smad3 deficiency attenuates bleomycin-induced pulmonary fibrosis in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 282, L585–L593 (2002).
赵,J.等人。 Smad3 缺陷可减轻博来霉素诱导的小鼠肺纤维化。是。 J.生理学。肺细胞分子。生理学。 282 ,L585–L593(2002)。Nakao, A. et al. Transient gene transfer and expression of Smad7 prevents bleomycin-induced lung fibrosis in mice. J. Clin. Invest 104, 5–11 (1999).
中尾,A.等人。 Smad7 的瞬时基因转移和表达可预防博莱霉素诱导的小鼠肺纤维化。 J.克林。投资104,5-11 (1999)。Yamamoto, T. et al. Expression of transforming growth factor-beta isoforms in human glomerular diseases. Kidney Int 49, 461–469 (1996).
山本,T.等人。转化生长因子-β亚型在人类肾小球疾病中的表达。肾脏国际49 , 461–469 (1996)。Shihab, F. S. et al. Transforming growth factor-beta and matrix protein expression in acute and chronic rejection of human renal allografts. J. Am. Soc. Nephrol. 6, 286–294 (1995).
Shihab,FS 等人。人肾同种异体移植物急性和慢性排斥反应中转化生长因子-β和基质蛋白的表达。 J. Am.苏克。肾病。 6,286-294 (1995)。Yoshioka, K. et al. Transforming growth factor-beta protein and mRNA in glomeruli in normal and diseased human kidneys. Lab Invest 68, 154–163 (1993).
吉冈,K.等人。正常和患病人类肾脏肾小球中的转化生长因子-β 蛋白和 mRNA。实验室投资68 , 154–163 (1993)。Iwano, M. et al. Intraglomerular expression of transforming growth factor-beta 1 (TGF-beta 1) mRNA in patients with glomerulonephritis: quantitative analysis by competitive polymerase chain reaction. Clin. Exp. Immunol. 97, 309–314 (1994).
岩野,M.等人。肾小球肾炎患者转化生长因子-β 1 (TGF-β 1) mRNA 的肾小球内表达:通过竞争性聚合酶链反应进行定量分析。临床。过期。免疫学。 97、309-314 (1994)。Grande, J., Melder, D., Zinsmeister, A. & Killen, P. Transforming growth factor-beta 1 induces collagen IV gene expression in NIH-3T3 cells. Lab Invest 69, 387–395 (1993).
Grande, J.、Melder, D.、Zinsmeister, A. 和 Killen, P. 转化生长因子-β 1 诱导 NIH-3T3 细胞中胶原蛋白 IV 基因表达。实验室投资69 , 387–395 (1993)。Nakamura, T., Miller, D., Ruoslahti, E. & Border, W. A. Production of extracellular matrix by glomerular epithelial cells is regulated by transforming growth factor-beta 1. Kidney Int 41, 1213–1221 (1992).
Nakamura, T.、Miller, D.、Ruoslahti, E. 和 Border, WA 肾小球上皮细胞产生的细胞外基质受转化生长因子-β 1 调节。Kidney Int 41 , 1213-1221 (1992)。Kagami, S. et al. Transforming growth factor-beta (TGF-beta) stimulates the expression of beta1 integrins and adhesion by rat mesangial cells. Exp. Cell Res 229, 1–6 (1996).
加贺美,S.等人。转化生长因子-β (TGF-β) 刺激 β1 整合素的表达和大鼠系膜细胞的粘附。过期。细胞研究229,1-6 (1996)。Okuda, S., Languino, L. R., Ruoslahti, E. & Border, W. A. Elevated expression of transforming growth factor-beta and proteoglycan production in experimental glomerulonephritis. Possible role in expansion of the mesangial extracellular matrix. J. Clin. Invest 86, 453–462 (1990).
Okuda, S.、Languino, LR、Ruoslahti, E. 和 Border, WA 实验性肾小球肾炎中转化生长因子-β 的表达升高和蛋白多糖的产生。可能在系膜细胞外基质扩张中发挥作用。 J.克林。投资86 , 453–462 (1990)。Marti, H. P., Lee, L., Kashgarian, M. & Lovett, D. H. Transforming growth factor-beta 1 stimulates glomerular mesangial cell synthesis of the 72-kd type IV collagenase. Am. J. Pathol. 144, 82–94 (1994).
Marti, HP、Lee, L.、Kashgarian, M. 和 Lovett, DH 转化生长因子-β 1 刺激肾小球系膜细胞合成 72-kd IV 型胶原酶。是。 J.帕索尔. 144 , 82–94 (1994)。Zeisberg, M., Maeshima, Y., Mosterman, B. & Kalluri, R. Renal fibrosis. Extracellular matrix microenvironment regulates migratory behavior of activated tubular epithelial cells. Am. J. Pathol. 160, 2001–2008 (2002).
Zeisberg, M.、Maeshima, Y.、Mosterman, B. 和 Kalluri, R. 肾纤维化。细胞外基质微环境调节活化的肾小管上皮细胞的迁移行为。是。 J.帕索尔. 160,2001-2008 (2002)。Yang, J. & Liu, Y. Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am. J. Pathol. 159, 1465–1475 (2001).
Yang, J. & Liu, Y. 肾小管上皮向肌成纤维细胞转变关键事件的剖析及其对肾间质纤维化的影响。是。 J.帕索尔. 159、1465–1475 (2001)。Kopp, J. B. et al. Transgenic mice with increased plasma levels of TGF-beta 1 develop progressive renal disease. Lab Invest 74, 991–1003 (1996).
科普,JB 等人。血浆 TGF-β1 水平升高的转基因小鼠会出现进行性肾病。实验室投资74 , 991–1003 (1996)。Mozes, M. M., Böttinger, E. P., Jacot, T. A. & Kopp, J. B. Renal expression of fibrotic matrix proteins and of transforming growth factor-beta (TGF-beta) isoforms in TGF-beta transgenic mice. J. Am. Soc. Nephrol. 10, 271–280 (1999).
Mozes, MM, Böttinger, EP, Jacot, TA & Kopp, JB TGF-β 转基因小鼠中纤维化基质蛋白和转化生长因子-β (TGF-β) 亚型的肾脏表达。 J. Am.苏克。肾病。 10、271-280 (1999)。Nagy, P., Schaff, Z. & Lapis, K. Immunohistochemical detection of transforming growth factor-beta 1 in fibrotic liver diseases. Hepatology 14, 269–273 (1991).
Nagy, P.、Schaff, Z. 和 Lapis, K. 纤维化肝病中转化生长因子-β 1 的免疫组织化学检测。肝病学14 , 269–273 (1991)。Castilla, A., Prieto, J. & Fausto, N. Transforming growth factors beta 1 and alpha in chronic liver disease. Effects of interferon alfa therapy. N. Engl. J. Med 324, 933–940 (1991).
卡斯蒂利亚,A.,普列托,J.和福斯托,N。慢性肝病中的转化生长因子β1和α。干扰素α治疗的效果。 N. 英格兰。医学杂志324 , 933–940 (1991)。Czaja, M. J. et al. In vitro and in vivo association of transforming growth factor-beta 1 with hepatic fibrosis. J. Cell Biol. 108, 2477–2482 (1989).
Czaja,MJ 等人。转化生长因子-β1 与肝纤维化的体外和体内关联。 J.细胞生物学。 108、2477-2482 (1989)。Sakaguchi, E. et al. Th1 down-regulation at the single-lymphocyte level in HCV-related liver cirrhosis and the effect of TGF-beta on Th1 response: possible implications for the development of hepatoma. Hepatol. Res 24, 282 (2002).
坂口,E.等人。 HCV 相关肝硬化中单淋巴细胞水平 Th1 下调以及 TGF-β 对 Th1 反应的影响:对肝癌发生的可能影响。肝醇。第 24、282号决议(2002 年)。Ueberham, E. et al. Conditional tetracycline-regulated expression of TGF-beta1 in liver of transgenic mice leads to reversible intermediary fibrosis. Hepatology 37, 1067–1078 (2003).
尤伯勒姆,E.等人。转基因小鼠肝脏中条件性四环素调节的 TGF-β1 表达导致可逆的中间纤维化。肝病学37 , 1067–1078 (2003)。Sanderson, N. et al. Hepatic expression of mature transforming growth factor beta 1 in transgenic mice results in multiple tissue lesions. Proc. Natl Acad. Sci. USA 92, 2572–2576 (1995).
桑德森,N.等人。转基因小鼠中成熟转化生长因子β1的肝脏表达导致多处组织损伤。过程。国家科学院。科学。美国92,2572–2576 (1995)。El-Youssef, M., Mu, Y., Huang, L., Stellmach, V. & Crawford, S. E. Increased expression of transforming growth factor-beta1 and thrombospondin-1 in congenital hepatic fibrosis: possible role of the hepatic stellate cell. J. Pediatr. Gastroenterol. Nutr. 28, 386–392 (1999).
El-Youssef, M.、Mu, Y.、Huang, L.、Stellmach, V. 和 Crawford, SE 先天性肝纤维化中转化生长因子-β1 和血小板反应蛋白-1 表达增加:肝星状细胞的可能作用。 J.儿科。胃肠病。营养。 28、386-392 (1999)。Teekakirikul, P. et al. Cardiac fibrosis in mice with hypertrophic cardiomyopathy is mediated by non-myocyte proliferation and requires Tgf-β. J. Clin. Invest 120, 3520–3529 (2010).
Teekakirikul,P. 等人。肥厚型心肌病小鼠的心脏纤维化是由非肌细胞增殖介导的,并且需要 Tgf-β。 J.克林。投资120 , 3520–3529 (2010)。Kania, G. et al. Heart-infiltrating prominin-1+/CD133+ progenitor cells represent the cellular source of transforming growth factor beta-mediated cardiac fibrosis in experimental autoimmune myocarditis. Circ. Res 105, 462–470 (2009).
卡尼亚,G.等人。心脏浸润的prominin-1+/CD133+祖细胞是实验性自身免疫性心肌炎中转化生长因子β介导的心脏纤维化的细胞来源。循环。第 105 号决议,462–470 (2009)。Zhao, W., Zhao, T., Chen, Y., Ahokas, R. A. & Sun, Y. Oxidative stress mediates cardiac fibrosis by enhancing transforming growth factor-beta1 in hypertensive rats. Mol. Cell Biochem 317, 43–50 (2008).
赵,W.,赵,T.,陈,Y.,阿霍卡斯,RA 和孙,Y。氧化应激通过增强高血压大鼠的转化生长因子-β1 介导心脏纤维化。摩尔。细胞生物化学317 , 43–50 (2008)。Lijnen, P. J., Petrov, V. V. & Fagard, R. H. Induction of cardiac fibrosis by transforming growth factor-beta(1). Mol. Genet Metab. 71, 418–435 (2000).
Lijnen, PJ, Petrov, VV 和 Fagard, RH 通过转化生长因子-β(1) 诱导心脏纤维化。摩尔。基因代谢物。 71、418-435 (2000)。Kuwahara, F. et al. Transforming growth factor-beta function blocking prevents myocardial fibrosis and diastolic dysfunction in pressure-overloaded rats. Circulation 106, 130–135 (2002).
桑原,F.等人。转化生长因子-β 功能阻断可预防压力超负荷大鼠的心肌纤维化和舒张功能障碍。流通量106 , 130–135 (2002)。Sakata, Y. et al. Transforming growth factor-beta receptor antagonism attenuates myocardial fibrosis in mice with cardiac-restricted overexpression of tumor necrosis factor. Basic Res Cardiol. 103, 60–68 (2008).
坂田,Y.等人。转化生长因子-β 受体拮抗作用可减轻肿瘤坏死因子心脏限制性过度表达小鼠的心肌纤维化。心脏基础研究。 103、60-68 (2008)。Hagler, M. A. et al. TGF-β signalling and reactive oxygen species drive fibrosis and matrix remodelling in myxomatous mitral valves. Cardiovasc Res 99, 175–184 (2013).
哈格勒,马萨诸塞州等人。 TGF-β 信号传导和活性氧促进粘液瘤二尖瓣的纤维化和基质重塑。心血管研究99 , 175–184 (2013)。Spriewald, B. M., Ensminger, S. M., Billing, J. S., Morris, P. J. & Wood, K. J. Increased expression of transforming growth factor-beta and eosinophil infiltration is associated with the development of transplant arteriosclerosis in long-term surviving cardiac allografts. Transplantation 76, 1105–1111 (2003).
Spriewald, BM, Ensminger, SM, Billing, JS, Morris, PJ & Wood, KJ 转化生长因子-β 表达增加和嗜酸性粒细胞浸润与长期存活的同种异体心脏移植物中移植动脉硬化的发生有关。移植76 , 1105–1111 (2003)。Bobik, A. et al. Distinct patterns of transforming growth factor-beta isoform and receptor expression in human atherosclerotic lesions. Colocalization implicates TGF-beta in fibrofatty lesion development. Circulation 99, 2883–2891 (1999).
博比克,A.等人。人类动脉粥样硬化病变中转化生长因子-β亚型和受体表达的独特模式。共定位表明 TGF-β 参与纤维脂肪病变的发展。流通量99 , 2883–2891 (1999)。Li, J. et al. Endothelial Cell Apoptosis Induces TGF-β Signaling-Dependent Host Endothelial-Mesenchymal Transition to Promote Transplant Arteriosclerosis. Am. J. Transpl. 15, 3095–3111 (2015).
李,J.等人。内皮细胞凋亡诱导 TGF-β 信号依赖性宿主内皮-间质转化,促进移植动脉硬化。是。 J.翻译。 15、3095-3111 (2015)。Chang, S. H. et al. Transforming growth factor-β-mediated CD44/STAT3 signaling contributes to the development of atrial fibrosis and fibrillation. Basic Res Cardiol. 112, 58 (2017).
张,SH 等人。转化生长因子-β 介导的 CD44/STAT3 信号传导有助于心房纤维化和颤动的发展。心脏基础研究。 112、58 (2017)。Seeland, U. et al. Myocardial fibrosis in transforming growth factor-beta(1) (TGF-beta(1)) transgenic mice is associated with inhibition of interstitial collagenase. Eur. J. Clin. Invest 32, 295–303 (2002).
Seeland,U.等人。转化生长因子-β(1) (TGF-β(1)) 转基因小鼠的心肌纤维化与间质胶原酶的抑制有关。欧元。 J.克林。投资32 , 295–303 (2002)。Cucoranu, I. et al. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ. Res 97, 900–907 (2005).
库科拉努,I.等人。 NAD(P)H 氧化酶 4 介导转化生长因子-β1 诱导的心脏成纤维细胞向肌成纤维细胞的分化。循环。第 97 号决议, 900–907 (2005)。Blyszczuk, P. et al. Transforming growth factor-β-dependent Wnt secretion controls myofibroblast formation and myocardial fibrosis progression in experimental autoimmune myocarditis. Eur. Heart J. 38, 1413–1425 (2017).
Blyszczuk,P.等人。转化生长因子-β 依赖性 Wnt 分泌控制实验性自身免疫性心肌炎中肌成纤维细胞的形成和心肌纤维化进展。欧元。 《心脏杂志》 38,1413–1425 (2017)。Song, S. et al. Foxm1 is a critical driver of TGF-β-induced EndMT in endothelial cells through Smad2/3 and binds to the Snail promoter. J. Cell Physiol. 234, 9052–9064 (2019).
宋,S.等人。 Foxm1 是内皮细胞中 TGF-β 通过 Smad2/3 诱导 EndMT 的关键驱动因素,并与 Snail 启动子结合。 J.细胞生理学。 234、9052–9064 (2019)。Zeisberg, E. M. et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med 13, 952–961 (2007).
蔡斯伯格,EM 等人。内皮向间质转化有助于心脏纤维化。纳特。医学13 , 952–961 (2007)。Varga, J., Rosenbloom, J. & Jimenez, S. A. Transforming growth factor beta (TGF beta) causes a persistent increase in steady-state amounts of type I and type III collagen and fibronectin mRNAs in normal human dermal fibroblasts. Biochem J. 247, 597–604 (1987).
Varga, J.、Rosenbloom, J. 和 Jimenez, SA 转化生长因子 β (TGF β) 导致正常人真皮成纤维细胞中 I 型和 III 型胶原蛋白和纤连蛋白 mRNA 的稳态量持续增加。生物化学杂志247 , 597–604 (1987)。Goto, K. et al. Development and progression of immobilization-induced skin fibrosis through overexpression of transforming growth factor-ß1 and hypoxic conditions in a rat knee joint contracture model. Connect Tissue Res 58, 586–596 (2017).
后藤,K.等人。在大鼠膝关节挛缩模型中,通过转化生长因子-β1 的过度表达和缺氧条件导致固定诱导的皮肤纤维化的发生和进展。连接组织研究58 , 586–596 (2017)。Zhang, X. et al. Roles of TGF-β/Smad signaling pathway in pathogenesis and development of gluteal muscle contracture. Connect Tissue Res 56, 9–17 (2015).
张,X.等人。 TGF-β/Smad信号通路在臀肌挛缩发病机制和发展中的作用。连接组织研究56 , 9–17 (2015)。Yoshikawa, H. et al. Role of TGF-beta1 in the development of pancreatic fibrosis in Otsuka Long-Evans Tokushima Fatty rats. Am. J. Physiol. Gastrointest. Liver Physiol. 282, G549–G558 (2002).
吉川,H.等人。 TGF-β1 在大冢 Long-Evans 德岛脂肪大鼠胰腺纤维化发展中的作用。是。 J.生理学。胃肠测试。肝脏生理学。 282 ,G549–G558(2002)。Vogelmann, R., Ruf, D., Wagner, M., Adler, G. & Menke, A. Effects of fibrogenic mediators on the development of pancreatic fibrosis in a TGF-beta1 transgenic mouse model. Am. J. Physiol. Gastrointest. Liver Physiol. 280, G164–G172 (2001).
Vogelmann, R.、Ruf, D.、Wagner, M.、Adler, G. 和 Menke, A. 纤维生成介质对 TGF-β1 转基因小鼠模型中胰腺纤维化发展的影响。是。 J.生理学。胃肠测试。肝脏生理学。 280 ,G164–G172(2001)。Van Laethem, J. L., Robberecht, P., Résibois, A. & Devière, J. Transforming growth factor beta promotes development of fibrosis after repeated courses of acute pancreatitis in mice. Gastroenterology 110, 576–582 (1996).
Van Laethem, JL、Robberecht, P.、Résibois, A. 和 Devière, J. 转化生长因子 β 可促进小鼠急性胰腺炎反复发作后纤维化的发展。胃肠病学110 , 576–582 (1996)。Shek, F. W. et al. Expression of transforming growth factor-beta 1 by pancreatic stellate cells and its implications for matrix secretion and turnover in chronic pancreatitis. Am. J. Pathol. 160, 1787–1798 (2002).
石,FW 等人。胰腺星状细胞转化生长因子-β1 的表达及其对慢性胰腺炎基质分泌和周转的影响。是。 J.帕索尔. 160、1787-1798 (2002)。Yao, J. C. et al. TGF-β signaling in myeloproliferative neoplasms contributes to myelofibrosis without disrupting the hematopoietic niche. J. Clin. Invest 132, e154092 (2022).
姚,JC 等。骨髓增生性肿瘤中的 TGF-β 信号传导可导致骨髓纤维化,但不会破坏造血生态位。 J.克林。投资132 ,e154092 (2022)。Ponce, C. C., de Lourdes, F. C. M., Ihara, S. S. & Silva, M. R. The relationship of the active and latent forms of TGF-β1 with marrow fibrosis in essential thrombocythemia and primary myelofibrosis. Med Oncol. 29, 2337–2344 (2012).
Ponce, CC, de Lourdes, FCM, Ihara, SS & Silva, MR TGF-β1 的活性和潜在形式与原发性血小板增多症和原发性骨髓纤维化中的骨髓纤维化的关系。医学肿瘤。 29、2337-2344 (2012)。Shen, M., Liu, X., Zhang, H. & Guo, S. W. Transforming growth factor β1 signaling coincides with epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation in the development of adenomyosis in mice. Hum. Reprod. 31, 355–369 (2016).
Shen,M.,Liu,X.,Zhang,H. 和Guo,SW 在小鼠子宫腺肌病的发展过程中,转化生长因子β1 信号传导与上皮-间质转化和成纤维细胞向肌成纤维细胞的转分化相一致。哼。重现。 31、355-369 (2016)。Koski, H., Konttinen, Y. T., Gu, X. H., Hietanen, J. & Malmström, M. Transforming growth factor beta 2 in labial salivary glands in Sjögren’s syndrome. Ann. Rheum. Dis. 54, 744–747 (1995).
Koski, H., Konttinen, YT, Gu, XH, Hietanen, J. & Malmström, M.干燥综合征唇唾液腺中的转化生长因子β2。安.感冒。迪斯。 54、744-747 (1995)。di Mola, F. F. et al. Transforming growth factor-betas and their signaling receptors are coexpressed in Crohn’s disease. Ann. Surg. 229, 67–75 (1999).
迪莫拉,FF 等人。转化生长因子-β 及其信号受体在克罗恩病中共表达。安.外科医生。 229 , 67–75 (1999)。Gómez-Bernal, F. et al. Transforming growth factor beta 1 is associated with subclinical carotid atherosclerosis in patients with systemic lupus erythematosus. Arthritis Res Ther. 25, 64 (2023).
戈麦斯-伯纳尔,F. 等人。转化生长因子β1与系统性红斑狼疮患者的亚临床颈动脉粥样硬化有关。关节炎研究。 25、64 (2023)。Liu, B. et al. Aberrant TGF-β1 signaling contributes to the development of primary biliary cirrhosis in murine model. World J. Gastroenterol. 19, 5828–5836 (2013).
刘,B.等人。异常的 TGF-β1 信号传导导致小鼠模型中原发性胆汁性肝硬化的发展。世界胃肠病学杂志。 19、5828-5836 (2013)。Chida, T. et al. Critical role of CREBH-mediated induction of transforming growth factor β2 by hepatitis C virus infection in fibrogenic responses in hepatic stellate cells. Hepatology 66, 1430–1443 (2017).
奇达,T.等人。 CREBH 介导的丙型肝炎病毒感染诱导转化生长因子 β2 在肝星状细胞纤维化反应中的关键作用。肝病学66 , 1430–1443 (2017)。Mehta, A. K., Doherty, T., Broide, D. & Croft, M. Tumor necrosis factor family member LIGHT acts with IL-1β and TGF-β to promote airway remodeling during rhinovirus infection. Allergy 73, 1415–1424 (2018).
Mehta, AK、Doherty, T.、Broide, D. 和 Croft, M. 肿瘤坏死因子家族成员 LIGHT 与 IL-1β 和 TGF-β 共同作用,促进鼻病毒感染期间的气道重塑。过敏73,1415–1424 (2018)。Ahodantin, J. et al. Type I interferons and TGF-β cooperate to induce liver fibrosis during HIV-1 infection under antiretroviral therapy. JCI Insight 7, e152738 (2022).
阿霍丹丁,J.等人。在抗逆转录病毒治疗下,HIV-1 感染期间,I 型干扰素和 TGF-β 协同诱导肝纤维化。 JCI 洞察7 ,e152738 (2022)。Huang, L. et al. CD8+ T cells with high TGF‑β1 expression cause lymph node fibrosis following HIV infection. Mol. Med Rep. 18, 77–86 (2018).
黄,L.等人。 TGF-β1 高表达的 CD8+ T 细胞在 HIV 感染后导致淋巴结纤维化。摩尔。医学报告18 , 77–86 (2018)。Kulkarni, A. B. et al. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc. Natl Acad. Sci. USA 90, 770–774 (1993).
库尔卡尼,AB 等人。小鼠转化生长因子β1无效突变会导致过度炎症反应和过早死亡。过程。国家科学院。科学。美国90,770–774 (1993)。Kulkarni, A. B. et al. Transforming growth factor-beta 1 null mice. An animal model for inflammatory disorders. Am. J. Pathol. 146, 264–275 (1995).
库尔卡尼,AB 等人。转化生长因子-β1缺失小鼠。炎症性疾病的动物模型。是。 J.帕索尔. 146、264-275 (1995)。Dang, H. et al. SLE-like autoantibodies and Sjögren’s syndrome-like lymphoproliferation in TGF-beta knockout mice. J. Immunol. 155, 3205–3212 (1995).
Dang,H.等人。 TGF-β 敲除小鼠中的 SLE 样自身抗体和干燥综合征样淋巴增殖。 J.免疫学。 155、3205-3212 (1995)。Laouar, Y. et al. TGF-beta signaling in dendritic cells is a prerequisite for the control of autoimmune encephalomyelitis. Proc. Natl Acad. Sci. USA 105, 10865–10870 (2008).
拉乌尔,Y.等人。树突状细胞中的 TGF-β 信号传导是控制自身免疫性脑脊髓炎的先决条件。过程。国家科学院。科学。美国105,10865–10870 (2008)。Hahm, K. B. et al. Loss of transforming growth factor beta signalling in the intestine contributes to tissue injury in inflammatory bowel disease. Gut 49, 190–198 (2001).
哈姆,KB 等人。肠道中转化生长因子β信号传导的丧失会导致炎症性肠病的组织损伤。肠道49 , 190–198 (2001)。Schramm, C. et al. Impairment of TGF-beta signaling in T cells increases susceptibility to experimental autoimmune hepatitis in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 284, G525–G535 (2003).
施拉姆,C.等人。 T 细胞中 TGF-β 信号传导受损会增加小鼠对实验性自身免疫性肝炎的易感性。是。 J.生理学。胃肠测试。肝脏生理学。 284 ,G525–G535(2003)。Ramalingam, R. et al. Dendritic cell-specific disruption of TGF-beta receptor II leads to altered regulatory T cell phenotype and spontaneous multiorgan autoimmunity. J. Immunol. 189, 3878–3893 (2012).
拉马林加姆,R.等人。树突状细胞特异性破坏 TGF-β 受体 II 会导致调节性 T 细胞表型改变和自发性多器官自身免疫。 J.免疫学。 189 , 3878–3893 (2012)。Ihara, S. et al. TGF-beta Signaling in Dendritic Cells Governs Colonic Homeostasis by Controlling Epithelial Differentiation and the Luminal Microbiota. J. Immunol. 196, 4603–4613 (2016).
Ihara,S.等人。树突状细胞中的 TGF-β 信号传导通过控制上皮分化和腔内微生物群来控制结肠稳态。 J.免疫学。 196 , 4603–4613 (2016)。Gorelik, L. & Flavell, R. A. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity 12, 171–181 (2000).
Gorelik, L. 和 Flavell, RA T 细胞中 TGFbeta 信号传导的废除会导致自发性 T 细胞分化和自身免疫性疾病。免疫12 , 171–181 (2000)。Turner, J. A. et al. Regulatory T Cell-Derived TGF-β1 Controls Multiple Checkpoints Governing Allergy and Autoimmunity. Immunity 53, 1202–1214.e1206 (2020).
特纳,JA 等人。调节性 T 细胞衍生的 TGF-β1 控制多个控制过敏和自身免疫的检查点。免疫53 , 1202–1214.e1206 (2020)。Hahm, K. B. et al. Loss of TGF-beta signaling contributes to autoimmune pancreatitis. J. Clin. Invest 105, 1057–1065 (2000).
哈姆,KB 等人。 TGF-β 信号传导的丧失会导致自身免疫性胰腺炎。 J.克林。投资105 、 1057–1065 (2000)。Gómez-Bernal, F. et al. Serum Levels of Transforming Growth Factor Beta 1 in Systemic Lupus Erythematosus Patients. Biomolecules 13, 73 (2022).
戈麦斯-伯纳尔,F. 等人。系统性红斑狼疮患者中转化生长因子β1的血清水平。生物分子13 , 73 (2022)。Manolova, I., Gerenova, J. & Ivanova, M. Serum levels of transforming growth factor-β1 (TGF-β1) in patients with systemic lupus erythematosus and Hashimoto’s thyroiditis. Eur. Cytokine Netw. 24, 69–74 (2013).
Manolova, I.、Gerenova, J. 和 Ivanova, M. 系统性红斑狼疮和桥本甲状腺炎患者转化生长因子-β1 (TGF-β1) 的血清水平。欧元。细胞因子网络。 24、69-74 (2013)。Becker-Merok, A., Eilertsen, G. & Nossent, J. C. Levels of transforming growth factor-beta are low in systemic lupus erythematosus patients with active disease. J. Rheumatol. 37, 2039–2045 (2010).
Becker-Merok, A.、Eilertsen, G. 和 Nossent, JC 患有活动性疾病的系统性红斑狼疮患者的转化生长因子-β 水平较低。 J.风湿病。 37、2039-2045 (2010)。Lomelí-Nieto, J. A. et al. Transforming growth factor beta isoforms and TGF-βR1 and TGF-βR2 expression in systemic sclerosis patients. Clin. Exp. Med 23, 471–481 (2023).
洛梅利-涅托,JA 等人。系统性硬化症患者中转化生长因子β亚型以及TGF-βR1和TGF-βR2的表达。临床。过期。医学23 , 471–481 (2023)。Dziadzio, M., Smith, R. E., Abraham, D. J., Black, C. M. & Denton, C. P. Circulating levels of active transforming growth factor beta1 are reduced in diffuse cutaneous systemic sclerosis and correlate inversely with the modified Rodnan skin score. Rheumatol. (Oxf.) 44, 1518–1524 (2005).
Dziadzio, M.、Smith, RE、Abraham, DJ、Black, CM 和 Denton, CP 活性转化生长因子 β1 的循环水平在弥漫性皮肤系统性硬化症中降低,并与改良的 Rodnan 皮肤评分成反比。风湿病。 (牛津大学) 44,1518-1524 (2005)。Kulozik, M., Hogg, A., Lankat-Buttgereit, B. & Krieg, T. Co-localization of transforming growth factor beta 2 with alpha 1(I) procollagen mRNA in tissue sections of patients with systemic sclerosis. J. Clin. Invest 86, 917–922 (1990).
Kulozik, M.、Hogg, A.、Lankat-Buttgereit, B. 和 Krieg, T. 系统性硬化症患者组织切片中转化生长因子 β 2 与 α 1(I) 前胶原 mRNA 的共定位。 J.克林。投资86 , 917–922 (1990)。Kubo, M., Ihn, H., Yamane, K. & Tamaki, K. Upregulated expression of transforming growth factor-beta receptors in dermal fibroblasts of skin sections from patients with systemic sclerosis. J. Rheumatol. 29, 2558–2564 (2002).
Kubo,M.,Ihn,H.,Yamane,K.和Tamaki,K.系统性硬化症患者皮肤切片的真皮成纤维细胞中转化生长因子-β受体的表达上调。 J.风湿病。 29、2558-2564 (2002)。Taketazu, F. et al. Enhanced expression of transforming growth factor-beta s and transforming growth factor-beta type II receptor in the synovial tissues of patients with rheumatoid arthritis. Lab Invest 70, 620–630 (1994).
竹田,F.等人。类风湿性关节炎患者滑膜组织中转化生长因子-β 和转化生长因子-β II 型受体的表达增强。实验室投资70 , 620–630 (1994)。Szekanecz, Z. et al. Increased synovial expression of transforming growth factor (TGF)-beta receptor endoglin and TGF-beta 1 in rheumatoid arthritis: possible interactions in the pathogenesis of the disease. Clin. Immunol. Immunopathol. 76, 187–194 (1995).
塞卡内茨,Z.等人。类风湿性关节炎中转化生长因子 (TGF)-β 受体内皮糖蛋白和 TGF-β1 滑膜表达增加:该疾病发病机制中可能存在相互作用。临床。免疫学。免疫病理。 76、187-194 (1995)。Mieliauskaite, D., Venalis, P., Dumalakiene, I., Venalis, A. & Distler, J. Relationship between serum levels of TGF-beta1 and clinical parameters in patients with rheumatoid arthritis and Sjögren’s syndrome secondary to rheumatoid arthritis. Autoimmunity 42, 356–358 (2009).
Mieliauskaite, D.、Venalis, P.、Dumalakiene, I.、Venalis, A. 和 Distler, J. 类风湿性关节炎和继发于类风湿性关节炎的干燥综合征患者血清 TGF-β1 水平与临床参数之间的关系。自身免疫42 , 356–358 (2009)。He, J. et al. Clinical significance of the expression levels of serum transforming growth factor-β and CXC type chemokine ligand 13 in primary Sjogren’s syndrome patients. Clin. Rheumatol. 42, 3283–3288 (2023).
他,J.等人。原发性干燥综合征患者血清转化生长因子-β和CXC型趋化因子配体13表达水平的临床意义临床。风湿病。 42 , 3283–3288 (2023)。Ogawa, N. et al. Analysis of transforming growth factor beta and other cytokines in autoimmune exocrinopathy (Sjögren’s syndrome). J. Interferon Cytokine Res 15, 759–767 (1995).
小川,N.等人。自身免疫性外分泌病(干燥综合征)中转化生长因子β和其他细胞因子的分析。 J.干扰素细胞因子研究15 , 759–767 (1995)。Kader, H. A. et al. Protein microarray analysis of disease activity in pediatric inflammatory bowel disease demonstrates elevated serum PLGF, IL-7, TGF-beta1, and IL-12p40 levels in Crohn’s disease and ulcerative colitis patients in remission versus active disease. Am. J. Gastroenterol. 100, 414–423 (2005).
卡德尔,HA 等人。对儿科炎症性肠病疾病活动性的蛋白质微阵列分析表明,与疾病活动期相比,克罗恩病和溃疡性结肠炎缓解期患者的血清 PLGF、IL-7、TGF-β1 和 IL-12p40 水平升高。是。 J.胃肠病学。 100 , 414–423 (2005)。Kanazawa, S. et al. VEGF, basic-FGF, and TGF-beta in Crohn’s disease and ulcerative colitis: a novel mechanism of chronic intestinal inflammation. Am. J. Gastroenterol. 96, 822–828 (2001).
金泽,S.等人。克罗恩病和溃疡性结肠炎中的 VEGF、碱性 FGF 和 TGF-β:慢性肠道炎症的新机制。是。 J.胃肠病学。 96、822-828 (2001)。Babyatsky, M. W., Rossiter, G. & Podolsky, D. K. Expression of transforming growth factors alpha and beta in colonic mucosa in inflammatory bowel disease. Gastroenterology 110, 975–984 (1996).
Babyatsky,MW,Rossiter,G. 和 Podolsky,DK 炎症性肠病结肠粘膜中转化生长因子 α 和 β 的表达。胃肠病学110 , 975–984 (1996)。Stadnicki, A., Machnik, G., Klimacka-Nawrot, E., Wolanska-Karut, A. & Labuzek, K. Transforming growth factor-beta1 and its receptors in patients with ulcerative colitis. Int Immunopharmacol. 9, 761–766 (2009).
Stadnicki, A.、Machnik, G.、Klimacka-Nawrot, E.、Wolanska-Karut, A. 和 Labuzek, K. 溃疡性结肠炎患者的转化生长因子-β1 及其受体。国际免疫药理学。 9、761-766 (2009)。Wiercińska-Drapało, A., Flisiak, R. & Prokopowicz, D. Effect of ulcerative colitis activity on plasma concentration of transforming growth factor beta1. Cytokine 14, 343–346 (2001).
Wiercińska-Drapało, A.、Flisiak, R. 和 Prokopowicz, D. 溃疡性结肠炎活动对转化生长因子 β1 血浆浓度的影响。细胞因子14 , 343–346 (2001)。Chowdhury, A., Fukuda, R. & Fukumoto, S. Growth factor mRNA expression in normal colorectal mucosa and in uninvolved mucosa from ulcerative colitis patients. J. Gastroenterol. 31, 353–360 (1996).
Chowdhury, A.、Fukuda, R. 和 Fukumoto, S.正常结直肠粘膜和溃疡性结肠炎患者未受累粘膜中生长因子 mRNA 的表达。 J.胃肠病学。 31、353-360 (1996)。Bayer, E. M. et al. Transforming growth factor-beta1 in autoimmune hepatitis: correlation of liver tissue expression and serum levels with disease activity. J. Hepatol. 28, 803–811 (1998).
拜耳,EM 等。自身免疫性肝炎中的转化生长因子-β1:肝组织表达和血清水平与疾病活动性的相关性。 J.肝素。 28、803-811 (1998)。Sakaguchi, K. et al. Serum level of transforming growth factor-beta1 (TGF-beta1) and the expression of TGF-beta receptor type II in peripheral blood mononuclear cells in patients with autoimmune hepatitis. Hepatogastroenterology 51, 1780–1783 (2004).
坂口,K.等人。自身免疫性肝炎患者血清转化生长因子-β1(TGF-β1)水平及外周血单个核细胞中TGF-β受体II型表达情况肝胃肠病学51 , 1780–1783 (2004)。Vural, P., Degirmencioglu, S., Erden, S. & Gelincik, A. The relationship between transforming growth factor-beta1, vascular endothelial growth factor, nitric oxide and Hashimoto’s thyroiditis. Int Immunopharmacol. 9, 212–215 (2009).
Vural, P.、Degirmencioglu, S.、Erden, S. 和 Gelincik, A. 转化生长因子-β1、血管内皮生长因子、一氧化氮和桥本甲状腺炎之间的关系。国际免疫药理学。 9、212-215 (2009)。Akinci, B. et al. Hashimoto’s thyroiditis, but not treatment of hypothyroidism, is associated with altered TGF-beta1 levels. Arch. Med Res 39, 397–401 (2008).
Akinci,B. 等人。桥本氏甲状腺炎与 TGF-β1 水平的改变有关,但与甲状腺功能减退症的治疗无关。拱。医学研究39 , 397–401 (2008)。Ohtsuka, K., Gray, J. D., Stimmler, M. M., Toro, B. & Horwitz, D. A. Decreased production of TGF-beta by lymphocytes from patients with systemic lupus erythematosus. J. Immunol. 160, 2539–2545 (1998).
Ohtsuka, K.、Gray, JD、Stimmler, MM、Toro, B. 和 Horwitz, DA 系统性红斑狼疮患者淋巴细胞产生的 TGF-β 减少。 J.免疫学。 160、2539-2545 (1998)。Del Zotto, B. et al. TGF-beta1 production in inflammatory bowel disease: differing production patterns in Crohn’s disease and ulcerative colitis. Clin. Exp. Immunol. 134, 120–126 (2003).
德尔佐托,B. 等人。炎症性肠病中 TGF-β1 的产生:克罗恩病和溃疡性结肠炎的不同产生模式。临床。过期。免疫学。 134、120-126 (2003)。Ohtsuka, K., Gray, J. D., Stimmler, M. M. & Horwitz, D. A. The relationship between defects in lymphocyte production of transforming growth factor-beta1 in systemic lupus erythematosus and disease activity or severity. Lupus 8, 90–94 (1999).
Ohtsuka, K.、Gray, JD、Stimmler, MM 和 Horwitz, DA 系统性红斑狼疮中转化生长因子-β1 淋巴细胞生成缺陷与疾病活动性或严重程度之间的关系。狼疮8 , 90–94 (1999)。Kotlarz, D. et al. Human TGF-β1 deficiency causes severe inflammatory bowel disease and encephalopathy. Nat. Genet 50, 344–348 (2018).
Kotlarz,D.等人。人类 TGF-β1 缺乏会导致严重的炎症性肠病和脑病。纳特。基因50,344–348 (2018)。Bai, B. et al. Molecular mechanism of the TGF‑β/Smad7 signaling pathway in ulcerative colitis. Mol. Med Rep. 25, 116 (2022).
Bai,B.等人。溃疡性结肠炎中TGF- β /Smad7信号通路的分子机制。摩尔。医学报告25 , 116 (2022)。Elbeldi-Ferchiou, A. et al. Resistance to exogenous TGF-β effects in patients with systemic lupus erythematosus. J. Clin. Immunol. 31, 574–583 (2011).
Elbeldi-Ferchiou,A. 等人。系统性红斑狼疮患者对外源性 TGF-β 作用的抵抗力。 J.克林。免疫学。 31、574-583 (2011)。Naviglio, S. et al. Severe inflammatory bowel disease associated with congenital alteration of transforming growth factor beta signaling. J. Crohns Colitis 8, 770–774 (2014).
纳维利奥,S.等人。与转化生长因子β信号先天性改变相关的严重炎症性肠病。 J. 克罗恩斯结肠炎8 , 770–774 (2014)。Peres, R. S. et al. TGF-β signalling defect is linked to low CD39 expression on regulatory T cells and methotrexate resistance in rheumatoid arthritis. J. Autoimmun. 90, 49–58 (2018).
佩雷斯,RS 等人。 TGF-β信号传导缺陷与调节性T细胞上CD39的低表达和类风湿性关节炎的甲氨蝶呤耐药性有关。 J.自身免疫。 90、49-58 (2018)。Rekik, R. et al. Impaired TGF-β signaling in patients with active systemic lupus erythematosus is associated with an overexpression of IL-22. Cytokine 108, 182–189 (2018).
雷基克,R.等人。活动性系统性红斑狼疮患者中 TGF-β 信号传导受损与 IL-22 过度表达相关。细胞因子108 , 182–189 (2018)。Yalcin, A. D., Bisgin, A. & Gorczynski, R. M. IL-8, IL-10, TGF-β, and GCSF levels were increased in severe persistent allergic asthma patients with the anti-IgE treatment. Mediators Inflamm. 2012, 720976 (2012).
在接受抗 IgE 治疗的严重持续性过敏性哮喘患者中,Yalcin, AD、Bisgin, A. 和 Gorczynski、RM IL-8、IL-10、TGF-β 和 GCSF 水平升高。炎症介质。 2012,720976 (2012)。Yamaguchi, M. et al. Sputum levels of transforming growth factor-beta1 in asthma: relation to clinical and computed tomography findings. J. Investig. Allergol. Clin. Immunol. 18, 202–206 (2008).
山口,M.等人。哮喘中转化生长因子-β1 的痰液水平:与临床和计算机断层扫描结果的关系。 J. 调查。过敏。临床。免疫学。 18、202-206 (2008)。Manuyakorn, W. et al. Serum TGF-beta1 in atopic asthma. Asian Pac. J. Allergy Immunol. 26, 185–189 (2008).
Manuyakorn,W. 等人。特应性哮喘中的血清 TGF-β1。亚洲太平洋地区。 J.过敏免疫学。 26、185-189 (2008)。Redington, A. E. et al. Transforming growth factor-beta 1 in asthma. Measurement in bronchoalveolar lavage fluid. Am. J. Respir. Crit. Care Med 156, 642–647 (1997).
雷丁顿,AE 等人。哮喘中的转化生长因子-β1。支气管肺泡灌洗液的测量。是。 J.呼吸。暴击。护理医学156 , 642–647 (1997)。Balzar, S. et al. Increased TGF-beta2 in severe asthma with eosinophilia. J. Allergy Clin. Immunol. 115, 110–117 (2005).
巴尔扎尔,S.等人。重度哮喘伴嗜酸性粒细胞增多时 TGF-β2 升高。 J.过敏临床。免疫学。 115、110-117 (2005)。Torrego, A., Hew, M., Oates, T., Sukkar, M. & Fan Chung, K. Expression and activation of TGF-beta isoforms in acute allergen-induced remodelling in asthma. Thorax 62, 307–313 (2007).
Torrego, A.、Hew, M.、Oates, T.、Sukkar, M. 和 Fan Chung, K. TGF-β 同种型在哮喘急性过敏原诱导的重塑中的表达和激活。胸部62 , 307–313 (2007)。Vignola, A. M. et al. Transforming growth factor-beta expression in mucosal biopsies in asthma and chronic bronchitis. Am. J. Respir. Crit. Care Med 156, 591–599 (1997).
维尼奥拉,AM 等人。哮喘和慢性支气管炎粘膜活检中转化生长因子-β的表达。是。 J.呼吸。暴击。护理医学156 , 591–599 (1997)。Jiang, K. et al. Changes in interleukin-17 and transforming growth factor beta 1 levels in serum and bronchoalveolar lavage fluid and their clinical significance among children with asthma. Transl. Pediatr. 2, 154–159 (2013).
江,K.等人。哮喘儿童血清和支气管肺泡灌洗液中白细胞介素17和转化生长因子β1水平的变化及其临床意义译。儿科。 2,154-159 (2013)。Vignola, A. M. et al. Release of transforming growth factor-beta (TGF-beta) and fibronectin by alveolar macrophages in airway diseases. Clin. Exp. Immunol. 106, 114–119 (1996).
维尼奥拉,AM 等人。气道疾病中肺泡巨噬细胞释放转化生长因子-β (TGF-β) 和纤连蛋白。临床。过期。免疫学。 106、114-119 (1996)。Minshall, E. M. et al. Eosinophil-associated TGF-beta1 mRNA expression and airways fibrosis in bronchial asthma. Am. J. Respir. Cell Mol. Biol. 17, 326–333 (1997).
明歇尔,EM 等人。支气管哮喘中嗜酸性粒细胞相关的 TGF-β1 mRNA 表达和气道纤维化。是。 J.呼吸。细胞分子。生物。 17、326-333 (1997)。Ohno, I. et al. Transforming growth factor beta 1 (TGF beta 1) gene expression by eosinophils in asthmatic airway inflammation. Am. J. Respir. Cell Mol. Biol. 15, 404–409 (1996).
他,我等人。哮喘气道炎症中嗜酸性粒细胞的转化生长因子 β 1 (TGF β 1) 基因表达。关于。 J.呼吸。摩尔细胞。生物。 15、404-409 (1996)。Xie, S., Sukkar, M. B., Issa, R., Khorasani, N. M. & Chung, K. F. Mechanisms of induction of airway smooth muscle hyperplasia by transforming growth factor-beta. Am. J. Physiol. Lung Cell Mol. Physiol. 293, L245–L253 (2007).
Xie, S.、Sukkar, MB、Issa, R.、Khorasani, NM 和 Chung, KF 通过转化生长因子-β 诱导气道平滑肌增生的机制。是。 J.生理学。肺细胞分子。生理学。 293 ,L245–L253(2007)。Berger, P. et al. Tryptase-stimulated human airway smooth muscle cells induce cytokine synthesis and mast cell chemotaxis. Faseb j. 17, 2139–2141 (2003).
伯杰,P.等人。类胰蛋白酶刺激的人气道平滑肌细胞诱导细胞因子合成和肥大细胞趋化性。法塞布 J. 17、2139-2141 (2003)。Janulaityte, I., Januskevicius, A., Kalinauskaite-Zukauske, V., Bajoriuniene, I. & Malakauskas, K. In Vivo Allergen-Activated Eosinophils Promote Collagen I and Fibronectin Gene Expression in Airway Smooth Muscle Cells via TGF-β1 Signaling Pathway in Asthma. Int J. Mol. Sci. 21, 1837 (2020).
Janulaityte, I.、Januskevicius, A.、Kalinauskaite-Zukauske, V.、Bajoriuniene, I. 和 Malakauskas, K. 体内过敏原激活的嗜酸性粒细胞通过 TGF-β1 信号通路促进气道平滑肌细胞中胶原蛋白 I 和纤连蛋白基因表达在哮喘中。国际 J. 摩尔。科学。 21 , 1837 (2020)。Januskevicius, A. et al. Eosinophils enhance WNT-5a and TGF-β1 genes expression in airway smooth muscle cells and promote their proliferation by increased extracellular matrix proteins production in asthma. BMC Pulm. Med 16, 94 (2016).
Januskevicius,A.等人。嗜酸性粒细胞增强哮喘气道平滑肌细胞中 WNT-5a 和 TGF-β1 基因的表达,并通过增加细胞外基质蛋白的产生来促进其增殖。 BMC 普尔。医学16 , 94 (2016)。Bottoms, S. E., Howell, J. E., Reinhardt, A. K., Evans, I. C. & McAnulty, R. J. Tgf-Beta isoform specific regulation of airway inflammation and remodelling in a murine model of asthma. PLoS One 5, e9674 (2010).
Bottoms, SE, Howell, JE, Reinhardt, AK, Evans, IC & McAnulty, RJ Tgf-Beta 异构体对哮喘小鼠模型中气道炎症和重塑的特异性调节。 PLoS One 5 ,e9674 (2010)。Gagliardo, R. et al. The role of transforming growth factor-β1 in airway inflammation of childhood asthma. Int J. Immunopathol. Pharm. 26, 725–738 (2013).
加利亚多,R.等人。转化生长因子-β1 在儿童哮喘气道炎症中的作用。 Int J.免疫病理学。医药。 26、725–738 (2013)。Eusebio, M., Kraszula, L., Kupczyk, M., Kuna, P. & Pietruczuk, M. The effects of interleukin-10 or TGF-beta on anti-CD3/CD28 induced activation of CD8+CD28- and CD8+CD28+ T cells in allergic asthma. J. Biol. Regul. Homeost. Agents 27, 681–692 (2013).
Eusebio, M., Kraszula, L., Kupczyk, M., Kuna, P. & Pietruczuk, M. 白细胞介素 10 或 TGF-β 对抗 CD3/CD28 诱导的 CD8+CD28- 和 CD8+ 激活的影响CD28+ T 细胞在过敏性哮喘中的作用。 J.Biol。规则。家乡。特工27 , 681–692 (2013)。Hung, C. H. et al. Altered pattern of monocyte differentiation and monocyte-derived TGF-β1 in severe asthma. Sci. Rep. 8, 919 (2018).
洪,CH 等人。严重哮喘中单核细胞分化和单核细胞衍生的 TGF-β1 模式的改变。科学。报告8 , 919 (2018)。Ma, Y. et al. Immunization against TGF-β1 reduces collagen deposition but increases sustained inflammation in a murine asthma model. Hum. Vaccin Immunother. 12, 1876–1885 (2016).
Ma,Y.等人。在小鼠哮喘模型中,针对 TGF-β1 的免疫可减少胶原蛋白沉积,但会增加持续炎症。哼。疫苗免疫疗法。 12、1876-1885 (2016)。Scherf, W., Burdach, S. & Hansen, G. Reduced expression of transforming growth factor beta 1 exacerbates pathology in an experimental asthma model. Eur. J. Immunol. 35, 198–206 (2005).
Scherf, W.、Burdach, S. 和 Hansen, G. 转化生长因子 β1 表达减少会加剧实验性哮喘模型的病理学。欧元。 J.免疫学。 35、198-206 (2005)。Luo, X. et al. In vivo disruption of TGF-beta signaling by Smad7 in airway epithelium alleviates allergic asthma but aggravates lung carcinogenesis in mouse. PLoS One 5, e10149 (2010).
罗,X.等人。 Smad7 在体内破坏气道上皮中的 TGF-β 信号传导可减轻小鼠过敏性哮喘,但会加剧肺癌的发生。 PLoS One 5 ,e10149 (2010)。Gao, P. et al. Functional effects of TGF-β1 on mesenchymal stem cell mobilization in cockroach allergen-induced asthma. J. Immunol. 192, 4560–4570 (2014).
高,P.等人。 TGF-β1 对蟑螂过敏原诱发哮喘中间充质干细胞动员的功能影响。 J.免疫学。 192、4560–4570 (2014)。Nemeth, K. et al. Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma. Proc. Natl Acad. Sci. USA 107, 5652–5657 (2010).
内梅斯,K.等人。骨髓基质细胞利用 TGF-β 来抑制豚草诱发哮喘小鼠模型的过敏反应。过程。国家科学院。科学。美国107,5652–5657 (2010)。Musiol, S. et al. TGF-β1 Drives Inflammatory Th Cell But Not Treg Cell Compartment Upon Allergen Exposure. Front Immunol. 12, 763243 (2021).
穆西奥尔,S.等人。接触过敏原后,TGF-β1 会驱动炎症性 Th 细胞,但不会驱动 Treg 细胞区室。前免疫学。 12、763243 (2021)。Whitehead, G. S. et al. A neutrophil/TGF-β axis limits the pathogenicity of allergen-specific CD4+ T cells. JCI Insight 7, e150251 (2022).
怀特海德,GS 等人。中性粒细胞/TGF-β轴限制了过敏原特异性CD4+ T细胞的致病性。 JCI 洞察7 ,e150251 (2022)。Yang, Z. C. et al. Transforming growth factor-β1 induces bronchial epithelial cells to mesenchymal transition by activating the Snail pathway and promotes airway remodeling in asthma. Mol. Med Rep. 8, 1663–1668 (2013).
杨,ZC 等。转化生长因子-β1 通过激活 Snail 通路诱导支气管上皮细胞向间质转化,促进哮喘气道重塑。摩尔。医学报告8,1663–1668 (2013)。Hackett, T. L. et al. Induction of epithelial-mesenchymal transition in primary airway epithelial cells from patients with asthma by transforming growth factor-beta1. Am. J. Respir. Crit. Care Med 180, 122–133 (2009).
哈克特,TL 等人。通过转化生长因子-β1 诱导哮喘患者原代气道上皮细胞上皮-间质转化。是。 J.呼吸。暴击。护理医学180 , 122–133 (2009)。Stumm, C. L. et al. Lung remodeling in a mouse model of asthma involves a balance between TGF-β1 and BMP-7. PLoS One 9, e95959 (2014).
Stumm,CL 等人。哮喘小鼠模型中的肺重塑涉及 TGF-β1 和 BMP-7 之间的平衡。 PLoS One 9 ,e95959 (2014)。Wnuk, D. et al. Enhanced asthma-related fibroblast to myofibroblast transition is the result of profibrotic TGF-β/Smad2/3 pathway intensification and antifibrotic TGF-β/Smad1/5/(8)9 pathway impairment. Sci. Rep. 10, 16492 (2020).
Wnuk,D.等人。哮喘相关成纤维细胞向肌成纤维细胞转变的增强是促纤维化 TGF-β/Smad2/3 通路强化和抗纤维化 TGF-β/Smad1/5/(8)9 通路损伤的结果。科学。报告10 , 16492 (2020)。Ciprandi, G., De Amici, M., Tosca, M. & Marseglia, G. Serum transforming growth factor-beta levels depend on allergen exposure in allergic rhinitis. Int Arch. Allergy Immunol. 152, 66–70 (2010).
Ciprandi, G.、De Amici, M.、Tosca, M. 和 Marseglia, G. 血清转化生长因子-β 水平取决于过敏性鼻炎中的过敏原暴露。国际拱门。过敏免疫学。 152、66-70 (2010)。Salib, R. J., Kumar, S., Wilson, S. J. & Howarth, P. H. Nasal mucosal immunoexpression of the mast cell chemoattractants TGF-beta, eotaxin, and stem cell factor and their receptors in allergic rhinitis. J. Allergy Clin. Immunol. 114, 799–806 (2004).
Salib, RJ, Kumar, S., Wilson, SJ & Howarth, PH 过敏性鼻炎中肥大细胞趋化剂 TGF-β、eotaxin 和干细胞因子及其受体的鼻粘膜免疫表达。 J.过敏临床。免疫学。 114、799–806 (2004)。Salib, R. J. Transforming growth factor-beta gene expression studies in nasal mucosal biopsies in naturally occurring allergic rhinitis. Ann. R. Coll. Surg. Engl. 89, 563–573 (2007).
Salib,RJ 在自然发生的过敏性鼻炎的鼻粘膜活检中进行转化生长因子-β 基因表达研究。安. R.科尔。外科医生。英语。 89、563-573 (2007)。Ouyang, Y., Nakao, A., Han, D. & Zhang, L. Transforming growth factor-β1 promotes nasal mucosal mast cell chemotaxis in murine experimental allergic rhinitis. ORL J. Otorhinolaryngol. Relat. Spec. 74, 117–123 (2012).
欧阳,Y.,中尾,A.,韩,D.和张,L。转化生长因子-β1促进小鼠实验性过敏性鼻炎的鼻粘膜肥大细胞趋化性。 ORL J.耳鼻喉科。相关。规格74、117-123 (2012)。Ouyang, Y. et al. TGF-beta signaling may play a role in the development of goblet cell hyperplasia in a mouse model of allergic rhinitis. Allergol. Int 59, 313–319 (2010).
欧阳,Y.等。 TGF-β信号传导可能在过敏性鼻炎小鼠模型杯状细胞增生的发生过程中发挥作用。过敏。 Int 59,313-319 (2010)。Wang, M., Gu, Z., Yang, J., Zhao, H. & Cao, Z. Changes among TGF-β1(+) Breg cells and helper T cell subsets in a murine model of allergic rhinitis with prolonged OVA challenge. Int Immunopharmacol. 69, 347–357 (2019).
Wang, M.、Gu, Z.、Yang, J.、Zhao, H. 和 Cao, Z. 长期 OVA 激发的过敏性鼻炎小鼠模型中 TGF-β1(+) Breg 细胞和辅助 T 细胞亚群的变化。国际免疫药理学。 69、347-357 (2019)。Yang, M., Yang, C. & Mine, Y. Multiple T cell epitope peptides suppress allergic responses in an egg allergy mouse model by the elicitation of forkhead box transcription factor 3- and transforming growth factor-beta-associated mechanisms. Clin. Exp. Allergy 40, 668–678 (2010).
Yang, M.、Yang, C. 和 Mine, Y. 多个 T 细胞表位肽通过引发叉头盒转录因子 3 和转化生长因子 β 相关机制来抑制鸡蛋过敏小鼠模型中的过敏反应。临床。过期。过敏40 , 668–678 (2010)。Barletta, B. et al. Probiotic VSL#3-induced TGF-β ameliorates food allergy inflammation in a mouse model of peanut sensitization through the induction of regulatory T cells in the gut mucosa. Mol. Nutr. Food Res 57, 2233–2244 (2013).
巴列塔,B.等人。益生菌 VSL#3 诱导的 TGF-β 通过诱导肠道粘膜中的调节性 T 细胞,改善花生过敏小鼠模型中的食物过敏炎症。摩尔。营养。食品研究57 , 2233–2244 (2013)。Pérez-Machado, M. A. et al. Reduced transforming growth factor-beta1-producing T cells in the duodenal mucosa of children with food allergy. Eur. J. Immunol. 33, 2307–2315 (2003).
佩雷斯-马查多,MA 等人。食物过敏儿童十二指肠粘膜中产生转化生长因子-β1 的 T 细胞减少。欧元。 J.免疫学。 33、2307-2315 (2003)。Park, H. H. et al. TGF-β secreted by human umbilical cord blood-derived mesenchymal stem cells ameliorates atopic dermatitis by inhibiting secretion of TNF-α and IgE. Stem Cells 38, 904–916 (2020).
帕克,HH 等人。人脐带血间充质干细胞分泌的TGF-β通过抑制TNF-α和IgE的分泌来改善特应性皮炎。干细胞38 , 904–916 (2020)。Sumiyoshi, K. et al. Transforming growth factor-beta1 suppresses atopic dermatitis-like skin lesions in NC/Nga mice. Clin. Exp. Allergy 32, 309–314 (2002).
住吉,K.等人。转化生长因子-β1 可抑制 NC/Nga 小鼠的特应性皮炎样皮肤病变。临床。过期。过敏症32 , 309–314 (2002)。Kim, H. S. et al. Human umbilical cord blood mesenchymal stem cell-derived PGE2 and TGF-β1 alleviate atopic dermatitis by reducing mast cell degranulation. Stem Cells 33, 1254–1266 (2015).
金,HS 等人。人脐带血间充质干细胞衍生的 PGE2 和 TGF-β1 通过减少肥大细胞脱颗粒来缓解特应性皮炎。干细胞33 , 1254–1266 (2015)。Lee, H. J., Lee, H. P., Ha, S. J., Byun, D. G. & Kim, J. W. Spontaneous expression of mRNA for IL-10, GM-CSF, TGF-beta, TGF-alpha, and IL-6 in peripheral blood mononuclear cells from atopic dermatitis. Ann. Allergy Asthma Immunol. 84, 553–558 (2000).
Lee, HJ, Lee, HP, Ha, SJ, Byun, DG & Kim, JW 外周血单核细胞中 IL-10、GM-CSF、TGF-β、TGF-α 和 IL-6 mRNA 的自发表达特应性皮炎。安.过敏哮喘免疫学。 84、553-558 (2000)。Peng, W. M., Maintz, L., Allam, J. P. & Novak, N. Attenuated TGF-β1 responsiveness of dendritic cells and their precursors in atopic dermatitis. Eur. J. Immunol. 43, 1374–1382 (2013).
Peng, WM, Maintz, L., Allam, JP & Novak, N. 特应性皮炎中树突状细胞及其前体的 TGF-β1 反应性减弱。欧元。 J.免疫学。 43、1374-1382 (2013)。Shafi, T. et al. Investigating dysregulation of TGF-β1/SMAD3 signaling in Atopic Dermatitis: A Molecular and Immunohistochemical Analysis. Clin. Exp. Immunol. https://doi.org/10.1093/cei/uxad130 (2023).
沙菲,T.等人。研究特应性皮炎中 TGF-β1/SMAD3 信号传导失调:分子和免疫组织化学分析。临床。过期。免疫学。 https://doi.org/10.1093/cei/uxad130(2023 )。Akbarshahi, H., Sam, A., Chen, C., Rosendahl, A. H. & Andersson, R. Early activation of pulmonary TGF-β1/Smad2 signaling in mice with acute pancreatitis-associated acute lung injury. Mediat. Inflamm. 2014, 148029 (2014).
Akbarshahi, H.、Sam, A.、Chen, C.、Rosendahl, AH 和 Andersson, R. 急性胰腺炎相关急性肺损伤小鼠肺部 TGF-β1/Smad2 信号的早期激活。媒体。发炎。 2014,148029 (2014)。Hori, Y. et al. Macrophage-derived transforming growth factor-beta1 induces hepatocellular injury via apoptosis in rat severe acute pancreatitis. Surgery 127, 641–649 (2000).
堀,Y.等人。巨噬细胞源性转化生长因子-β1 通过细胞凋亡诱导大鼠重症急性胰腺炎肝细胞损伤。外科127 , 641–649 (2000)。van Laethem, J. L. et al. Localization of transforming growth factor beta 1 and its latent binding protein in human chronic pancreatitis. Gastroenterology 108, 1873–1881 (1995).
范·莱瑟姆,JL 等人。转化生长因子β1及其潜在结合蛋白在人类慢性胰腺炎中的定位。胃肠病学108,1873–1881 (1995)。Kanamaru, Y. et al. Blockade of TGF-beta signaling in T cells prevents the development of experimental glomerulonephritis. J. Immunol. 166, 2818–2823 (2001).
Kanamaru,Y.等人。阻断 T 细胞中的 TGF-β 信号传导可防止实验性肾小球肾炎的发生。 J.免疫学。 166、2818-2823 (2001)。Kitamura, M., Sütö, T., Yokoo, T., Shimizu, F. & Fine, L. G. Transforming growth factor-beta 1 is the predominant paracrine inhibitor of macrophage cytokine synthesis produced by glomerular mesangial cells. J. Immunol. 156, 2964–2971 (1996).
Kitamura, M.、Sütö, T.、Yokoo, T.、Shimizu, F. 和 Fine, LG 转化生长因子-β 1 是肾小球系膜细胞产生的巨噬细胞细胞因子合成的主要旁分泌抑制剂。 J.免疫学。 156、2964-2971 (1996)。Zhang, W. et al. Staphylococcus aureus Infection Initiates Hypoxia-Mediated Transforming Growth Factor-β1 Upregulation to Trigger Osteomyelitis. mSystems 7, e0038022 (2022).
张,W.等人。金黄色葡萄球菌感染引发缺氧介导的转化生长因子-β1 上调,引发骨髓炎。 mSystems 7 ,e0038022 (2022)。Brandes, M. E., Allen, J. B., Ogawa, Y. & Wahl, S. M. Transforming growth factor beta 1 suppresses acute and chronic arthritis in experimental animals. J. Clin. Invest 87, 1108–1113 (1991).
Brandes, ME、Allen, JB、Okawa, Y. 和 Wahl, SM 转化生长因子 β 1 可抑制实验动物的急性和慢性关节炎。 J.克林。投资87 , 1108–1113 (1991)。Yan, J. et al. Obesity- and aging-induced excess of central transforming growth factor-β potentiates diabetic development via an RNA stress response. Nat. Med 20, 1001–1008 (2014).
严,J.等人。肥胖和衰老引起的中心转化生长因子-β 过量会通过 RNA 应激反应促进糖尿病的发生。纳特。医学20 , 1001–1008 (2014)。Weiss, R., Lifshitz, V. & Frenkel, D. TGF-β1 affects endothelial cell interaction with macrophages and T cells leading to the development of cerebrovascular amyloidosis. Brain Behav. Immun. 25, 1017–1024 (2011).
Weiss, R.、Lifshitz, V. 和 Frenkel, D. TGF-β1 影响内皮细胞与巨噬细胞和 T 细胞的相互作用,导致脑血管淀粉样变性的发生。大脑行为。免疫。 25、1017-1024 (2011)。Grammas, P. & Ovase, R. Cerebrovascular transforming growth factor-beta contributes to inflammation in the Alzheimer’s disease brain. Am. J. Pathol. 160, 1583–1587 (2002).
Grammas, P. 和 Ovase, R. 脑血管转化生长因子-β 会导致阿尔茨海默病大脑中的炎症。是。 J.帕索尔. 160、1583-1587 (2002)。Rendón-Ramirez, E. J. et al. TGF-β Blood Levels Distinguish Between Influenza A (H1N1)pdm09 Virus Sepsis and Sepsis due to Other Forms of Community-Acquired Pneumonia. Viral Immunol. 28, 248–254 (2015).
伦东-拉米雷斯,EJ 等人。 TGF-β 血液水平可区分甲型 (H1N1)pdm09 流感病毒脓毒症和其他形式的社区获得性肺炎引起的脓毒症。病毒免疫学。 28、248-254 (2015)。Carlson, C. M. et al. Transforming growth factor-β: activation by neuraminidase and role in highly pathogenic H5N1 influenza pathogenesis. PLoS Pathog. 6, e1001136 (2010).
卡尔森,CM 等人。转化生长因子-β:神经氨酸酶激活及其在高致病性 H5N1 流感发病机制中的作用。 PLoS Pathog。 6 、e1001136 (2010)。Furuya, Y. et al. Prevention of Influenza Virus-Induced Immunopathology by TGF-β Produced during Allergic Asthma. PLoS Pathog. 11, e1005180 (2015).
古谷,Y.等人。通过过敏性哮喘期间产生的 TGF-β 预防流感病毒诱导的免疫病理学。 PLoS Pathog。 11 、e1005180 (2015)。Richer, M. J., Straka, N., Fang, D., Shanina, I. & Horwitz, M. S. Regulatory T-cells protect from type 1 diabetes after induction by coxsackievirus infection in the context of transforming growth factor-beta. Diabetes 57, 1302–1311 (2008).
Richer, MJ、Straka, N.、Fang, D.、Shanina, I. 和 Horwitz, MS 在转化生长因子-β 的背景下,通过柯萨奇病毒感染诱导后,调节性 T 细胞可以预防 1 型糖尿病。糖尿病57 , 1302–1311 (2008)。Shi, Y. et al. Regulatory T cells protect mice against coxsackievirus-induced myocarditis through the transforming growth factor beta-coxsackie-adenovirus receptor pathway. Circulation 121, 2624–2634 (2010).
石,Y.等人。调节性 T 细胞通过转化生长因子 β-柯萨奇腺病毒受体途径保护小鼠免受柯萨奇病毒诱导的心肌炎。流通量121 , 2624–2634 (2010)。Beckham, J. D., Tuttle, K. & Tyler, K. L. Reovirus activates transforming growth factor beta and bone morphogenetic protein signaling pathways in the central nervous system that contribute to neuronal survival following infection. J. Virol. 83, 5035–5045 (2009).
Beckham, JD、Tuttle, K. 和 Tyler, KL 呼肠孤病毒激活中枢神经系统中的转化生长因子 β 和骨形态发生蛋白信号通路,有助于感染后神经元的存活。 J.维罗尔。 83、5035–5045 (2009)。Stanifer, M. L. et al. Reovirus intermediate subviral particles constitute a strategy to infect intestinal epithelial cells by exploiting TGF-β dependent pro-survival signaling. Cell Microbiol 18, 1831–1845 (2016).
斯坦尼弗,ML 等人。呼肠孤病毒中间亚病毒颗粒构成了一种通过利用 TGF-β 依赖性促生存信号传导来感染肠上皮细胞的策略。细胞微生物学18,1831–1845 (2016)。Chen, Y. et al. Mechanism of exosomes from adipose-derived mesenchymal stem cells on sepsis-induced acute lung injury by promoting TGF-β secretion in macrophages. Surgery 174, 1208–1219 (2023).
陈,Y.等人。脂肪间充质干细胞外泌体通过促进巨噬细胞中 TGF-β 分泌对脓毒症诱导的急性肺损伤的机制。外科174 , 1208–1219 (2023)。Sanfilippo, A. M., Furuya, Y., Roberts, S., Salmon, S. L. & Metzger, D. W. Allergic Lung Inflammation Reduces Tissue Invasion and Enhances Survival from Pulmonary Pneumococcal Infection in Mice, Which Correlates with Increased Expression of Transforming Growth Factor β1 and SiglecF(low) Alveolar Macrophages. Infect. Immun. 83, 2976–2983 (2015).
Sanfilippo, AM、Furuya, Y.、Roberts, S.、Salmon, SL 和 Metzger, DW 过敏性肺部炎症可减少组织侵袭并提高小鼠肺部肺炎球菌感染的存活率,这与转化生长因子 β1 和 SiglecF 表达的增加相关(低)肺泡巨噬细胞。感染。免疫。 83、2976-2983 (2015)。Wang, B. et al. Induction of TGF-beta1 and TGF-beta1-dependent predominant Th17 differentiation by group A streptococcal infection. Proc. Natl Acad. Sci. USA 107, 5937–5942 (2010).
王,B.等人。 A 组链球菌感染诱导 TGF-β1 和 TGF-β1 依赖性主要 Th17 分化。过程。国家科学院。科学。美国107 , 5937–5942 (2010)。Nakane, A. et al. Transforming growth factor beta is protective in host resistance against Listeria monocytogenes infection in mice. Infect. Immun. 64, 3901–3904 (1996).
Nakane,A.等人。转化生长因子β可保护宿主抵抗小鼠单核细胞增生李斯特氏菌感染。感染。免疫。 64、3901-3904 (1996)。Zhong, Y., Cantwell, A. & Dube, P. H. Transforming growth factor beta and CD25 are important for controlling systemic dissemination following Yersinia enterocolitica infection of the gut. Infect. Immun. 78, 3716–3725 (2010).
Chung, Y.、Cantwell, A. 和 Dube, PH 转化生长因子 β 和 CD25 对于控制肠道小肠结肠炎耶尔森菌感染后的全身传播非常重要。感染。免疫。 78、3716-3725 (2010)。Shao, X., Rivera, J., Niang, R., Casadevall, A. & Goldman, D. L. A dual role for TGF-beta1 in the control and persistence of fungal pneumonia. J. Immunol. 175, 6757–6763 (2005).
Shao, X.、Rivera, J.、Niang, R.、Casadevall, A. 和 Goldman, DL TGF-β1 在控制和持续真菌性肺炎中的双重作用。 J.免疫学。 175、6757–6763 (2005)。Omer, F. M. & Riley, E. M. Transforming growth factor beta production is inversely correlated with severity of murine malaria infection. J. Exp. Med 188, 39–48 (1998).
Omer, FM 和 Riley, EM 转化生长因子 β 的产生与鼠疟疾感染的严重程度呈负相关。 J.Exp。医学188 , 39–48 (1998)。Namangala, B., Sugimoto, C. & Inoue, N. Effects of exogenous transforming growth factor beta on Trypanosoma congolense infection in mice. Infect. Immun. 75, 1878–1885 (2007).
Namangala,B.,Sugimoto,C. 和 Inoue,N. 外源转化生长因子 β 对小鼠刚果锥虫感染的影响。感染。免疫。 75,1878-1885 (2007)。Buzoni-Gatel, D. et al. Murine ileitis after intracellular parasite infection is controlled by TGF-beta-producing intraepithelial lymphocytes. Gastroenterology 120, 914–924 (2001).
布佐尼-盖特尔,D. 等人。细胞内寄生虫感染后的小鼠回肠炎由产生 TGF-β 的上皮内淋巴细胞控制。胃肠病学120 , 914–924 (2001)。Cekanaviciute, E. et al. Astrocytic TGF-β signaling limits inflammation and reduces neuronal damage during central nervous system Toxoplasma infection. J. Immunol. 193, 139–149 (2014).
Cekanaviciute,E.等人。星形胶质细胞 TGF-β 信号传导可限制中枢神经系统弓形虫感染期间的炎症并减少神经元损伤。 J.免疫学。 193、139-149 (2014)。Zhao, M. et al. The Effect of TGF-β on Treg Cells in Adverse Pregnancy Outcome upon Toxoplasma gondii Infection. Front Microbiol 8, 901 (2017).
赵,M.等人。 TGF-β 对弓形虫感染不良妊娠结局中 Treg 细胞的影响。微生物前沿8 , 901 (2017)。Xu, X. et al. TGF-β1 improving abnormal pregnancy outcomes induced by Toxoplasma gondii infection: Regulating NKG2D/DAP10 and killer subset of decidual NK cells. Cell Immunol. 317, 9–17 (2017).
徐,X.等人。 TGF-β1 改善弓形虫感染引起的异常妊娠结局:调节 NKG2D/DAP10 和蜕膜 NK 细胞的杀伤子集。细胞免疫学。 317,9-17 (2017)。Heitmann, L. et al. TGF-β-responsive myeloid cells suppress type 2 immunity and emphysematous pathology after hookworm infection. Am. J. Pathol. 181, 897–906 (2012).
海特曼,L.等人。钩虫感染后,TGF-β 反应性骨髓细胞抑制 2 型免疫和肺气肿病理。是。 J.帕索尔. 181 , 897–906 (2012)。Wu, H. P. et al. Plasma transforming growth factor-beta1 level in patients with severe community-acquired pneumonia and association with disease severity. J. Formos. Med Assoc. 108, 20–27 (2009).
吴,惠普等人。重症社区获得性肺炎患者血浆转化生长因子-β1 水平及其与疾病严重程度的关系。 J.福莫斯。医学协会。 108、20-27 (2009)。de Pablo, R. et al. Sepsis-induced acute respiratory distress syndrome with fatal outcome is associated to increased serum transforming growth factor beta-1 levels. Eur. J. Intern Med 23, 358–362 (2012).
德巴勃罗,R.等人。脓毒症引起的致命性急性呼吸窘迫综合征与血清转化生长因子 β-1 水平升高有关。欧元。实习医学杂志23 , 358–362 (2012)。Gauthier, T. et al. TGF-β uncouples glycolysis and inflammation in macrophages and controls survival during sepsis. Sci. Signal 16, eade0385 (2023).
高蒂尔,T.等人。 TGF-β 解偶联巨噬细胞中的糖酵解和炎症,并控制脓毒症期间的存活。科学。信号16 ,eade0385 (2023)。Ahmad, S., Choudhry, M. A., Shankar, R. & Sayeed, M. M. Transforming growth factor-beta negatively modulates T-cell responses in sepsis. FEBS Lett. 402, 213–218 (1997).
Ahmad, S.、Choudhry, MA、Shankar, R. 和 Sayeed, MM 转化生长因子-β 负向调节脓毒症中的 T 细胞反应。 FEBS 快报。 402、213-218 (1997)。Lowrance, J. H., O’Sullivan, F. X., Caver, T. E., Waegell, W. & Gresham, H. D. Spontaneous elaboration of transforming growth factor beta suppresses host defense against bacterial infection in autoimmune MRL/lpr mice. J. Exp. Med 180, 1693–1703 (1994).
Lowrance, JH, O'Sullivan, FX, Caver, TE, Waegell, W. & Gresham, HD 在自身免疫 MRL/lpr 小鼠中,转化生长因子 β 的自发阐述可抑制宿主对细菌感染的防御。 J.Exp。医学180 , 1693–1703 (1994)。Li, N. et al. Influenza viral neuraminidase primes bacterial coinfection through TGF-β-mediated expression of host cell receptors. Proc. Natl Acad. Sci. USA 112, 238–243 (2015).
李,N.等人。流感病毒神经氨酸酶通过 TGF-β 介导的宿主细胞受体表达引发细菌共感染。过程。国家科学院。科学。美国112 , 238–243 (2015)。Zhang, M. et al. TGF-β1 promoted the infection of bovine mammary epithelial cells by Staphylococcus aureus through increasing expression of cells’ fibronectin and integrin β1. Vet. Microbiol 237, 108420 (2019).
张,M.等人。 TGF-β1通过增加细胞纤连蛋白和整合素β1的表达来促进金黄色葡萄球菌对牛乳腺上皮细胞的感染。兽医。微生物学237 , 108420 (2019)。Owyang, S. Y. et al. Dendritic cell-derived TGF-β mediates the induction of mucosal regulatory T-cell response to Helicobacter infection essential for maintenance of immune tolerance in mice. Helicobacter 25, e12763 (2020).
欧阳,SY 等人。树突状细胞衍生的 TGF-β 介导诱导粘膜调节 T 细胞对螺杆菌感染的反应,这对于维持小鼠的免疫耐受至关重要。螺杆菌25 ,e12763 (2020)。Beswick, E. J., Pinchuk, I. V., Earley, R. B., Schmitt, D. A. & Reyes, V. E. Role of gastric epithelial cell-derived transforming growth factor beta in reduced CD4+ T cell proliferation and development of regulatory T cells during Helicobacter pylori infection. Infect. Immun. 79, 2737–2745 (2011).
Beswick,EJ,Pinchuk,IV,Earley,RB,Schmitt,DA 和 Reyes,VE 幽门螺杆菌感染期间胃上皮细胞衍生的转化生长因子β在减少 CD4+ T 细胞增殖和调节性 T 细胞发育中的作用。感染。免疫。 79、2737-2745 (2011)。Liu, Y., Islam, E. A., Jarvis, G. A., Gray-Owen, S. D. & Russell, M. W. Neisseria gonorrhoeae selectively suppresses the development of Th1 and Th2 cells, and enhances Th17 cell responses, through TGF-β-dependent mechanisms. Mucosal. Immunol. 5, 320–331 (2012).
Liu, Y., Islam, EA, Jarvis, GA, Gray-Owen, SD 和 Russell, MW 淋病奈瑟菌通过 TGF-β 依赖性机制选择性抑制 Th1 和 Th2 细胞的发育,并增强 Th17 细胞反应。粘膜。免疫学。 5、320-331 (2012)。Balkhi, M. Y., Sinha, A. & Natarajan, K. Dominance of CD86, transforming growth factor- beta 1, and interleukin-10 in Mycobacterium tuberculosis secretory antigen-activated dendritic cells regulates T helper 1 responses to mycobacterial antigens. J. Infect. Dis. 189, 1598–1609 (2004).
Balkhi, MY, Sinha, A. & Natarajan, K. 结核分枝杆菌分泌抗原激活树突状细胞中 CD86、转化生长因子-β1 和白细胞介素 10 的优势调节 T 辅助细胞 1 对分枝杆菌抗原的反应。 J.感染。迪斯。 189、1598-1609 (2004)。Roberts, T., Beyers, N., Aguirre, A. & Walzl, G. Immunosuppression during active tuberculosis is characterized by decreased interferon- gamma production and CD25 expression with elevated forkhead box P3, transforming growth factor- beta, and interleukin-4 mRNA levels. J. Infect. Dis. 195, 870–878 (2007).
Roberts, T.、Beyers, N.、Aguirre, A. 和 Walzl, G. 活动性结核病期间的免疫抑制的特点是干扰素 γ 产生和 CD25 表达减少,叉头盒 P3、转化生长因子 - β 和白细胞介素 4 升高mRNA 水平。 J.感染。迪斯。 195、870–878 (2007)。Denney, L., Branchett, W., Gregory, L. G., Oliver, R. A. & Lloyd, C. M. Epithelial-derived TGF-β1 acts as a pro-viral factor in the lung during influenza A infection. Mucosal. Immunol. 11, 523–535 (2018).
Denney, L.、Branchett, W.、Gregory, LG、Oliver, RA 和 Lloyd, CM 上皮源性 TGF-β1 在甲型流感感染期间在肺部充当促病毒因子。粘膜。免疫学。 11 , 523–535 (2018)。Thomas, B. J. et al. Transforming growth factor-beta enhances rhinovirus infection by diminishing early innate responses. Am. J. Respir. Cell Mol. Biol. 41, 339–347 (2009).
托马斯,BJ 等人。转化生长因子-β 通过减少早期先天反应来增强鼻病毒感染。是。 J.呼吸。细胞分子。生物。 41、339-347 (2009)。Lewis, G. M., Macal, M., Hesser, C. R. & Zuñiga, E. I. Constitutive but not inducible attenuation of transforming growth factor β signaling increases natural killer cell responses without directly affecting dendritic cells early after persistent viral infection. J. Virol. 89, 3343–3355 (2015).
Lewis, GM、Macal, M.、Hesser, CR 和 Zuñiga, EI 转化生长因子 β 信号传导的组成型而非诱导性减弱可增加自然杀伤细胞反应,而不会在持续病毒感染后早期直接影响树突状细胞。 J.维罗尔。 89、3343–3355 (2015)。Marcoe, J. P. et al. TGF-β is responsible for NK cell immaturity during ontogeny and increased susceptibility to infection during mouse infancy. Nat. Immunol. 13, 843–850 (2012).
马科,JP 等人。 TGF-β 导致个体发育期间 NK 细胞不成熟,并增加小鼠婴儿期感染的易感性。纳特。免疫学。 13、843-850 (2012)。Kekow, J. et al. Transforming growth factor beta and noncytopathic mechanisms of immunodeficiency in human immunodeficiency virus infection. Proc. Natl Acad. Sci. USA 87, 8321–8325 (1990).
Kekow,J.等人。人类免疫缺陷病毒感染中转化生长因子β和免疫缺陷的非细胞病变机制。过程。国家科学院。科学。美国87 , 8321–8325 (1990)。Kekow, J. et al. Transforming growth factor-beta and suppression of humoral immune responses in HIV infection. J. Clin. Invest 87, 1010–1016 (1991).
Kekow,J.等人。 HIV 感染中转化生长因子-β 和体液免疫反应的抑制。 J.克林。投资87 , 1010–1016 (1991)。Bedke, N. et al. Transforming growth factor-beta promotes rhinovirus replication in bronchial epithelial cells by suppressing the innate immune response. PLoS One 7, e44580 (2012).
Bedke,N.等人。转化生长因子-β 通过抑制先天免疫反应来促进支气管上皮细胞中鼻病毒的复制。 PLoS One 7 ,e44580 (2012)。Grunwell, J. R. et al. TGF-β1 Suppresses the Type I IFN Response and Induces Mitochondrial Dysfunction in Alveolar Macrophages. J. Immunol. 200, 2115–2128 (2018).
格伦威尔,JR 等人。 TGF-β1 抑制 I 型 IFN 反应并诱导肺泡巨噬细胞线粒体功能障碍。 J.免疫学。 200 , 2115–2128 (2018)。Witkowski, M. et al. Untimely TGFβ responses in COVID-19 limit antiviral functions of NK cells. Nature 600, 295–301 (2021).
维特科夫斯基,M.等人。 COVID-19 中不合时宜的 TGFβ 反应限制了 NK 细胞的抗病毒功能。自然600 , 295–301 (2021)。Moriuchi, M. & Moriuchi, H. Cell-type-dependent effect of transforming growth factor beta, a major cytokine in breast milk, on human immunodeficiency virus type 1 infection of mammary epithelial MCF-7 cells or macrophages. J. Virol. 78, 13046–13052 (2004).
Moriuchi, M. & Moriuchi, H. 转化生长因子β(母乳中的主要细胞因子)对乳腺上皮 MCF-7 细胞或巨噬细胞的人类免疫缺陷病毒 1 型感染的细胞类型依赖性作用。 J.维罗尔。 78、13046-13052 (2004)。Yim, L. Y. et al. Transforming Growth Factor β Signaling Promotes HIV-1 Infection in Activated and Resting Memory CD4(+) T Cells. J. Virol. 97, e0027023 (2023).
伊姆,LY 等人。转化生长因子 β 信号传导促进激活和静息记忆 CD4(+) T 细胞中的 HIV-1 感染。 J.维罗尔。 97 、e0027023 (2023)。Cheung, K. W. et al. α(4)β(7)(+) CD4(+) Effector/Effector Memory T Cells Differentiate into Productively and Latently Infected Central Memory T Cells by Transforming Growth Factor β1 during HIV-1 Infection. J. Virol. 92, e01510–e01517 (2018).
张,KW 等人。 HIV-1 感染期间,α(4)β(7)(+) CD4(+) 效应/效应记忆 T 细胞通过转化生长因子 β1 分化为高效和潜伏感染的中央记忆 T 细胞。 J.维罗尔。 92 ,e01510–e01517(2018)。Moriuchi, M. & Moriuchi, H. Transforming growth factor-beta enhances human T-cell leukemia virus type I infection. J. Med Virol. 67, 427–430 (2002).
Moriuchi, M. & Moriuchi, H. 转化生长因子-β 增强人类 T 细胞白血病病毒 I 型感染。 J. Med Virol。 67、427-430 (2002)。Lin, W. et al. HIV increases HCV replication in a TGF-beta1-dependent manner. Gastroenterology 134, 803–811 (2008).
林,W.等人。 HIV 以 TGF-β1 依赖性方式增加 HCV 复制。胃肠病学134 , 803–811 (2008)。Trinh, Q. D. et al. TGF-β1 Promotes Zika Virus Infection in Immortalized Human First-Trimester Trophoblasts via the Smad Pathway. Cells 11, 3026 (2022).
Trinh,QD 等。 TGF-β1 通过 Smad 途径促进永生化人类妊娠早期滋养细胞中的寨卡病毒感染。细胞11 , 3026 (2022)。Pham, N. T. K. et al. The Epithelial-to-Mesenchymal Transition-Like Process Induced by TGF-β1 Enhances Rubella Virus Binding and Infection in A549 Cells via the Smad Pathway. Microorganisms 9, 662 (2021).
范,NTK 等人。 TGF-β1 诱导的上皮间质转化样过程通过 Smad 途径增强 A549 细胞中风疹病毒的结合和感染。微生物9 , 662 (2021)。Flynn, R. J. & Mulcahy, G. The roles of IL-10 and TGF-beta in controlling IL-4 and IFN-gamma production during experimental Fasciola hepatica infection. Int J. Parasitol. 38, 1673–1680 (2008).
Flynn, RJ 和 Mulcahy, G。IL-10 和 TGF-β 在实验性肝片形吸虫感染期间控制 IL-4 和 IFN-γ 产生的作用。 Int J. Parasitol。 38、1673-1680 (2008)。Pang, N. et al. TGF-β/Smad signaling pathway regulates Th17/Treg balance during Echinococcus multilocularis infection. Int Immunopharmacol. 20, 248–257 (2014).
庞,N.等人。 TGF-β/Smad信号通路在多房棘球绦虫感染过程中调节Th17/Treg平衡。国际免疫药理学。 20、248-257 (2014)。Barbosa, B. F. et al. IL10, TGF beta1, and IFN gamma modulate intracellular signaling pathways and cytokine production to control Toxoplasma gondii infection in BeWo trophoblast cells. Biol. Reprod. 92, 82 (2015).
巴博萨,BF 等人。 IL10、TGF beta1 和 IFN gamma 调节细胞内信号传导途径和细胞因子的产生,以控制 BeWo 滋养层细胞中的弓形虫感染。生物。重现。 92、82 (2015)。Barral-Netto, M. et al. Transforming growth factor-beta in leishmanial infection: a parasite escape mechanism. Science 257, 545–548 (1992).
巴拉尔-内托,M.等人。利什曼原虫感染中的转化生长因子-β:寄生虫逃逸机制。科学257 , 545–548 (1992)。Walther, M. et al. Upregulation of TGF-beta, FOXP3, and CD4+CD25+ regulatory T cells correlates with more rapid parasite growth in human malaria infection. Immunity 23, 287–296 (2005).
瓦尔特,M.等人。 TGF-β、FOXP3 和 CD4+CD25+ 调节性 T 细胞的上调与人类疟疾感染中寄生虫的更快生长相关。免疫23 , 287–296 (2005)。Tsutsui, N. & Kamiyama, T. Transforming growth factor beta-induced failure of resistance to infection with blood-stage Plasmodium chabaudi in mice. Infect. Immun. 67, 2306–2311 (1999).
Tsutsui, N. & Kamiyama, T. 转化生长因子β诱导小鼠对血期恰鲍迪疟原虫感染的抵抗力失败。感染。免疫。 67、2306-2311 (1999)。Akhurst, R. J., Fee, F. & Balmain, A. Localized production of TGF-beta mRNA in tumour promoter-stimulated mouse epidermis. Nature 331, 363–365 (1988).
Akhurst, RJ, Fee, F. 和 Balmain, A. 肿瘤启动子刺激的小鼠表皮中 TGF-β mRNA 的局部产生。自然331 , 363–365 (1988)。Cui, W. et al. Concerted action of TGF-beta 1 and its type II receptor in control of epidermal homeostasis in transgenic mice. Genes Dev. 9, 945–955 (1995).
崔,W.等人。 TGF-β1 及其 II 型受体在控制转基因小鼠表皮稳态中的协同作用。基因开发。 9、945-955 (1995)。Fowlis, D. J., Cui, W., Johnson, S. A., Balmain, A. & Akhurst, R. J. Altered epidermal cell growth control in vivo by inducible expression of transforming growth factor beta 1 in the skin of transgenic mice. Cell Growth Differ. 7, 679–687 (1996).
Fowlis, DJ、Cui, W.、Johnson, SA、Balmain, A. 和 Akhurst, RJ 通过在转基因小鼠皮肤中诱导表达转化生长因子 β 1 来改变体内表皮细胞生长控制。细胞生长不同。 7、679-687 (1996)。Boulanger, C. A. & Smith, G. H. Reducing mammary cancer risk through premature stem cell senescence. Oncogene 20, 2264–2272 (2001).
Boulanger, CA 和 Smith, GH 通过干细胞过早衰老降低乳腺癌风险。癌基因20 , 2264–2272 (2001)。Siegel, P. M., Shu, W., Cardiff, R. D., Muller, W. J. & Massagué, J. Transforming growth factor beta signaling impairs Neu-induced mammary tumorigenesis while promoting pulmonary metastasis. Proc. Natl Acad. Sci. USA 100, 8430–8435 (2003).
Siegel, PM、Shu, W.、Cardiff, RD、Muller, WJ 和 Massagué, J。转化生长因子 β 信号传导会损害 Neu 诱导的乳腺肿瘤发生,同时促进肺转移。过程。国家科学院。科学。美国100,8430–8435 (2003)。Weeks, B. H., He, W., Olson, K. L. & Wang, X. J. Inducible expression of transforming growth factor beta1 in papillomas causes rapid metastasis. Cancer Res 61, 7435–7443 (2001).
Weeks,BH,He,W.,Olson,KL 和 Wang,XJ 乳头状瘤中转化生长因子β1 的诱导表达导致快速转移。癌症研究61 , 7435–7443 (2001)。Gobbi, H. et al. Transforming growth factor-beta and breast cancer risk in women with mammary epithelial hyperplasia. J. Natl Cancer Inst. 91, 2096–2101 (1999).
戈比,H.等人。乳腺上皮增生女性的转化生长因子-β 和乳腺癌风险。 J.国家癌症研究所。 91、2096-2101 (1999)。Goudie, D. R. et al. Multiple self-healing squamous epithelioma is caused by a disease-specific spectrum of mutations in TGFBR1. Nat. Genet 43, 365–369 (2011).
古迪,DR 等人。多发性自愈性鳞状上皮瘤是由 TGFBR1 的一系列疾病特异性突变引起的。纳特。基因43 , 365–369 (2011)。Goudie, D. Multiple Self-Healing Squamous Epithelioma (MSSE): A Digenic Trait Associated with Loss of Function Mutations in TGFBR1 and Variants at a Second Linked Locus on the Long Arm of Chromosome 9. Genes (Basel) 11, 1410 (2020).
Goudie, D. 多发性自愈鳞状上皮瘤 (MSSE):与 TGFBR1 和 9 号染色体长臂第二连锁位点变异体功能丧失突变相关的双基因特征。基因 (巴塞尔) 11 , 1410 (2020) 。Lu, S. L. et al. HNPCC associated with germline mutation in the TGF-beta type II receptor gene. Nat. Genet 19, 17–18 (1998).
卢,SL 等。 HNPCC 与 TGF-β II 型受体基因的种系突变有关。纳特。基因19 , 17–18 (1998)。Woodford-Richens, K. et al. Analysis of genetic and phenotypic heterogeneity in juvenile polyposis. Gut 46, 656–660 (2000).
伍德福德-里琴斯 (Woodford-Richens),K. 等人。幼年性息肉病的遗传和表型异质性分析。肠道46 , 656–660 (2000)。Howe, J. R. et al. The prevalence of MADH4 and BMPR1A mutations in juvenile polyposis and absence of BMPR2, BMPR1B, and ACVR1 mutations. J. Med Genet 41, 484–491 (2004).
豪,JR 等人。幼年性息肉病中 MADH4 和 BMPR1A 突变的患病率,以及 BMPR2、BMPR1B 和 ACVR1 突变的缺失。 J. Med Genet 41 , 484–491 (2004)。Engle, S. J. et al. Transforming growth factor beta1 suppresses nonmetastatic colon cancer at an early stage of tumorigenesis. Cancer Res 59, 3379–3386 (1999).
恩格尔,SJ 等人。转化生长因子β1 可在肿瘤发生的早期阶段抑制非转移性结肠癌。癌症研究59 , 3379–3386 (1999)。Glick, A. B. et al. Loss of expression of transforming growth factor beta in skin and skin tumors is associated with hyperproliferation and a high risk for malignant conversion. Proc. Natl Acad. Sci. USA 90, 6076–6080 (1993).
格利克,AB 等人。皮肤和皮肤肿瘤中转化生长因子β表达的丧失与过度增殖和恶性转化的高风险有关。过程。国家科学院。科学。美国90,6076–6080 (1993)。Forrester, E. et al. Effect of conditional knockout of the type II TGF-beta receptor gene in mammary epithelia on mammary gland development and polyomavirus middle T antigen induced tumor formation and metastasis. Cancer Res 65, 2296–2302 (2005).
福雷斯特,E.等人。乳腺上皮II型TGF-β受体基因条件性敲除对乳腺发育和多瘤病毒中T抗原诱导肿瘤形成和转移的影响。癌症研究65 , 2296–2302 (2005)。Muñoz, N. M. et al. Transforming growth factor beta receptor type II inactivation induces the malignant transformation of intestinal neoplasms initiated by Apc mutation. Cancer Res 66, 9837–9844 (2006).
穆尼奥斯,NM 等人。转化生长因子β受体II型失活诱导Apc突变引发的肠道肿瘤恶性转化。癌症研究66 , 9837–9844 (2006)。Ijichi, H. et al. Aggressive pancreatic ductal adenocarcinoma in mice caused by pancreas-specific blockade of transforming growth factor-beta signaling in cooperation with active Kras expression. Genes Dev. 20, 3147–3160 (2006).
Ijichi,H.等人。由胰腺特异性阻断转化生长因子-β信号传导与活跃的 Kras 表达引起的小鼠侵袭性胰腺导管腺癌。基因开发。 20、3147-3160 (2006)。Lu, S. L. et al. Loss of transforming growth factor-beta type II receptor promotes metastatic head-and-neck squamous cell carcinoma. Genes Dev. 20, 1331–1342 (2006).
卢,SL 等。转化生长因子-β II 型受体的丧失会促进转移性头颈鳞状细胞癌。基因开发。 20、1331-1342 (2006)。Bian, Y. et al. Progressive tumor formation in mice with conditional deletion of TGF-beta signaling in head and neck epithelia is associated with activation of the PI3K/Akt pathway. Cancer Res 69, 5918–5926 (2009).
卞,Y.等人。在头颈部上皮细胞中条件性删除 TGF-β 信号传导的小鼠中,进行性肿瘤的形成与 PI3K/Akt 通路的激活有关。癌症研究69 , 5918–5926 (2009)。Guasch, G. et al. Loss of TGFbeta signaling destabilizes homeostasis and promotes squamous cell carcinomas in stratified epithelia. Cancer Cell 12, 313–327 (2007).
Guasch,G.等人。 TGFbeta 信号传导的丧失会破坏体内平衡并促进复层上皮中的鳞状细胞癌。癌细胞12 , 313–327 (2007)。Go, C. et al. Aberrant cell cycle progression contributes to the early-stage accelerated carcinogenesis in transgenic epidermis expressing the dominant negative TGFbetaRII. Oncogene 19, 3623–3631 (2000).
去,C.等人。异常的细胞周期进程导致表达显性失活 TGFbetaRII 的转基因表皮的早期加速致癌作用。癌基因19 , 3623–3631 (2000)。Amendt, C., Schirmacher, P., Weber, H. & Blessing, M. Expression of a dominant negative type II TGF-beta receptor in mouse skin results in an increase in carcinoma incidence and an acceleration of carcinoma development. Oncogene 17, 25–34 (1998).
Amendt, C.、Schirmacher, P.、Weber, H. 和 Blessing, M。小鼠皮肤中显性失活 II 型 TGF-β 受体的表达导致癌症发病率增加和癌症发展加速。癌基因17 , 25–34 (1998)。Kanzler, S. et al. Hepatocellular expression of a dominant-negative mutant TGF-beta type II receptor accelerates chemically induced hepatocarcinogenesis. Oncogene 20, 5015–5024 (2001).
Kanzler,S.等人。肝细胞表达显性失活突变型 TGF-β II 型受体可加速化学诱导的肝癌发生。癌基因20 , 5015–5024 (2001)。Hahm, K. B. et al. Conditional loss of TGF-beta signalling leads to increased susceptibility to gastrointestinal carcinogenesis in mice. Aliment Pharm. Ther. 16, 115–127 (2002).
哈姆,KB 等人。 TGF-β信号有条件丧失会导致小鼠胃肠道癌变的易感性增加。营养制药。瑟尔。 16、115-127 (2002)。Biswas, S. et al. Transforming growth factor beta receptor type II inactivation promotes the establishment and progression of colon cancer. Cancer Res 64, 4687–4692 (2004).
比斯瓦斯,S.等人。转化生长因子β受体II型失活促进结肠癌的形成和进展。癌症研究64 , 4687–4692 (2004)。Gorska, A. E. et al. Transgenic mice expressing a dominant-negative mutant type II transforming growth factor-beta receptor exhibit impaired mammary development and enhanced mammary tumor formation. Am. J. Pathol. 163, 1539–1549 (2003).
戈尔斯卡,AE 等人。表达显性失活突变型 II 型转化生长因子-β 受体的转基因小鼠表现出乳腺发育受损和乳腺肿瘤形成增强。是。 J.帕索尔. 163、1539-1549 (2003)。Zhu, Y., Richardson, J. A., Parada, L. F. & Graff, J. M. Smad3 mutant mice develop metastatic colorectal cancer. Cell 94, 703–714 (1998).
Zhu, Y.、Richardson, JA、Parada、LF 和 Graff、JM Smad3 突变小鼠患上转移性结直肠癌。细胞94 , 703–714 (1998)。Wolfraim, L. A. et al. Loss of Smad3 in acute T-cell lymphoblastic leukemia. N. Engl. J. Med 351, 552–559 (2004).
沃尔夫莱姆,洛杉矶等人。急性 T 细胞淋巴细胞白血病中 Smad3 的缺失。 N. 英格兰。医学杂志351 , 552–559 (2004)。Takaku, K. et al. Gastric and duodenal polyps in Smad4 (Dpc4) knockout mice. Cancer Res 59, 6113–6117 (1999).
高久,K.等人。 Smad4 (Dpc4) 敲除小鼠的胃和十二指肠息肉。癌症研究59 , 6113–6117 (1999)。Bardeesy, N. et al. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev. 20, 3130–3146 (2006).
巴迪西,N.等人。 Smad4 对于正常胰腺发育是可有可无的,但对于胰腺癌的进展和肿瘤生物学至关重要。基因开发。 20、3130-3146 (2006)。Alberici, P. et al. Smad4 haploinsufficiency in mouse models for intestinal cancer. Oncogene 25, 1841–1851 (2006).
阿尔贝里奇,P.等人。肠癌小鼠模型中的 Smad4 单倍体不足。致癌基因25,1841–1851 (2006)。Xu, X. et al. Haploid loss of the tumor suppressor Smad4/Dpc4 initiates gastric polyposis and cancer in mice. Oncogene 19, 1868–1874 (2000).
徐,X.等人。肿瘤抑制因子 Smad4/Dpc4 的单倍体缺失会引发小鼠胃息肉病和癌症。癌基因19,1868-1874 (2000)。Braun, L., Dürst, M., Mikumo, R., Crowley, A. & Robinson, M. Regulation of growth and gene expression in human papillomavirus-transformed keratinocytes by transforming growth factor-beta: implications for the control of papillomavirus infection. Mol. Carcinog. 6, 100–111 (1992).
Braun,L.,Dürst,M.,Mikumo,R.,Crowley,A.&Robinson,M。通过转化生长因子-β调节人乳头瘤病毒转化的角质形成细胞的生长和基因表达:对控制乳头瘤病毒感染的影响。摩尔。致癌。 6,100-111 (1992)。Souza, R. F. et al. A transforming growth factor beta 1 receptor type II mutation in ulcerative colitis-associated neoplasms. Gastroenterology 112, 40–45 (1997).
苏扎,RF 等人。溃疡性结肠炎相关肿瘤中转化生长因子β1受体II型突变。胃肠病学112 , 40–45 (1997)。Kim, B. G. et al. Smad4 signalling in T cells is required for suppression of gastrointestinal cancer. Nature 441, 1015–1019 (2006).
金,BG 等人。 T 细胞中的 Smad4 信号传导是抑制胃肠道癌症所必需的。自然441 , 1015–1019 (2006)。Matsuzaki, K. et al. Chronic inflammation associated with hepatitis C virus infection perturbs hepatic transforming growth factor beta signaling, promoting cirrhosis and hepatocellular carcinoma. Hepatology 46, 48–57 (2007).
松崎,K.等人。与丙型肝炎病毒感染相关的慢性炎症扰乱肝脏转化生长因子β信号传导,促进肝硬化和肝细胞癌。肝病学46 , 48–57 (2007)。Achyut, B. R. et al. Inflammation-mediated genetic and epigenetic alterations drive cancer development in the neighboring epithelium upon stromal abrogation of TGF-β signaling. PLoS Genet 9, e1003251 (2013).
Achyut,BR 等人。当间质消除 TGF-β 信号传导时,炎症介导的遗传和表观遗传改变会驱动邻近上皮细胞发生癌症。 PLoS Genet 9 ,e1003251 (2013)。Glick, A. B., Weinberg, W. C., Wu, I. H., Quan, W. & Yuspa, S. H. Transforming growth factor beta 1 suppresses genomic instability independent of a G1 arrest, p53, and Rb. Cancer Res 56, 3645–3650 (1996).
Glick, AB、Weinberg, WC、Wu, IH、Quan, W. 和 Yuspa, SH 转化生长因子 beta 1 可抑制基因组不稳定性,与 G1 停滞、p53 和 Rb 无关。癌症研究56 , 3645–3650 (1996)。Katakura, Y., Nakata, E., Miura, T. & Shirahata, S. Transforming growth factor beta triggers two independent-senescence programs in cancer cells. Biochem Biophys. Res Commun. 255, 110–115 (1999).
Katakura,Y.,Nakata,E.,Miura,T.和Shirahata,S。转化生长因子β触发癌细胞中的两个独立的衰老程序。生物化学生物物理学。资源通讯。 255、110-115 (1999)。Bhowmick, N. A. et al. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science 303, 848–851 (2004).
博米克,NA 等人。成纤维细胞中的 TGF-β 信号传导调节邻近上皮细胞的致癌潜力。科学303 , 848–851 (2004)。Moustakas, A. & Kardassis, D. Regulation of the human p21/WAF1/Cip1 promoter in hepatic cells by functional interactions between Sp1 and Smad family members. Proc. Natl Acad. Sci. USA 95, 6733–6738 (1998).
Moustakas, A. & Kardassis, D. 通过 Sp1 和 Smad 家族成员之间的功能相互作用调节肝细胞中的人类 p21/WAF1/Cip1 启动子。过程。国家科学院。科学。美国95,6733–6738 (1998)。Kang, S. H. et al. Rapid induction of p21WAF1 but delayed down-regulation of Cdc25A in the TGF-beta-induced cell cycle arrest of gastric carcinoma cells. Br. J. Cancer 80, 1144–1149 (1999).
康,SH 等人。在 TGF-β 诱导的胃癌细胞细胞周期停滞中,p21WAF1 快速诱导,但延迟 Cdc25A 下调。 Br。 J. 癌症80 , 1144–1149 (1999)。Damdinsuren, B. et al. TGF-beta1-induced cell growth arrest and partial differentiation is related to the suppression of Id1 in human hepatoma cells. Oncol. Rep. 15, 401–408 (2006).
达姆丁苏伦 (Damdinsuren),B. 等人。 TGF-β1 诱导的细胞生长停滞和部分分化与人肝癌细胞中 Id1 的抑制有关。安科尔。报告15 , 401–408 (2006)。Hishikawa, K. et al. Connective tissue growth factor induces apoptosis in human breast cancer cell line MCF-7. J. Biol. Chem. 274, 37461–37466 (1999).
菱川,K.等人。结缔组织生长因子诱导人乳腺癌细胞系 MCF-7 凋亡。 J.Biol。化学。 274、37461–37466 (1999)。Zhang, H. et al. Involvement of programmed cell death 4 in transforming growth factor-beta1-induced apoptosis in human hepatocellular carcinoma. Oncogene 25, 6101–6112 (2006).
张,H.等人。程序性细胞死亡 4 参与转化生长因子-β1 诱导的人肝细胞癌细胞凋亡。癌基因25 , 6101–6112 (2006)。Kim, S. G. et al. Transforming growth factor-beta 1 induces apoptosis through Fas ligand-independent activation of the Fas death pathway in human gastric SNU-620 carcinoma cells. Mol. Biol. Cell 15, 420–434 (2004).
金,SG 等人。转化生长因子-β 1 通过 Fas 配体独立激活人胃 SNU-620 癌细胞中的 Fas 死亡途径来诱导细胞凋亡。摩尔。生物。细胞15 , 420–434 (2004)。Jang, C. W. et al. TGF-beta induces apoptosis through Smad-mediated expression of DAP-kinase. Nat. Cell Biol. 4, 51–58 (2002).
张,CW 等人。 TGF-β 通过 Smad 介导的 DAP 激酶表达诱导细胞凋亡。纳特。细胞生物学。 4、51-58 (2002)。David, C. J. et al. TGF-β Tumor Suppression through a Lethal EMT. Cell 164, 1015–1030 (2016).
大卫,CJ 等人。通过致命的 EMT 抑制 TGF-β 肿瘤。单元164,1015–1030 (2016)。Tachibana, I. et al. Overexpression of the TGFbeta-regulated zinc finger encoding gene, TIEG, induces apoptosis in pancreatic epithelial cells. J. Clin. Invest 99, 2365–2374 (1997).
立花,I.等人。 TGFbeta 调节的锌指编码基因 TIEG 的过度表达可诱导胰腺上皮细胞凋亡。 J.克林。投资99 , 2365–2374 (1997)。Kim, B. C., Mamura, M., Choi, K. S., Calabretta, B. & Kim, S. J. Transforming growth factor beta 1 induces apoptosis through cleavage of BAD in a Smad3-dependent mechanism in FaO hepatoma cells. Mol. Cell Biol. 22, 1369–1378 (2002).
Kim, BC、Mamura, M.、Choi, KS、Calabretta, B. 和 Kim, SJ 在 FaO 肝癌细胞中,转化生长因子 beta 1 通过 Smad3 依赖机制中的 BAD 裂解诱导细胞凋亡。摩尔。细胞生物学。 22、1369-1378 (2002)。Chipuk, J. E., Bhat, M., Hsing, A. Y., Ma, J. & Danielpour, D. Bcl-xL blocks transforming growth factor-beta 1-induced apoptosis by inhibiting cytochrome c release and not by directly antagonizing Apaf-1-dependent caspase activation in prostate epithelial cells. J. Biol. Chem. 276, 26614–26621 (2001).
Chipuk, JE, Bhat, M., Hsing, AY, Ma, J. & Danielpour, D. Bcl-xL 通过抑制细胞色素 c 释放而不是直接拮抗 Apaf-1 依赖性来阻断转化生长因子-β 1 诱导的细胞凋亡前列腺上皮细胞中半胱天冬酶的激活。 J.Biol。化学。 276、26614–26621 (2001)。Spender, L. C. et al. TGF-beta induces apoptosis in human B cells by transcriptional regulation of BIK and BCL-XL. Cell Death Differ. 16, 593–602 (2009).
斯彭德,LC 等人。 TGF-β 通过 BIK 和 BCL-XL 的转录调节诱导人 B 细胞凋亡。细胞死亡不同。 16、593-602 (2009)。Ohgushi, M. et al. Transforming growth factor beta-dependent sequential activation of Smad, Bim, and caspase-9 mediates physiological apoptosis in gastric epithelial cells. Mol. Cell Biol. 25, 10017–10028 (2005).
Ohgushi,M.等人。转化生长因子 β 依赖性的 Smad、Bim 和 caspase-9 的连续激活介导胃上皮细胞的生理性凋亡。摩尔。细胞生物学。 25、10017-10028 (2005)。Saltzman, A. et al. Transforming growth factor-beta-mediated apoptosis in the Ramos B-lymphoma cell line is accompanied by caspase activation and Bcl-XL downregulation. Exp. Cell Res 242, 244–254 (1998).
萨尔茨曼,A.等人。 Ramos B 淋巴瘤细胞系中转化生长因子-β 介导的细胞凋亡伴随着 caspase 激活和 Bcl-XL 下调。过期。细胞研究242 , 244–254 (1998)。Kanamaru, C., Yasuda, H. & Fujita, T. Involvement of Smad proteins in TGF-beta and activin A-induced apoptosis and growth inhibition of liver cells. Hepatol. Res 23, 211–219 (2002).
Kanamaru, C.、Yasuda, H. 和 Fujita, T. Smad 蛋白参与 TGF-β 和激活素 A 诱导的肝细胞凋亡和生长抑制。肝醇。第 23 号决议,211-219(2002 年)。Bakhshayesh, M., Zaker, F., Hashemi, M., Katebi, M. & Solaimani, M. TGF- β1-mediated apoptosis associated with SMAD-dependent mitochondrial Bcl-2 expression. Clin. Lymphoma Myeloma Leuk. 12, 138–143 (2012).
Bakhshayesh, M.、Zaker, F.、Hashemi, M.、Katebi, M. & Solaimani, M. TGF-β1 介导的细胞凋亡与 SMAD 依赖性线粒体 Bcl-2 表达相关。临床。淋巴瘤骨髓瘤白细胞。 12、138-143 (2012)。Cui, W., Kemp, C. J., Duffie, E., Balmain, A. & Akhurst, R. J. Lack of transforming growth factor-beta 1 expression in benign skin tumors of p53null mice is prognostic for a high risk of malignant conversion. Cancer Res 54, 5831–5836 (1994).
Cui, W.、Kemp, CJ、Duffie, E.、Balmain, A. 和 Akhurst, RJ p53null 小鼠良性皮肤肿瘤中转化生长因子-β 1 表达的缺乏预示着恶性转化的高风险。癌症研究54 , 5831–5836 (1994)。Cui, W. et al. TGFbeta1 inhibits the formation of benign skin tumors, but enhances progression to invasive spindle carcinomas in transgenic mice. Cell 86, 531–542 (1996).
崔,W.等人。 TGFbeta1 抑制良性皮肤肿瘤的形成,但在转基因小鼠中促进侵袭性纺锤体癌的进展。细胞86 , 531–542 (1996)。Capocasale, R. J. et al. Reduced surface expression of transforming growth factor beta receptor type II in mitogen-activated T cells from Sézary patients. Proc. Natl Acad. Sci. USA 92, 5501–5505 (1995).
卡波卡萨莱,RJ 等人。 Sézary 患者的有丝分裂原激活 T 细胞中 II 型转化生长因子 β 受体的表面表达减少。过程。国家科学院。科学。美国92,5501–5505 (1995)。Kadin, M. E. et al. Loss of receptors for transforming growth factor beta in human T-cell malignancies. Proc. Natl Acad. Sci. USA 91, 6002–6006 (1994).
卡丁,ME 等人。人类 T 细胞恶性肿瘤中转化生长因子 β 受体的丧失。过程。国家科学院。科学。美国91,6002–6006 (1994)。Fukai, Y. et al. Reduced expression of transforming growth factor-beta receptors is an unfavorable prognostic factor in human esophageal squamous cell carcinoma. Int J. Cancer 104, 161–166 (2003).
Fukai,Y.等人。转化生长因子-β受体表达降低是人食管鳞状细胞癌的不利预后因素。国际癌症杂志104 , 161–166 (2003)。Tanaka, S., Mori, M., Mafune, K., Ohno, S. & Sugimachi, K. A dominant negative mutation of transforming growth factor-beta receptor type II gene in microsatellite stable oesophageal carcinoma. Br. J. Cancer 82, 1557–1560 (2000).
Tanaka, S.、Mori, M.、Mafune, K.、Ohno, S. 和 Sugimachi, K. 微卫星稳定食管癌中转化生长因子-β 受体 II 型基因的显性负突变。 Br。 J. 癌症82 , 1557–1560 (2000)。Souza, R. F. et al. Alterations of transforming growth factor-beta 1 receptor type II occur in ulcerative colitis-associated carcinomas, sporadic colorectal neoplasms, and esophageal carcinomas, but not in gastric neoplasms. Hum. Cell 9, 229–236 (1996).
苏扎,RF 等人。 II 型转化生长因子-β1 受体的改变发生在溃疡性结肠炎相关癌、散发性结直肠肿瘤和食管癌中,但不发生在胃肿瘤中。哼。细胞9,229-236 (1996)。Myeroff, L. L. et al. A transforming growth factor beta receptor type II gene mutation common in colon and gastric but rare in endometrial cancers with microsatellite instability. Cancer Res 55, 5545–5547 (1995).
迈耶罗夫,LL 等人。转化生长因子β受体II型基因突变常见于结肠和胃,但在具有微卫星不稳定性的子宫内膜癌中罕见。癌症研究55 , 5545–5547 (1995)。Grady, W. M. et al. Mutation of the type II transforming growth factor-beta receptor is coincident with the transformation of human colon adenomas to malignant carcinomas. Cancer Res 58, 3101–3104 (1998).
格雷迪,WM 等人。 II型转化生长因子-β受体的突变与人类结肠腺瘤向恶性肿瘤的转化同时发生。癌症研究58 , 3101–3104 (1998)。Salovaara, R. et al. Frequent loss of SMAD4/DPC4 protein in colorectal cancers. Gut 51, 56–59 (2002).
萨洛瓦拉,R.等人。结直肠癌中 SMAD4/DPC4 蛋白频繁丢失。肠道51 , 56–59 (2002)。Takagi, Y. et al. Somatic alterations of the DPC4 gene in human colorectal cancers in vivo. Gastroenterology 111, 1369–1372 (1996).
高木,Y.等人。人结直肠癌体内 DPC4 基因的体细胞改变。胃肠病学111 , 1369–1372 (1996)。Goggins, M. et al. Genetic alterations of the transforming growth factor beta receptor genes in pancreatic and biliary adenocarcinomas. Cancer Res 58, 5329–5332 (1998).
戈金斯,M.等人。胰腺癌和胆道腺癌中转化生长因子β受体基因的遗传改变。癌症研究58 , 5329–5332 (1998)。Venkatasubbarao, K. et al. Novel mutations in the polyadenine tract of the transforming growth factor beta type II receptor gene are found in a subpopulation of human pancreatic adenocarcinomas. Genes Chromosomes Cancer 22, 138–144 (1998).
Venkatasubbarao,K. 等人。在人类胰腺癌亚群中发现了转化生长因子β II 型受体基因聚腺嘌呤区的新突变。基因染色体癌症22 , 138–144 (1998)。Imai, Y., Tsurutani, N., Oda, H., Inoue, T. & Ishikawa, T. Genetic instability and mutation of the TGF-beta-receptor-II gene in ampullary carcinomas. Int J. Cancer 76, 407–411 (1998).
Imai, Y.、Tsurutani, N.、Oda, H.、Inoue, T. 和 Ishikawa, T.壶腹癌中 TGF-β-受体-II 基因的遗传不稳定性和突变。国际癌症杂志76 , 407–411 (1998)。Lazzereschi, D. et al. Human malignant thyroid tumors displayed reduced levels of transforming growth factor beta receptor type II messenger RNA and protein. Cancer Res 57, 2071–2076 (1997).
拉泽雷斯基 (Lazzereschi),D. 等人。人类恶性甲状腺肿瘤显示转化生长因子β受体II型信使RNA和蛋白质水平降低。癌症研究57,2071–2076 (1997)。Kim, I. Y. et al. Loss of expression of transforming growth factor-beta receptors is associated with poor prognosis in prostate cancer patients. Clin. Cancer Res 4, 1625–1630 (1998).
金,IY 等人。转化生长因子-β受体表达的丧失与前列腺癌患者的不良预后相关。临床。癌症研究4,1625–1630 (1998)。Guo, Y., Jacobs, S. C. & Kyprianou, N. Down-regulation of protein and mRNA expression for transforming growth factor-beta (TGF-beta1) type I and type II receptors in human prostate cancer. Int J. Cancer 71, 573–579 (1997).
Guo,Y.,Jacobs,SC 和 Kyprianou,N。人前列腺癌中转化生长因子-β (TGF-β1) I 型和 II 型受体的蛋白质和 mRNA 表达下调。国际癌症杂志71 , 573–579 (1997)。Gobbi, H. et al. Loss of expression of transforming growth factor beta type II receptor correlates with high tumour grade in human breast in-situ and invasive carcinomas. Histopathology 36, 168–177 (2000).
戈比,H.等人。转化生长因子β II 型受体表达的缺失与人乳腺原位癌和浸润性癌的高肿瘤级别相关。组织病理学36 , 168–177 (2000)。Lynch, M. A. et al. Mutational analysis of the transforming growth factor beta receptor type II gene in human ovarian carcinoma. Cancer Res 58, 4227–4232 (1998).
林奇,马萨诸塞州等人。人卵巢癌转化生长因子β受体II型基因的突变分析。癌症研究58 , 4227–4232 (1998)。Chen, T. et al. Novel inactivating mutations of transforming growth factor-beta type I receptor gene in head-and-neck cancer metastases. Int J. Cancer 93, 653–661 (2001).
陈,T.等人。头颈癌转移中转化生长因子-β I 型受体基因的新型失活突变。国际癌症杂志93 , 653–661 (2001)。Wang, D. et al. Mutation and downregulation of the transforming growth factor beta type II receptor gene in primary squamous cell carcinomas of the head and neck. Carcinogenesis 18, 2285–2290 (1997).
王,D.等人。头颈原发性鳞状细胞癌中转化生长因子βII型受体基因的突变和下调。癌变18,2285–2290 (1997)。Qiu, W., Schönleben, F., Li, X. & Su, G. H. Disruption of transforming growth factor beta-Smad signaling pathway in head and neck squamous cell carcinoma as evidenced by mutations of SMAD2 and SMAD4. Cancer Lett. 245, 163–170 (2007).
Qiu, W.、Schönleben, F.、Li, X. 和 Su, GH 头颈鳞状细胞癌中转化生长因子 β-Smad 信号通路的破坏,由 SMAD2 和 SMAD4 突变证明。癌症快报。 245 , 163–170 (2007)。Xie, W. et al. Frequent alterations of Smad signaling in human head and neck squamous cell carcinomas: a tissue microarray analysis. Oncol. Res 14, 61–73 (2003).
谢,W.等人。人头颈鳞状细胞癌中 Smad 信号的频繁改变:组织微阵列分析。安科尔。第 14 号决议,61-73 (2003)。Sun, L. et al. Expression of transforming growth factor beta type II receptor leads to reduced malignancy in human breast cancer MCF-7 cells. J. Biol. Chem. 269, 26449–26455 (1994).
孙,L.等人。转化生长因子β II 型受体的表达可降低人乳腺癌MCF-7 细胞的恶性程度。 J.Biol。化学。 269、26449–26455 (1994)。Steiner, M. S. & Barrack, E. R. Transforming growth factor-beta 1 overproduction in prostate cancer: effects on growth in vivo and in vitro. Mol. Endocrinol. 6, 15–25 (1992).
Steiner,MS 和 Barrack,ER 前列腺癌中转化生长因子-β 1 的过量产生:对体内和体外生长的影响。摩尔。内分泌。 6、15-25 (1992)。Chang, H. L. et al. Increased transforming growth factor beta expression inhibits cell proliferation in vitro, yet increases tumorigenicity and tumor growth of Meth A sarcoma cells. Cancer Res 53, 4391–4398 (1993).
张,HL 等人。转化生长因子β表达增加可抑制体外细胞增殖,但会增加 Meth A 肉瘤细胞的致瘤性和肿瘤生长。癌症研究53 , 4391–4398 (1993)。Kleeff, J. et al. The TGF-beta signaling inhibitor Smad7 enhances tumorigenicity in pancreatic cancer. Oncogene 18, 5363–5372 (1999).
克莱夫,J.等人。 TGF-β 信号抑制剂 Smad7 增强胰腺癌的致瘤性。癌基因18 , 5363–5372 (1999)。Datto, M. B., Hu, P. P., Kowalik, T. F., Yingling, J. & Wang, X. F. The viral oncoprotein E1A blocks transforming growth factor beta-mediated induction of p21/WAF1/Cip1 and p15/INK4B. Mol. Cell Biol. 17, 2030–2037 (1997).
Datto, MB, Hu, PP, Kowalik, TF, Yingling, J. & Wang, XF 病毒癌蛋白 E1A 阻断转化生长因子 β 介导的 p21/WAF1/Cip1 和 p15/INK4B 诱导。摩尔。细胞生物学。 17、2030-2037 (1997)。Missero, C. Ramon y Cajal, S. & Dotto, G. P. Escape from transforming growth factor beta control and oncogene cooperation in skin tumor development. Proc. Natl Acad. Sci. USA 88, 9613–9617 (1991).
Missero, C. Ramon y Cajal, S. 和 Dotto, GP 摆脱皮肤肿瘤发展中转化生长因子 β 的控制和癌基因的合作。过程。国家科学院。科学。美国88 , 9613–9617 (1991)。Kurokawa, M. et al. The oncoprotein Evi-1 represses TGF-beta signalling by inhibiting Smad3. Nature 394, 92–96 (1998).
黑川,M.等人。癌蛋白 Evi-1 通过抑制 Smad3 来抑制 TGF-β 信号传导。自然394 , 92–96 (1998)。Alexandrow, M. G., Kawabata, M., Aakre, M. & Moses, H. L. Overexpression of the c-Myc oncoprotein blocks the growth-inhibitory response but is required for the mitogenic effects of transforming growth factor beta 1. Proc. Natl Acad. Sci. USA 92, 3239–3243 (1995).
Alexandrow, MG、Kawabata, M.、Aakre, M. 和 Moses, HL c-Myc 癌蛋白的过度表达会阻断生长抑制反应,但对于转化生长因子 β 1 的有丝分裂作用是必需的。国家科学院。科学。美国92,3239–3243 (1995)。Schlegel, N. C. et al. Id2 suppression of p15 counters TGF-beta-mediated growth inhibition of melanoma cells. Pigment Cell Melanoma Res 22, 445–453 (2009).
北卡罗来纳州施莱格尔等人。 Id2 对 p15 的抑制可以对抗 TGF-β 介导的黑色素瘤细胞生长抑制。色素细胞黑色素瘤研究22 , 445–453 (2009)。Ewen, M. E., Oliver, C. J., Sluss, H. K., Miller, S. J. & Peeper, D. S. p53-dependent repression of CDK4 translation in TGF-beta-induced G1 cell-cycle arrest. Genes Dev. 9, 204–217 (1995).
Ewen, ME, Oliver, CJ, Sluss, HK, Miller, SJ & Peeper, DS 在 TGF-β 诱导的 G1 细胞周期停滞中 p53 依赖性 CDK4 翻译抑制。基因开发。 9、204-217 (1995)。Kretzschmar, M., Doody, J., Timokhina, I. & Massague, J. A mechanism of repression of TGFbeta/ Smad signaling by oncogenic Ras. Genes Dev. 13, 804–816 (1999).
Kretzschmar, M.、Doody, J.、Timokhina, I. 和 Massague, J. 致癌 Ras 抑制 TGFbeta/Smad 信号传导的机制。基因开发。 13、804-816 (1999)。Dalal, B. I., Keown, P. A. & Greenberg, A. H. Immunocytochemical localization of secreted transforming growth factor-beta 1 to the advancing edges of primary tumors and to lymph node metastases of human mammary carcinoma. Am. J. Pathol. 143, 381–389 (1993).
Dalal, BI, Keown, PA 和 Greenberg, AH 分泌型转化生长因子-β1 免疫细胞化学定位至原发肿瘤的前进边缘和人乳腺癌的淋巴结转移。是。 J.帕索尔. 143、381-389 (1993)。Walker, R. A. & Dearing, S. J. Transforming growth factor beta 1 in ductal carcinoma in situ and invasive carcinomas of the breast. Eur. J. Cancer 28, 641–644 (1992).
Walker, RA 和 Dearing, SJ 转化生长因子 β1 在导管原位癌和浸润性乳腺癌中的作用。欧元。 J. 癌症28 , 641–644 (1992)。Friess, H. et al. Enhanced expression of transforming growth factor beta isoforms in pancreatic cancer correlates with decreased survival. Gastroenterology 105, 1846–1856 (1993).
弗里斯,H.等人。胰腺癌中转化生长因子β亚型的表达增强与生存率降低相关。胃肠病学105,1846–1856 (1993)。Wagner, M., Kleeff, J., Friess, H., Büchler, M. W. & Korc, M. Enhanced expression of the type II transforming growth factor-beta receptor is associated with decreased survival in human pancreatic cancer. Pancreas 19, 370–376 (1999).
Wagner, M.、Kleeff, J.、Friess, H.、Büchler, MW 和 Korc, M. II 型转化生长因子-β 受体表达增强与人类胰腺癌生存率降低相关。胰腺19 , 370–376 (1999)。Tateishi, M. et al. The progression of invasiveness regarding the role of transforming growth factor beta receptor type II in gastric cancer. Eur. J. Surg. Oncol. 26, 377–380 (2000).
立石,M.等人。关于 II 型转化生长因子β受体在胃癌中的作用的侵袭性进展。欧元。 J.外科医生。安科尔。 26 , 377–380 (2000)。Welch, D. R., Fabra, A. & Nakajima, M. Transforming growth factor beta stimulates mammary adenocarcinoma cell invasion and metastatic potential. Proc. Natl Acad. Sci. USA 87, 7678–7682 (1990).
Welch, DR、Fabra, A. 和 Nakajima, M。转化生长因子 β 刺激乳腺癌细胞侵袭和转移潜力。过程。国家科学院。科学。美国87 , 7678–7682 (1990)。Tang, B. et al. TGF-beta switches from tumor suppressor to prometastatic factor in a model of breast cancer progression. J. Clin. Invest 112, 1116–1124 (2003).
唐,B.等人。在乳腺癌进展模型中,TGF-β 从肿瘤抑制因子转变为促转移因子。 J.克林。投资112 , 1116–1124 (2003)。Oft, M., Heider, K. H. & Beug, H. TGFbeta signaling is necessary for carcinoma cell invasiveness and metastasis. Curr. Biol. 8, 1243–1252 (1998).
Oft, M., Heider, KH 和 Beug, H. TGFbeta 信号传导对于癌细胞的侵袭和转移是必需的。电流。生物。 8、1243-1252 (1998)。Han, G. et al. Distinct mechanisms of TGF-beta1-mediated epithelial-to-mesenchymal transition and metastasis during skin carcinogenesis. J. Clin. Invest 115, 1714–1723 (2005).
汉,G.等人。皮肤癌发生过程中 TGF-β1 介导的上皮间质转化和转移的独特机制。 J.克林。投资115 , 1714–1723 (2005)。Caulín, C., Scholl, F. G., Frontelo, P., Gamallo, C. & Quintanilla, M. Chronic exposure of cultured transformed mouse epidermal cells to transforming growth factor-beta 1 induces an epithelial-mesenchymal transdifferentiation and a spindle tumoral phenotype. Cell Growth Differ. 6, 1027–1035 (1995).
Caulín, C.、Scholl, FG、Frontelo, P.、Gamallo, C. 和 Quintanilla, M. 培养的转化小鼠表皮细胞长期暴露于转化生长因子-β 1 会诱导上皮-间质转分化和纺锤体肿瘤表型。细胞生长不同。 6、1027-1035 (1995)。Cheng, J. C., Auersperg, N. & Leung, P. C. TGF-beta induces serous borderline ovarian tumor cell invasion by activating EMT but triggers apoptosis in low-grade serous ovarian carcinoma cells. PLoS One 7, e42436 (2012).
Cheng, JC, Auersperg, N. 和 Leung, PC TGF-β 通过激活 EMT 诱导浆液性交界性卵巢肿瘤细胞侵袭,但引发低级别浆液性卵巢癌细胞凋亡。 PLoS One 7 ,e42436 (2012)。Qiao, B., Johnson, N. W. & Gao, J. Epithelial-mesenchymal transition in oral squamous cell carcinoma triggered by transforming growth factor-beta1 is Snail family-dependent and correlates with matrix metalloproteinase-2 and -9 expressions. Int J. Oncol. 37, 663–668 (2010).
Qiao,B.,Johnson,NW 和Gao,J。转化生长因子-β1 触发的口腔鳞状细胞癌中的上皮-间质转化是 Snail 家族依赖性的,并且与基质金属蛋白酶-2 和 -9 的表达相关。 Int J.Oncol。 37、663-668 (2010)。Vincent, T. et al. A SNAIL1-SMAD3/4 transcriptional repressor complex promotes TGF-beta mediated epithelial-mesenchymal transition. Nat. Cell Biol. 11, 943–950 (2009).
文森特,T.等人。 SNAIL1-SMAD3/4 转录抑制复合物促进 TGF-β 介导的上皮间质转化。纳特。细胞生物学。 11 , 943–950 (2009)。Arrick, B. A. et al. Altered metabolic and adhesive properties and increased tumorigenesis associated with increased expression of transforming growth factor beta 1. J. Cell Biol. 118, 715–726 (1992).
阿里克,BA 等。代谢和粘附特性的改变以及与转化生长因子 β 1 表达增加相关的肿瘤发生增加。 J. Cell Biol。 118、715–726 (1992)。Tu, W. H. et al. The loss of TGF-beta signaling promotes prostate cancer metastasis. Neoplasia 5, 267–277 (2003).
涂,WH 等人。 TGF-β信号传导的丧失会促进前列腺癌的转移。肿瘤5 , 267–277 (2003)。Huntley, S. P. et al. Attenuated type II TGF-beta receptor signalling in human malignant oral keratinocytes induces a less differentiated and more aggressive phenotype that is associated with metastatic dissemination. Int J. Cancer 110, 170–176 (2004).
亨特利,SP 等人。人类恶性口腔角质形成细胞中 II 型 TGF-β 受体信号减弱会诱导与转移性播散相关的分化程度较低且更具侵袭性的表型。国际癌症杂志110 , 170–176 (2004)。Hoosein, N. M. et al. Differential sensitivity of subclasses of human colon carcinoma cell lines to the growth inhibitory effects of transforming growth factor-beta 1. Exp. Cell Res 181, 442–453 (1989).
Hoosein,NM 等人。人结肠癌细胞系亚类对转化生长因子-β 1 的生长抑制作用的不同敏感性。实验。细胞研究181 , 442–453 (1989)。Takaku, K. et al. Intestinal tumorigenesis in compound mutant mice of both Dpc4 (Smad4) and Apc genes. Cell 92, 645–656 (1998).
高久,K.等人。 Dpc4 (Smad4) 和 Apc 基因复合突变小鼠的肠道肿瘤发生。细胞92,645–656 (1998)。Lewis, M. P. et al. Tumour-derived TGF-beta1 modulates myofibroblast differentiation and promotes HGF/SF-dependent invasion of squamous carcinoma cells. Br. J. Cancer 90, 822–832 (2004).
刘易斯,议员等。肿瘤来源的 TGF-β1 调节肌成纤维细胞分化并促进鳞状癌细胞的 HGF/SF 依赖性侵袭。 Br。癌症杂志90 , 822–832 (2004)。Stuelten, C. H. et al. Transient tumor-fibroblast interactions increase tumor cell malignancy by a TGF-Beta mediated mechanism in a mouse xenograft model of breast cancer. PLoS One 5, e9832 (2010).
斯图尔滕,CH 等人。在小鼠乳腺癌异种移植模型中,短暂的肿瘤-成纤维细胞相互作用通过 TGF-β 介导的机制增加了肿瘤细胞的恶性程度。 PLoS One 5 ,e9832 (2010)。Löhr, M. et al. Transforming growth factor-beta1 induces desmoplasia in an experimental model of human pancreatic carcinoma. Cancer Res 61, 550–555 (2001).
勒尔,M.等人。转化生长因子-β1 在人胰腺癌实验模型中诱导结缔组织形成。癌症研究61 , 550–555 (2001)。Igarashi, A., Okochi, H., Bradham, D. M. & Grotendorst, G. R. Regulation of connective tissue growth factor gene expression in human skin fibroblasts and during wound repair. Mol. Biol. Cell 4, 637–645 (1993).
Igarashi, A.、Okochi, H.、Bradham, DM 和 Grotendorst, GR 人皮肤成纤维细胞和伤口修复过程中结缔组织生长因子基因表达的调节。摩尔。生物。细胞4,637–645 (1993)。Chantry, D., Turner, M., Abney, E. & Feldmann, M. Modulation of cytokine production by transforming growth factor-beta. J. Immunol. 142, 4295–4300 (1989).
Chantry, D.、Turner, M.、Abney, E. 和 Feldmann, M. 通过转化生长因子-β 调节细胞因子的产生。 J.免疫学。 142、4295–4300 (1989)。Sieuwerts, A. M., Klijn, J. G., Henzen-Logmans, S. C. & Foekens, J. A. Cytokine-regulated urokinase-type-plasminogen-activator (uPA) production by human breast fibroblasts in vitro. Breast Cancer Res Treat. 55, 9–20 (1999).
Sieuwerts,AM,Klijn,JG,Henzen-Logmans,SC 和 Foekens,JA 人乳腺成纤维细胞体外产生细胞因子调节的尿激酶型纤溶酶原激活剂 (uPA)。乳腺癌研究治疗。 55、9-20 (1999)。Cheng, N. et al. Loss of TGF-beta type II receptor in fibroblasts promotes mammary carcinoma growth and invasion through upregulation of TGF-alpha-, MSP- and HGF-mediated signaling networks. Oncogene 24, 5053–5068 (2005).
程,N.等人。成纤维细胞中 TGF-β II 型受体的缺失会通过上调 TGF-α、MSP 和 HGF 介导的信号网络促进乳腺癌生长和侵袭。癌基因24 , 5053–5068 (2005)。Zeisberg, E. M., Potenta, S., Xie, L., Zeisberg, M. & Kalluri, R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res 67, 10123–10128 (2007).
Zeisberg, EM、Potenta, S.、Xie, L.、Zeisberg, M. 和 Kalluri, R. 发现内皮到间质转化作为癌症相关成纤维细胞的来源。癌症研究67 , 10123–10128 (2007)。Go, C., Li, P. & Wang, X. J. Blocking transforming growth factor beta signaling in transgenic epidermis accelerates chemical carcinogenesis: a mechanism associated with increased angiogenesis. Cancer Res 59, 2861–2868 (1999).
Go, C.、Li, P. 和 Wang, XJ 阻断转基因表皮中的转化生长因子 β 信号传导会加速化学致癌作用:一种与血管生成增加相关的机制。癌症研究59 , 2861–2868 (1999)。Ueki, N. et al. Excessive production of transforming growth-factor beta 1 can play an important role in the development of tumorigenesis by its action for angiogenesis: validity of neutralizing antibodies to block tumor growth. Biochim Biophys. Acta 1137, 189–196 (1992).
植木,N.等人。转化生长因子β1的过量产生可以通过其血管生成作用在肿瘤发生的发展中发挥重要作用:中和抗体阻止肿瘤生长的有效性。生物化学生物物理学。 Acta 1137 , 189–196 (1992)。Padua, D. et al. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell 133, 66–77 (2008).
帕多瓦,D.等人。 TGFbeta 通过血管生成素样启动乳腺肿瘤的肺转移播种 4. Cell 133 , 66–77 (2008)。Haak-Frendscho, M., Wynn, T. A., Czuprynski, C. J. & Paulnock, D. Transforming growth factor-beta 1 inhibits activation of macrophage cell line RAW 264.7 for cell killing. Clin. Exp. Immunol. 82, 404–410 (1990).
Haak-Frendscho, M.、Wynn, TA、Czuprynski, CJ 和 Paulnock, D. 转化生长因子-β 1 抑制巨噬细胞系 RAW 264.7 的活化以杀死细胞。临床。过期。免疫学。 82、404-410 (1990)。Torre-Amione, G. et al. A highly immunogenic tumor transfected with a murine transforming growth factor type beta 1 cDNA escapes immune surveillance. Proc. Natl Acad. Sci. USA 87, 1486–1490 (1990).
托雷-阿米奥内,G.等人。用鼠转化生长因子 β1 型 cDNA 转染的高免疫原性肿瘤可以逃避免疫监视。过程。国家科学院。科学。美国87 , 1486–1490 (1990)。Donkor, M. K., Sarkar, A. & Li, M. O. Tgf-β1 produced by activated CD4(+) T Cells Antagonizes T Cell Surveillance of Tumor Development. Oncoimmunology 1, 162–171 (2012).
Donkor, MK、Sarkar, A. 和 Li, MO 由激活的 CD4(+) T 细胞产生的 Tgf-β1 拮抗 T 细胞对肿瘤发展的监视。肿瘤免疫学1 , 162–171 (2012)。Sarkar, A., Donkor, M. K. & Li, M. O. T cell- but not tumor cell-produced TGF-β1 promotes the development of spontaneous mammary cancer. Oncotarget 2, 1339–1351 (2011).
Sarkar, A., Donkor, MK & Li, MO T 细胞而非肿瘤细胞产生的 TGF-β1 促进自发性乳腺癌的发展。 Oncotarget 2,1339–1351 (2011)。Arteaga, C. L. et al. Anti-transforming growth factor (TGF)-beta antibodies inhibit breast cancer cell tumorigenicity and increase mouse spleen natural killer cell activity. Implications for a possible role of tumor cell/host TGF-beta interactions in human breast cancer progression. J. Clin. Invest 92, 2569–2576 (1993).
Arteaga,CL 等人。抗转化生长因子 (TGF)-β 抗体可抑制乳腺癌细胞致瘤性并增加小鼠脾脏自然杀伤细胞活性。肿瘤细胞/宿主 TGF-β 相互作用在人类乳腺癌进展中可能发挥的作用。 J.克林。投资92 , 2569–2576 (1993)。Ghiringhelli, F. et al. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J. Exp. Med 202, 919–929 (2005).
吉林盖利,F.等人。肿瘤细胞将未成熟的骨髓树突状细胞转化为 TGF-β 分泌细胞,诱导 CD4+CD25+ 调节性 T 细胞增殖。 J.Exp。医学202 , 919–929 (2005)。Hsiao, Y. W. et al. Interactions of host IL-6 and IFN-gamma and cancer-derived TGF-beta1 on MHC molecule expression during tumor spontaneous regression. Cancer Immunol. Immunother. 57, 1091–1104 (2008).
萧,YW 等人。肿瘤自发消退过程中宿主 IL-6 和 IFN-γ 以及癌症衍生的 TGF-β1 对 MHC 分子表达的相互作用。癌症免疫学。免疫疗法。 57、1091-1104 (2008)。Czarniecki, C. W., Chiu, H. H., Wong, G. H., McCabe, S. M. & Palladino, M. A. Transforming growth factor-beta 1 modulates the expression of class II histocompatibility antigens on human cells. J. Immunol. 140, 4217–4223 (1988).
Czarniecki, CW, Chiu, HH, Wong, GH, McCabe, SM 和 Palladino, MA 转化生长因子-β 1 调节人类细胞上 II 类组织相容性抗原的表达。 J.免疫学。 140、4217-4223 (1988)。Ma, D. & Niederkorn, J. Y. Transforming growth factor-beta down-regulates major histocompatibility complex class I antigen expression and increases the susceptibility of uveal melanoma cells to natural killer cell-mediated cytolysis. Immunology 86, 263–269 (1995).
Ma, D. 和 Niederkorn, JY 转化生长因子-β 下调主要组织相容性复合物 I 类抗原表达,并增加葡萄膜黑色素瘤细胞对自然杀伤细胞介导的细胞溶解作用的敏感性。免疫学86 , 263–269 (1995)。Bogdahn, U. et al. Targeted therapy for high-grade glioma with the TGF-beta2 inhibitor trabedersen: results of a randomized and controlled phase IIb study. Neuro Oncol. 13, 132–142 (2011).
博格达恩,U.等人。使用 TGF-β2 抑制剂 Trabedersen 靶向治疗高级别胶质瘤:随机对照 IIb 期研究的结果。神经肿瘤。 13、132-142 (2011)。Niu, N. K. et al. Novel targeting of PEGylated liposomes for codelivery of TGF-β1 siRNA and four antitubercular drugs to human macrophages for the treatment of mycobacterial infection: a quantitative proteomic study. Drug Des. Devel Ther. 9, 4441–4470 (2015).
牛,NK 等人。用于将 TGF-β1 siRNA 和四种抗结核药物共同递送至人巨噬细胞以治疗分枝杆菌感染的聚乙二醇化脂质体的新靶向:定量蛋白质组学研究。药物设计。开发Ther。 9、4441-4470 (2015)。Yang, Z. et al. Preparation, characterization, and in-vitro cytotoxicity of nanoliposomes loaded with anti-tubercular drugs and TGF-β1 siRNA for improving spinal tuberculosis therapy. BMC Infect. Dis. 22, 824 (2022).
杨,Z.等人。负载抗结核药物和 TGF-β1 siRNA 的纳米脂质体的制备、表征和体外细胞毒性,用于改善脊柱结核治疗。 BMC 感染。迪斯。 22、824 (2022)。Guoyou, Z. et al. Modulation of transforming growth factor-beta1 production by vector-based RNAi in hypertrophic scar fibroblasts: a therapeutic potential strategy for hypertrophic scar. J. Dermatol Sci. 48, 67–70 (2007).
郭友,Z.等。通过基于载体的 RNAi 调节肥厚性疤痕成纤维细胞中转化生长因子-β1 的产生:肥厚性疤痕的潜在治疗策略。 J. Dermatol Sci。 48、67-70 (2007)。Loiselle, A. E. et al. Development of antisense oligonucleotide (ASO) technology against Tgf-β signaling to prevent scarring during flexor tendon repair. J. Orthop. Res 33, 859–866 (2015).
洛伊塞尔,AE 等人。开发针对 Tgf-β 信号传导的反义寡核苷酸 (ASO) 技术,以防止屈肌腱修复过程中形成疤痕。 J.奥索普。第 33 号决议,859–866(2015 年)。Jeong, H. S. et al. Effect of antisense TGF-beta1 oligodeoxynucleotides in streptozotocin- induced diabetic rat kidney. J. Korean Med Sci. 19, 374–383 (2004).
郑,HS 等人。反义TGF-β1寡脱氧核苷酸对链脲佐菌素诱导的糖尿病大鼠肾的影响。 J.韩国医学科学。 19、374-383 (2004)。Isaka, Y. et al. Transforming growth factor-beta 1 antisense oligodeoxynucleotides block interstitial fibrosis in unilateral ureteral obstruction. Kidney Int 58, 1885–1892 (2000).
Isaka,Y. 等人。转化生长因子-β1 反义寡脱氧核苷酸可阻断单侧输尿管梗阻中的间质纤维化。肾脏国际58,1885-1892 (2000)。Han, D. C., Hoffman, B. B., Hong, S. W., Guo, J. & Ziyadeh, F. N. Therapy with antisense TGF-beta1 oligodeoxynucleotides reduces kidney weight and matrix mRNAs in diabetic mice. Am. J. Physiol. Ren. Physiol. 278, F628–F634 (2000).
Han,DC,Hoffman,BB,Hong,SW,Guo,J. 和 Ziyadeh,FN 使用反义 TGF-β1 寡脱氧核苷酸治疗可减轻糖尿病小鼠的肾脏重量和基质 mRNA。是。 J.生理学。任。生理学。 278 ,F628–F634(2000)。Akagi, Y. et al. Inhibition of TGF-beta 1 expression by antisense oligonucleotides suppressed extracellular matrix accumulation in experimental glomerulonephritis. Kidney Int 50, 148–155 (1996).
赤城,Y.等人。反义寡核苷酸抑制 TGF-β1 表达可抑制实验性肾小球肾炎中细胞外基质的积累。肾脏国际杂志50 , 148–155 (1996)。Giaccone, G. et al. A phase III study of belagenpumatucel-L, an allogeneic tumour cell vaccine, as maintenance therapy for non-small cell lung cancer. Eur. J. Cancer 51, 2321–2329 (2015).
贾科内,G.等人。 belagenpumatucel-L(一种同种异体肿瘤细胞疫苗)作为非小细胞肺癌维持治疗的 III 期研究。欧元。 J. 癌症51 , 2321–2329 (2015)。Olivares, J. et al. Phase I trial of TGF-beta 2 antisense GM-CSF gene-modified autologous tumor cell (TAG) vaccine. Clin. Cancer Res 17, 183–192 (2011).
奥利瓦雷斯,J.等人。 TGF-β2 反义 GM-CSF 基因修饰自体肿瘤细胞 (TAG) 疫苗的 I 期试验。临床。癌症研究17 , 183–192 (2011)。Senzer, N. et al. Phase I trial of “bi-shRNAi(furin)/GMCSF DNA/autologous tumor cell” vaccine (FANG) in advanced cancer. Mol. Ther. 20, 679–686 (2012).
森泽,N.等人。 “bi-shRNAi(弗林蛋白酶)/GMCSF DNA/自体肿瘤细胞”疫苗(FANG)治疗晚期癌症的 I 期试验。摩尔。瑟尔。 20、679-686 (2012)。Rocconi, R. P. et al. Gemogenovatucel-T (Vigil) immunotherapy demonstrates clinical benefit in homologous recombination proficient (HRP) ovarian cancer. Gynecol. Oncol. 161, 676–680 (2021).
罗科尼,RP 等人。 Gemogenovatucel-T (Vigil) 免疫疗法在同源重组 (HRP) 卵巢癌中显示出临床益处。妇科。安科尔。 161 , 676–680 (2021)。Martin, C. J. et al. Selective inhibition of TGFbeta1 activation overcomes primary resistance to checkpoint blockade therapy by altering tumor immune landscape. Sci. Transl. Med 12, eaay8456 (2020).
马丁,CJ 等人。选择性抑制 TGFbeta1 激活可通过改变肿瘤免疫景观来克服对检查点阻断治疗的主要耐药性。科学。译。医学12 ,eaay8456 (2020)。Welsh, B. T. et al. Nonclinical Development of SRK-181: An Anti-Latent TGFbeta1 Monoclonal Antibody for the Treatment of Locally Advanced or Metastatic Solid Tumors. Int J. Toxicol. 40, 226–241 (2021).
威尔士,英国电信等。 SRK-181 的非临床开发:一种抗潜伏 TGFbeta1 单克隆抗体,用于治疗局部晚期或转移性实体瘤。 Int J.Toxicol。 40 , 226–241 (2021)。Li, A. et al. Selective targeting of GARP-LTGFbeta axis in the tumor microenvironment augments PD-1 blockade via enhancing CD8(+) T cell antitumor immunity. J. Immunother. Cancer 10, e005433 (2022).
李,A.等人。选择性靶向肿瘤微环境中的 GARP-LTGFbeta 轴可通过增强 CD8(+) T 细胞抗肿瘤免疫来增强 PD-1 阻断。 J.免疫瑟。癌症10 ,e005433 (2022)。Elez, E. et al. Abituzumab combined with cetuximab plus irinotecan versus cetuximab plus irinotecan alone for patients with KRAS wild-type metastatic colorectal cancer: the randomised phase I/II POSEIDON trial. Ann. Oncol. 26, 132–140 (2015).
埃莱兹,E.等人。阿比珠单抗联合西妥昔单抗加伊立替康与单独西妥昔单抗加伊立替康治疗 KRAS 野生型转移性结直肠癌患者:随机 I/II 期 POSEIDON 试验。安.安科尔。 26、132-140 (2015)。Hussain, M. et al. Differential Effect on Bone Lesions of Targeting Integrins: Randomized Phase II Trial of Abituzumab in Patients with Metastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res 22, 3192–3200 (2016).
侯赛因,M.等人。靶向整合素对骨病变的不同作用:阿比珠单抗在转移性去势抵抗性前列腺癌患者中的随机 II 期试验。临床。癌症研究22 , 3192–3200 (2016)。Khanna, D. et al. STRATUS: A Phase II Study of Abituzumab in Patients With Systemic Sclerosis-associated Interstitial Lung Disease. J. Rheumatol. 48, 1295–1298 (2021).
卡纳,D.等人。 STRATUS:阿比珠单抗治疗系统性硬化症相关间质性肺病患者的 II 期研究。 J.风湿病。 48、1295–1298 (2021)。Uhl, W., Zuhlsdorf, M., Koernicke, T., Forssmann, U. & Kovar, A. Safety, tolerability, and pharmacokinetics of the novel alphav-integrin antibody EMD 525797 (DI17E6) in healthy subjects after ascending single intravenous doses. Invest N. Drugs 32, 347–354 (2014).
Uhl, W.、Zuhlsdorf, M.、Koernicke, T.、Forssmann, U. 和 Kovar, A. 新型 αv 整合素抗体 EMD 525797 (DI17E6) 在健康受试者中单次静脉剂量上升后的安全性、耐受性和药代动力学。投资 N. 药物32 , 347–354 (2014)。Manegold, C. et al. Randomized phase II study of three doses of the integrin inhibitor cilengitide versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer. Invest N. Drugs 31, 175–182 (2013).
Manegold,C. 等人。三种剂量的整合素抑制剂西仑吉肽与多西紫杉醇作为晚期非小细胞肺癌患者二线治疗的随机 II 期研究。投资 N. 药物31 , 175–182 (2013)。Vansteenkiste, J. et al. Cilengitide combined with cetuximab and platinum-based chemotherapy as first-line treatment in advanced non-small-cell lung cancer (NSCLC) patients: results of an open-label, randomized, controlled phase II study (CERTO). Ann. Oncol. 26, 1734–1740 (2015).
Vansteenkiste,J. 等人。西仑吉肽联合西妥昔单抗和铂类化疗作为晚期非小细胞肺癌 (NSCLC) 患者的一线治疗:一项开放标签、随机、对照 II 期研究 (CERTO) 的结果。安.安科尔。 26,1734-1740 (2015)。Vermorken, J. B. et al. Cisplatin, 5-fluorouracil, and cetuximab (PFE) with or without cilengitide in recurrent/metastatic squamous cell carcinoma of the head and neck: results of the randomized phase I/II ADVANTAGE trial (phase II part). Ann. Oncol. 25, 682–688 (2014).
Vermorken,JB 等人。顺铂、5-氟尿嘧啶和西妥昔单抗 (PFE) 联合或不联合西仑吉肽治疗头颈部复发/转移性鳞状细胞癌:随机 I/II 期 ADVANTAGE 试验的结果(II 期部分)。安.安科尔。 25、682-688 (2014)。Stupp, R. et al. Phase I/IIa study of cilengitide and temozolomide with concomitant radiotherapy followed by cilengitide and temozolomide maintenance therapy in patients with newly diagnosed glioblastoma. J. Clin. Oncol. 28, 2712–2718 (2010).
Stupp,R.等人。对新诊断的胶质母细胞瘤患者进行西仑吉肽和替莫唑胺联合放疗,然后进行西仑吉肽和替莫唑胺维持治疗的 I/IIa 期研究。 J.克林。安科尔。 28、2712-2718 (2010)。Reardon, D. A. et al. Randomized phase II study of cilengitide, an integrin-targeting arginine-glycine-aspartic acid peptide, in recurrent glioblastoma multiforme. J. Clin. Oncol. 26, 5610–5617 (2008).
里尔登,DA 等人。西仑吉肽(一种整合素靶向精氨酸-甘氨酸-天冬氨酸肽)治疗复发性多形性胶质母细胞瘤的随机 II 期研究。 J.克林。安科尔。 26、5610-5617 (2008)。Nabors, L. B. et al. A safety run-in and randomized phase 2 study of cilengitide combined with chemoradiation for newly diagnosed glioblastoma (NABTT 0306). Cancer 118, 5601–5607 (2012).
纳伯斯,LB 等人。西仑吉肽联合放化疗治疗新诊断胶质母细胞瘤的安全性磨合和随机 2 期研究 (NABTT 0306)。癌症118 , 5601–5607 (2012)。Gilbert, M. R. et al. Cilengitide in patients with recurrent glioblastoma: the results of NABTC 03-02, a phase II trial with measures of treatment delivery. J. Neurooncol 106, 147–153 (2012).
吉尔伯特先生等人。西仑吉肽治疗复发性胶质母细胞瘤患者:NABTC 03-02 的结果,这是一项关于治疗实施措施的 II 期试验。 J. Neurooncol 106 , 147–153 (2012)。MacDonald, T. J. et al. Phase II study of cilengitide in the treatment of refractory or relapsed high-grade gliomas in children: a report from the Children’s Oncology Group. Neuro Oncol. 15, 1438–1444 (2013).
麦克唐纳,TJ 等人。西仑吉肽治疗儿童难治性或复发性高级别神经胶质瘤的 II 期研究:来自儿童肿瘤学组的报告。神经肿瘤。 15、1438-1444 (2013)。Nabors, L. B. et al. Two cilengitide regimens in combination with standard treatment for patients with newly diagnosed glioblastoma and unmethylated MGMT gene promoter: results of the open-label, controlled, randomized phase II CORE study. Neuro Oncol. 17, 708–717 (2015).
纳伯斯,LB 等人。两种西仑吉肽方案联合标准治疗治疗新诊断的胶质母细胞瘤和未甲基化 MGMT 基因启动子的患者:开放标签、对照、随机 II 期 CORE 研究的结果。神经肿瘤。 17、708-717 (2015)。Khasraw, M. et al. Cilengitide with metronomic temozolomide, procarbazine, and standard radiotherapy in patients with glioblastoma and unmethylated MGMT gene promoter in ExCentric, an open-label phase II trial. J. Neurooncol 128, 163–171 (2016).
Khasraw,M.等人。在 ExCentric(一项开放标签 II 期试验)中,西仑吉肽联合节拍替莫唑胺、丙卡巴肼和标准放疗治疗胶质母细胞瘤和未甲基化 MGMT 基因启动子的患者。 J. Neurooncol 128 , 163–171 (2016)。Stupp, R. et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 15, 1100–1108 (2014).
Stupp,R.等人。西仑吉肽联合标准治疗治疗新诊断的具有甲基化 MGMT 启动子的胶质母细胞瘤患者(CENTRIC EORTC 26071-22072 研究):一项多中心、随机、开放标签的 3 期试验。柳叶刀 Oncol。 15、1100-1108 (2014)。Kim, K. B. et al. A randomized phase II study of cilengitide (EMD 121974) in patients with metastatic melanoma. Melanoma Res 22, 294–301 (2012).
金,KB 等人。西仑吉肽 (EMD 121974) 在转移性黑色素瘤患者中的一项随机 II 期研究。黑色素瘤研究22 , 294–301 (2012)。Friess, H. et al. A randomized multi-center phase II trial of the angiogenesis inhibitor Cilengitide (EMD 121974) and gemcitabine compared with gemcitabine alone in advanced unresectable pancreatic cancer. BMC Cancer 6, 285 (2006).
弗里斯,H.等人。血管生成抑制剂西仑吉肽 (EMD 121974) 和吉西他滨与单用吉西他滨治疗晚期不可切除胰腺癌的随机多中心 II 期试验。 BMC 癌症6 , 285 (2006)。Bradley, D. A. et al. Cilengitide (EMD 121974, NSC 707544) in asymptomatic metastatic castration resistant prostate cancer patients: a randomized phase II trial by the prostate cancer clinical trials consortium. Invest N. Drugs 29, 1432–1440 (2011).
布拉德利,DA 等人。西仑吉肽(EMD 121974,NSC 707544)治疗无症状转移性去势抵抗性前列腺癌患者:前列腺癌临床试验联盟的一项随机 II 期试验。投资 N. 药物29 , 1432–1440 (2011)。Alva, A. et al. Phase II study of cilengitide (EMD 121974, NSC 707544) in patients with non-metastatic castration resistant prostate cancer, NCI-6735. A study by the DOD/PCF prostate cancer clinical trials consortium. Invest N. Drugs 30, 749–757 (2012).
阿尔瓦,A.等人。西仑吉肽(EMD 121974,NSC 707544)在非转移性去势抵抗性前列腺癌(NCI-6735)患者中的 II 期研究。 DOD/PCF 前列腺癌临床试验联盟的一项研究。投资 N. 药物30 , 749–757 (2012)。Li, C. et al. Increased activation of latent TGF-β1 by αVβ3 in human Crohn’s disease and fibrosis in TNBS colitis can be prevented by cilengitide. Inflamm. Bowel Dis. 19, 2829–2839 (2013).
李,C.等人。西仑吉肽可以预防人类克罗恩病中 αVβ3 对潜在 TGF-β1 的激活增加以及 TNBS 结肠炎中的纤维化。发炎。肠道疾病。 19、2829-2839 (2013)。Bagnato, G. L. et al. Dual αvβ3 and αvβ5 blockade attenuates fibrotic and vascular alterations in a murine model of systemic sclerosis. Clin. Sci. (Lond.) 132, 231–242 (2018).
巴尼亚托,GL 等人。 αvβ3 和 αvβ5 双重阻断可减轻系统性硬化症小鼠模型中的纤维化和血管改变。临床。科学。 (伦敦) 132、231-242 (2018)。MacDonald, W. J. et al. Broad spectrum integrin inhibitor GLPG-0187 bypasses immune evasion in colorectal cancer by TGF-β signaling mediated downregulation of PD-L1. Am. J. Cancer Res 13, 2938–2947 (2023).
麦克唐纳,WJ 等人。广谱整合素抑制剂 GLPG-0187 通过 TGF-β 信号介导的 PD-L1 下调绕过结直肠癌的免疫逃避。是。癌症研究杂志13,2938–2947 (2023)。Verschleiser, B. et al. Pan-integrin inhibitor GLPG-0187 promotes T-cell killing of mismatch repair-deficient colorectal cancer cells by suppression of SMAD/TGF-β signaling. Am. J. Cancer Res 13, 2878–2885 (2023).
Verschleiser,B. 等人。泛整合素抑制剂 GLPG-0187 通过抑制 SMAD/TGF-β 信号传导促进 T 细胞杀死错配修复缺陷的结直肠癌细胞。是。 J. 癌症研究13 , 2878–2885 (2023)。Belmadani, S. et al. A thrombospondin-1 antagonist of transforming growth factor-beta activation blocks cardiomyopathy in rats with diabetes and elevated angiotensin II. Am. J. Pathol. 171, 777–789 (2007).
贝尔玛达尼,S.等人。转化生长因子-β 激活的血小板反应蛋白-1 拮抗剂可阻断糖尿病和血管紧张素 II 升高大鼠的心肌病。是。 J.帕索尔. 171 , 777–789 (2007)。Ruschkowski, B. A. et al. Thrombospondin-1 Plays a Major Pathogenic Role in Experimental and Human Bronchopulmonary Dysplasia. Am. J. Respir. Crit. Care Med 205, 685–699 (2022).
鲁什科夫斯基,BA 等。 Thrombospondin-1 在实验性和人类支气管肺发育不良中发挥主要致病作用。是。 J.呼吸。暴击。护理医学205 , 685–699 (2022)。Song, S. et al. Sestrin2 remedies podocyte injury via orchestrating TSP-1/TGF-β1/Smad3 axis in diabetic kidney disease. Cell Death Dis. 13, 663 (2022).
宋,S.等人。 Sestrin2 通过协调 TSP-1/TGF-β1/Smad3 轴在糖尿病肾病中修复足细胞损伤。细胞死亡疾病。 13、663 (2022)。Xie, X. S. et al. LSKL, a peptide antagonist of thrombospondin-1, attenuates renal interstitial fibrosis in rats with unilateral ureteral obstruction. Arch. Pharm. Res 33, 275–284 (2010).
谢XS等。 LSKL 是一种血小板反应蛋白-1 的肽拮抗剂,可减轻单侧输尿管梗阻大鼠的肾间质纤维化。拱。医药。第 33 号决议,275–284 (2010)。Lu, A., Miao, M., Schoeb, T. R., Agarwal, A. & Murphy-Ullrich, J. E. Blockade of TSP1-dependent TGF-β activity reduces renal injury and proteinuria in a murine model of diabetic nephropathy. Am. J. Pathol. 178, 2573–2586 (2011).
Lu, A.、Miao, M.、Schoeb, TR、Agarwal, A. 和 Murphy-Ullrich, JE 阻断 TSP1 依赖性 TGF-β 活性可减少糖尿病肾病小鼠模型中的肾损伤和蛋白尿。是。 J.帕索尔. 178、2573–2586 (2011)。Zhang, Y. et al. P2Y4/TSP-1/TGF-β1/pSmad2/3 pathway contributes to acute generalized seizures induced by kainic acid. Brain Res Bull. 149, 106–119 (2019).
张,Y.等人。 P2Y4/TSP-1/TGF-β1/pSmad2/3 通路有助于红藻氨酸诱导的急性全身性癫痫发作。大脑资源公牛。 149、106-119 (2019)。Liao, F. et al. LSKL peptide alleviates subarachnoid fibrosis and hydrocephalus by inhibiting TSP1-mediated TGF-β1 signaling activity following subarachnoid hemorrhage in rats. Exp. Ther. Med 12, 2537–2543 (2016).
廖,F.等人。 LSKL 肽通过抑制大鼠蛛网膜下腔出血后 TSP1 介导的 TGF-β1 信号活性来减轻蛛网膜下腔纤维化和脑积水。过期。瑟尔。医学12 , 2537–2543 (2016)。Jiang, N. et al. Blockade of thrombospondin-1 ameliorates high glucose-induced peritoneal fibrosis through downregulation of TGF-β1/Smad3 signaling pathway. J. Cell Physiol. 235, 364–379 (2020).
江,N.等人。阻断血小板反应蛋白-1 可通过下调 TGF-β1/Smad3 信号通路改善高糖诱导的腹膜纤维化。 J.细胞生理学。 235 , 364–379 (2020)。Narmada, B. C., Chia, S. M., Tucker-Kellogg, L. & Yu, H. HGF regulates the activation of TGF-β1 in rat hepatocytes and hepatic stellate cells. J. Cell Physiol. 228, 393–401 (2013).
Narmada, BC, Chia, SM, Tucker-Kellogg, L. 和 Yu, H. HGF 调节大鼠肝细胞和肝星状细胞中 TGF-β1 的激活。 J.细胞生理学。 228、393-401 (2013)。Kondou, H. et al. A blocking peptide for transforming growth factor-beta1 activation prevents hepatic fibrosis in vivo. J. Hepatol. 39, 742–748 (2003).
Kondou,H.等人。用于转化生长因子-β1 激活的阻断肽可预防体内肝纤维化。 J.肝素。 39、742-748 (2003)。Xu, X. et al. Investigating the potential of LSKL peptide as a novel hypertrophic scar treatment. Biomed. Pharmacother. 124, 109824 (2020).
徐,X.等人。研究 LSKL 肽作为新型增生性疤痕治疗方法的潜力。生物医学。药剂师。 124、109824 (2020)。Kuroki, H. et al. Effect of LSKL peptide on thrombospondin 1-mediated transforming growth factor β signal activation and liver regeneration after hepatectomy in an experimental model. Br. J. Surg. 102, 813–825 (2015).
黑木,H.等人。在实验模型中,LSKL 肽对肝切除术后血小板反应蛋白 1 介导的转化生长因子 β 信号激活和肝再生的影响。 Br。 J.外科医生。 102、813–825 (2015)。Lu, A. et al. Inhibition of Transforming Growth Factor-β Activation Diminishes Tumor Progression and Osteolytic Bone Disease in Mouse Models of Multiple Myeloma. Am. J. Pathol. 186, 678–690 (2016).
卢,A.等人。抑制转化生长因子-β 激活可减少多发性骨髓瘤小鼠模型中的肿瘤进展和溶骨性骨疾病。是。 J.帕索尔. 186、678–690 (2016)。Fu, P. Y. et al. Far upstream element-binding protein 1 facilitates hepatocellular carcinoma invasion and metastasis. Carcinogenesis 41, 950–960 (2020).
傅,PY 等人。远上游元件结合蛋白1促进肝细胞癌的侵袭和转移。致癌作用41 , 950–960 (2020)。Gu, J. et al. Irradiation induces DJ-1 secretion from esophageal squamous cell carcinoma cells to accelerate metastasis of bystander cells via a TGF-β1 positive feedback loop. J. Exp. Clin. Cancer Res 41, 259 (2022).
顾,J.等人。辐射诱导食管鳞状细胞癌细胞分泌 DJ-1,通过 TGF-β1 正反馈环加速旁观细胞的转移。 J.Exp。临床。癌症研究41 , 259 (2022)。Daniel, C. et al. Antisense oligonucleotides against thrombospondin-1 inhibit activation of tgf-beta in fibrotic renal disease in the rat in vivo. Am. J. Pathol. 163, 1185–1192 (2003).
丹尼尔,C. 等人。针对血小板反应蛋白-1 的反义寡核苷酸可抑制大鼠体内纤维化肾病中 tgf-β 的激活。是。 J.帕索尔. 163、1185-1192 (2003)。Agin, M., Yucel, A., Gumus, M., Yuksekkaya, H. A. & Tumgor, G. The Effect of Enteral Nutrition Support Rich in TGF-β in the Treatment of Inflammatory Bowel Disease in Childhood. Med. (Kaunas.) 55, 620 (2019).
Agin, M.、Yucel, A.、Gumus, M.、Yuksekkaya, HA 和 Tumgor, G. 富含 TGF-β 的肠内营养支持在治疗儿童炎症性肠病中的作用。医学。 (考纳斯。) 55、620 (2019)。Fell, J. M. et al. Mucosal healing and a fall in mucosal pro-inflammatory cytokine mRNA induced by a specific oral polymeric diet in paediatric Crohn’s disease. Aliment Pharm. Ther. 14, 281–289 (2000).
菲尔,JM 等人。儿童克罗恩病中特定口服聚合物饮食诱导的粘膜愈合和粘膜促炎细胞因子 mRNA 的下降。营养制药。瑟尔。 14、281-289 (2000)。Hartman, C. et al. Nutritional supplementation with polymeric diet enriched with transforming growth factor-beta 2 for children with Crohn’s disease. Isr. Med Assoc. J. 10, 503–507 (2008).
哈特曼,C.等人。为患有克罗恩病的儿童提供富含转化生长因子-β 2 的聚合饮食的营养补充。 Isr。医学协会。 J.10,503-507 ( 2008 )。Ferreira, T. M. R. et al. Effect of Oral Nutrition Supplements and TGF-β2 on Nutrition and Inflammatory Patterns in Patients With Active Crohn’s Disease. Nutr. Clin. Pr. 35, 885–893 (2020).
费雷拉,TMR 等人。口服营养补充剂和 TGF-β2 对活动性克罗恩病患者营养和炎症模式的影响。营养。临床。 Pr。 35 , 885–893 (2020)。Borrelli, O. et al. Polymeric diet alone versus corticosteroids in the treatment of active pediatric Crohn’s disease: a randomized controlled open-label trial. Clin. Gastroenterol. Hepatol. 4, 744–753 (2006).
博雷利,O.等人。单独使用聚合物饮食与皮质类固醇治疗活动性儿科克罗恩病:一项随机对照开放标签试验。临床。胃肠病。肝醇。 4、744-753 (2006)。Pigneur, B. et al. Mucosal Healing and Bacterial Composition in Response to Enteral Nutrition Vs Steroid-based Induction Therapy-A Randomised Prospective Clinical Trial in Children With Crohn’s Disease. J. Crohns Colitis 13, 846–855 (2019).
Pigneur,B.等人。肠内营养与类固醇诱导治疗反应的粘膜愈合和细菌组成——克罗恩病儿童的随机前瞻性临床试验。 J.克罗恩斯结肠炎13 , 846–855 (2019)。Beaupel, N. et al. Preoperative oral polymeric diet enriched with transforming growth factor-beta 2 (Modulen) could decrease postoperative morbidity after surgery for complicated ileocolonic Crohn’s disease. Scand. J. Gastroenterol. 52, 5–10 (2017).
博佩尔,N.等人。术前口服富含转化生长因子-β 2 (Modulen) 的聚合饮食可以降低复杂性回结肠克罗恩病术后的发病率。扫描。 J.胃肠病学。 52、5-10 (2017)。Okamoto, A. et al. Suppression of serum IgE response and systemic anaphylaxis in a food allergy model by orally administered high-dose TGF-beta. Int Immunol. 17, 705–712 (2005).
冈本,A.等人。口服高剂量 TGF-β 可抑制食物过敏模型中的血清 IgE 反应和全身过敏反应。国际免疫学。 17、705-712 (2005)。Rekima, A. et al. Long-term reduction in food allergy susceptibility in mice by combining breastfeeding-induced tolerance and TGF-β-enriched formula after weaning. Clin. Exp. Allergy 47, 565–576 (2017).
Rekima,A.等人。通过将母乳喂养诱导的耐受性与断奶后富含 TGF-β 的配方奶相结合,长期降低小鼠食物过敏易感性。临床。过期。过敏47 , 565–576 (2017)。Penttila, I. Effects of transforming growth factor-beta and formula feeding on systemic immune responses to dietary beta-lactoglobulin in allergy-prone rats. Pediatr. Res 59, 650–655 (2006).
Penttila,I. 转化生长因子-β 和配方奶喂养对易过敏大鼠的膳食 β-乳球蛋白系统免疫反应的影响。儿科。第 59 号决议,650–655(2006 年)。Verhasselt, V. Neonatal tolerance under breastfeeding influence: the presence of allergen and transforming growth factor-beta in breast milk protects the progeny from allergic asthma. J. Pediatr. 156, S16–S20 (2010).
Verhasselt, V. 母乳喂养影响下的新生儿耐受性:母乳中过敏原和转化生长因子-β 的存在可保护后代免受过敏性哮喘的影响。 J.儿科。 156 ,S16-S20(2010)。Morita, Y. et al. TGF-β Concentration in Breast Milk is Associated With the Development of Eczema in Infants. Front Pediatr. 6, 162 (2018).
森田,Y.等人。母乳中的 TGF-β 浓度与婴儿湿疹的发生有关。前儿科。 6、162 (2018)。Saarinen, K. M., Vaarala, O., Klemetti, P. & Savilahti, E. Transforming growth factor-beta1 in mothers’ colostrum and immune responses to cows’ milk proteins in infants with cows’ milk allergy. J. Allergy Clin. Immunol. 104, 1093–1098 (1999).
Saarinen, KM、Vaarala, O.、Klemetti, P. 和 Savilahti, E. 母亲初乳中的转化生长因子-β1 以及牛奶过敏婴儿对牛奶蛋白的免疫反应。 J.过敏临床。免疫学。 104、1093-1098 (1999)。Ferguson, M. W. et al. Prophylactic administration of avotermin for improvement of skin scarring: three double-blind, placebo-controlled, phase I/II studies. Lancet 373, 1264–1274 (2009).
弗格森,MW 等人。预防性施用阿伏特明以改善皮肤疤痕:三项双盲、安慰剂对照、I/II 期研究。柳叶刀373 , 1264–1274 (2009)。So, K. et al. Avotermin for scar improvement following scar revision surgery: a randomized, double-blind, within-patient, placebo-controlled, phase II clinical trial. Plast. Reconstr. Surg. 128, 163–172 (2011).
所以,K.等人。 Avotermin 用于改善疤痕修复手术后的疤痕:一项随机、双盲、患者内部、安慰剂对照、II 期临床试验。塑料。重建。外科医生。 128、163-172 (2011)。Bush, J. et al. Scar-improving efficacy of avotermin administered into the wound margins of skin incisions as evaluated by a randomized, double-blind, placebo-controlled, phase II clinical trial. Plast. Reconstr. Surg. 126, 1604–1615 (2010).
布什,J.等人。通过一项随机、双盲、安慰剂对照、II 期临床试验评估了阿伏特明注射到皮肤切口伤口边缘的疤痕改善功效。塑料。重建。外科医生。 126、1604-1615 (2010)。McCollum, P. T. et al. Randomized phase II clinical trial of avotermin versus placebo for scar improvement. Br. J. Surg. 98, 925–934 (2011).
麦科勒姆,PT 等人。阿沃特明与安慰剂改善疤痕的随机 II 期临床试验。 Br。 J.外科医生。 98、925–934 (2011)。Robson, M. C. et al. Safety and effect of transforming growth factor-beta(2) for treatment of venous stasis ulcers. Wound Repair Regen. 3, 157–167 (1995).
罗布森,MC 等人。转化生长因子-β(2)治疗静脉淤滞性溃疡的安全性和效果。伤口修复再生。 3,157-167 (1995)。Wang, X. et al. Demineralized bone matrix combined bone marrow mesenchymal stem cells, bone morphogenetic protein-2 and transforming growth factor-β3 gene promoted pig cartilage defect repair. PLoS One 9, e116061 (2014).
王X.等。脱矿骨基质联合骨髓间充质干细胞、骨形态发生蛋白2和转化生长因子β3基因促进猪软骨缺损修复。 PLoS One 9 ,e116061 (2014)。Luo, Z. et al. Mechano growth factor (MGF) and transforming growth factor (TGF)-β3 functionalized silk scaffolds enhance articular hyaline cartilage regeneration in rabbit model. Biomaterials 52, 463–475 (2015).
罗,Z.等人。机械生长因子 (MGF) 和转化生长因子 (TGF)-β3 功能化丝支架可增强兔模型中的关节透明软骨再生。生物材料52 , 463–475 (2015)。Kuruvilla, A. P. et al. Protective effect of transforming growth factor beta 1 on experimental autoimmune diseases in mice. Proc. Natl Acad. Sci. USA 88, 2918–2921 (1991).
库鲁维拉,美联社等人。转化生长因子β1对小鼠实验性自身免疫性疾病的保护作用。过程。国家科学院。科学。美国88,2918-2921 (1991)。Srivastava, V., Khanna, M., Sharma, S. & Kumar, B. Resolution of immune response by recombinant transforming growth factor-beta (rTGF-β) during influenza A virus infection. Indian J. Med Res 136, 641–648 (2012).
Srivastava, V.、Khanna, M.、Sharma, S. 和 Kumar, B. 在甲型流感病毒感染期间通过重组转化生长因子-β (rTGF-β) 解决免疫反应。印度医学研究杂志136 , 641–648 (2012)。Morris, J. C. et al. Phase I study of GC1008 (fresolimumab): a human anti-transforming growth factor-beta (TGFbeta) monoclonal antibody in patients with advanced malignant melanoma or renal cell carcinoma. PLoS One 9, e90353 (2014).
莫里斯,JC 等人。 GC1008(fresolimumab)的 I 期研究:一种人抗转化生长因子-β(TGFβ)单克隆抗体,用于治疗晚期恶性黑色素瘤或肾细胞癌患者。 PLoS One 9 ,e90353 (2014)。Formenti, S. C. et al. Focal Irradiation and Systemic TGFbeta Blockade in Metastatic Breast Cancer. Clin. Cancer Res 24, 2493–2504 (2018).
福门蒂,SC 等人。转移性乳腺癌的局部照射和全身 TGFbeta 阻断。临床。癌症研究24 , 2493–2504 (2018)。Rice, L. M. et al. Fresolimumab treatment decreases biomarkers and improves clinical symptoms in systemic sclerosis patients. J. Clin. Invest 125, 2795–2807 (2015).
赖斯,LM 等人。 Fresolimumab 治疗可降低系统性硬化症患者的生物标志物并改善临床症状。 J.克林。投资125,2795–2807 (2015)。Trachtman, H. et al. A phase 1, single-dose study of fresolimumab, an anti-TGF-beta antibody, in treatment-resistant primary focal segmental glomerulosclerosis. Kidney Int 79, 1236–1243 (2011).
特拉赫特曼,H.等人。 fresolimumab(一种抗 TGF-β 抗体)治疗难治性原发性局灶节段性肾小球硬化症的 1 期单剂量研究。肾脏国际79 , 1236–1243 (2011)。Lacouture, M. E. et al. Cutaneous keratoacanthomas/squamous cell carcinomas associated with neutralization of transforming growth factor beta by the monoclonal antibody fresolimumab (GC1008). Cancer Immunol. Immunother. 64, 437–446 (2015).
拉库图尔,ME 等人。与单克隆抗体 fresolimumab (GC1008) 中和转化生长因子 β 相关的皮肤角化棘皮瘤/鳞状细胞癌。癌症免疫学。免疫疗法。 64、437-446 (2015)。Bauer, T. M. et al. Phase I/Ib, open-label, multicenter, dose-escalation study of the anti-TGF-β monoclonal antibody, NIS793, in combination with spartalizumab in adult patients with advanced tumors. J. Immunother. Cancer 11, e007353 (2023).
鲍尔,TM 等人。抗 TGF-β 单克隆抗体 NIS793 与斯帕他珠单抗联合治疗晚期肿瘤成年患者的 I/Ib 期、开放标签、多中心、剂量递增研究。 J.免疫瑟。癌症11 ,e007353 (2023)。Denton, C. P. et al. Recombinant human anti-transforming growth factor beta1 antibody therapy in systemic sclerosis: a multicenter, randomized, placebo-controlled phase I/II trial of CAT-192. Arthritis Rheum. 56, 323–333 (2007).
丹顿,CP 等人。重组人抗转化生长因子β1抗体治疗系统性硬化症:CAT-192的多中心、随机、安慰剂对照I/II期试验。关节炎大黄。 56、323-333 (2007)。Voelker, J. et al. Anti-TGF-β1 Antibody Therapy in Patients with Diabetic Nephropathy. J. Am. Soc. Nephrol. 28, 953–962 (2017).
沃克,J.等人。糖尿病肾病患者的抗 TGF-β1 抗体治疗。 J. Am.苏克。肾病。 28 , 953–962 (2017)。Mascarenhas, J. et al. A Phase Ib Trial of AVID200, a TGFβ 1/3 Trap, in Patients with Myelofibrosis. Clin. Cancer Res 29, 3622–3632 (2023).
马斯卡雷尼亚斯,J.等人。 AVID200(一种 TGFβ 1/3 陷阱)在骨髓纤维化患者中的 Ib 期试验。临床。癌症研究29 , 3622–3632 (2023)。Li, Y. et al. Neutralization of excessive levels of active TGF-β1 reduces MSC recruitment and differentiation to mitigate peritendinous adhesion. Bone Res 11, 24 (2023).
李,Y.等人。中和过量的活性 TGF-β1 会减少 MSC 的募集和分化,从而减轻腱周粘附。骨骼研究11 , 24 (2023)。Lu, L. et al. The temporal effects of anti-TGF-beta1, 2, and 3 monoclonal antibody on wound healing and hypertrophic scar formation. J. Am. Coll. Surg. 201, 391–397 (2005).
卢,L.等人。抗 TGF-β1、2 和 3 单克隆抗体对伤口愈合和肥厚性疤痕形成的时间影响。 J. Am.科尔。外科医生。 201 , 391–397 (2005)。Wang, L. et al. Aberrant Transforming Growth Factor-β Activation Recruits Mesenchymal Stem Cells During Prostatic Hyperplasia. Stem Cells Transl. Med 6, 394–404 (2017).
王,L.等人。异常的转化生长因子-β 激活在前列腺增生期间招募间充质干细胞。干细胞翻译。医学6 , 394–404 (2017)。Deng, S., Zhang, H., Han, W., Guo, C. & Deng, C. Transforming Growth Factor-β-Neutralizing Antibodies Improve Alveolarization in the Oxygen-Exposed Newborn Mouse Lung. J. Interferon Cytokine Res 39, 106–116 (2019).
Deng, S.、Zhang, H.、Han, W.、Guo, C. 和 Deng, C. 转化生长因子-β-中和抗体可改善暴露于氧气的新生小鼠肺中的肺泡化。 J. 干扰素细胞因子研究39 , 106–116 (2019)。Nelson, C. A. et al. Inhibiting TGF-β activity improves respiratory function in mdx mice. Am. J. Pathol. 178, 2611–2621 (2011).
尼尔森,加利福尼亚州等人。抑制 TGF-β 活性可改善 mdx 小鼠的呼吸功能。是。 J.帕索尔. 178、2611-2621 (2011)。Cook, J. R. et al. Dimorphic effects of transforming growth factor-β signaling during aortic aneurysm progression in mice suggest a combinatorial therapy for Marfan syndrome. Arterioscler Thromb. Vasc. Biol. 35, 911–917 (2015).
库克,JR 等人。转化生长因子-β信号在小鼠主动脉瘤进展过程中的二态性效应提示了马凡综合征的联合治疗。动脉硬化血栓。瓦斯克。生物。 35、911-917 (2015)。Wang, X. et al. Aberrant TGF-β activation in bone tendon insertion induces enthesopathy-like disease. J. Clin. Invest 128, 846–860 (2018).
王X.等。骨腱插入过程中异常的 TGF-β 激活会诱发类似附着点病的疾病。 J.克林。投资128 , 846–860 (2018)。Becerikli, M. et al. TGF-beta pathway inhibition as the therapeutic acceleration of diabetic bone regeneration. J. Orthop. Res 40, 1810–1826 (2022).
Becerikli,M.等人。抑制 TGF-β 通路可加速糖尿病骨再生的治疗。 J.奥索普。第 40 号决议,1810–1826 (2022)。Sahbani, K., Cardozo, C. P., Bauman, W. A. & Tawfeek, H. A. Inhibition of TGF-β Signaling Attenuates Disuse-induced Trabecular Bone Loss After Spinal Cord Injury in Male Mice. Endocrinology 163, bqab230 (2022).
Sahbani, K.、Cardozo, CP、Bauman, WA 和 Tawfeek, HA 抑制 TGF-β 信号传导可减轻雄性小鼠脊髓损伤后废用性诱导的小梁骨丢失。内分泌学163 ,bqab230 (2022)。Grafe, I. et al. Excessive transforming growth factor-β signaling is a common mechanism in osteogenesis imperfecta. Nat. Med 20, 670–675 (2014).
格拉夫,I.等人。过度的转化生长因子-β信号传导是成骨不全症的常见机制。纳特。医学20 , 670–675 (2014)。Wahl, S. M., Allen, J. B., Costa, G. L., Wong, H. L. & Dasch, J. R. Reversal of acute and chronic synovial inflammation by anti-transforming growth factor beta. J. Exp. Med 177, 225–230 (1993).
Wahl, SM, Allen, JB, Costa, GL, Wong, HL & Dasch, JR 通过抗转化生长因子β逆转急性和慢性滑膜炎症。 J.Exp。医学177 , 225–230 (1993)。Xie, L. et al. Systemic neutralization of TGF-β attenuates osteoarthritis. Ann. N. Y Acad. Sci. 1376, 53–64 (2016).
谢,L.等人。 TGF-β 的全身中和可减轻骨关节炎。安.纽约学院。科学。 1376 , 53–64 (2016)。Ferreira, R. R. et al. In Chagas disease, transforming growth factor beta neutralization reduces Trypanosoma cruzi infection and improves cardiac performance. Front Cell Infect. Microbiol 12, 1017040 (2022).
费雷拉,RR 等人。在恰加斯病中,转化生长因子β中和可减少克氏锥虫感染并改善心脏功能。前细胞感染。微生物学12 , 1017040 (2022)。Ravi, R. et al. Bifunctional immune checkpoint-targeted antibody-ligand traps that simultaneously disable TGFbeta enhance the efficacy of cancer immunotherapy. Nat. Commun. 9, 741 (2018).
拉维,R.等人。双功能免疫检查点靶向抗体配体陷阱可同时禁用 TGFbeta,从而增强癌症免疫治疗的功效。纳特。交流。 9、741 (2018)。Paz-Ares, L. et al. Bintrafusp Alfa, a Bifunctional Fusion Protein Targeting TGF-beta and PD-L1, in Second-Line Treatment of Patients With NSCLC: Results From an Expansion Cohort of a Phase 1 Trial. J. Thorac. Oncol. 15, 1210–1222 (2020).
帕兹-阿雷斯,L.等人。 Bintrafusp Alfa 是一种靶向 TGF-β 和 PD-L1 的双功能融合蛋白,用于 NSCLC 患者的二线治疗:1 期试验扩展队列的结果。 J·索拉克。安科尔。 15、1210-1222 (2020)。Kang, Y. K. et al. Safety and Tolerability of Bintrafusp Alfa, a Bifunctional Fusion Protein Targeting TGFbeta and PD-L1, in Asian Patients with Pretreated Recurrent or Refractory Gastric Cancer. Clin. Cancer Res 26, 3202–3210 (2020).
康,YK 等人。 Bintrafusp Alfa(一种靶向 TGFbeta 和 PD-L1 的双功能融合蛋白)在亚洲经治复发性或难治性胃癌患者中的安全性和耐受性。临床。癌症研究26 , 3202–3210 (2020)。Yoo, C. et al. Phase I study of bintrafusp alfa, a bifunctional fusion protein targeting TGF-beta and PD-L1, in patients with pretreated biliary tract cancer. J. Immunother. Cancer 8, e000564 (2020).
尤,C.等人。 bintrafusp alfa(一种靶向 TGF-β 和 PD-L1 的双功能融合蛋白)在接受过治疗的胆道癌患者中进行的 I 期研究。 J.免疫瑟。癌症8 ,e000564 (2020)。Redman, J. M. et al. Enhanced neoepitope-specific immunity following neoadjuvant PD-L1 and TGF-beta blockade in HPV-unrelated head and neck cancer. J. Clin. Invest 132, e161400 (2022).
雷德曼,JM 等人。在与 HPV 无关的头颈癌中,新辅助 PD-L1 和 TGF-β 阻断后新表位特异性免疫增强。 J.克林。投资132 ,e161400 (2022)。Strauss, J. et al. Phase I Trial of M7824 (MSB0011359C), a Bifunctional Fusion Protein Targeting PD-L1 and TGFbeta, in Advanced Solid Tumors. Clin. Cancer Res 24, 1287–1295 (2018).
施特劳斯,J.等人。 M7824 (MSB0011359C) 是一种针对 PD-L1 和 TGFbeta 的双功能融合蛋白,在晚期实体瘤中的 I 期试验。临床。癌症研究24 , 1287–1295 (2018)。Wu, Z. H. et al. Development of the Novel Bifunctional Fusion Protein BR102 That Simultaneously Targets PD-L1 and TGF-β for Anticancer Immunotherapy. Cancers (Basel) 14, 4964 (2022).
吴,ZH 等人。开发同时靶向 PD-L1 和 TGF-β 的新型双功能融合蛋白 BR102,用于抗癌免疫治疗。癌症(巴塞尔) 14,4964 (2022)。Chen, X. et al. Secretion of bispecific protein of anti-PD-1 fused with TGF-beta trap enhances antitumor efficacy of CAR-T cell therapy. Mol. Ther. Oncolyt. 21, 144–157 (2021).
陈,X.等人。分泌与 TGF-β 陷阱融合的抗 PD-1 双特异性蛋白可增强 CAR-T 细胞疗法的抗肿瘤功效。摩尔。瑟尔。 Oncolyt。 21 , 144–157 (2021)。Fukushima, K. et al. The use of an antifibrosis agent to improve muscle recovery after laceration. Am. J. Sports Med 29, 394–402 (2001).
福岛,K.等人。使用抗纤维化剂来改善撕裂后的肌肉恢复。是。 J. 运动医学29 , 394–402 (2001)。Ruehle, M. A. et al. Decorin-supplemented collagen hydrogels for the co-delivery of bone morphogenetic protein-2 and microvascular fragments to a composite bone-muscle injury model with impaired vascularization. Acta Biomater. 93, 210–221 (2019).
鲁尔,马萨诸塞州等人。补充核心蛋白聚糖的胶原水凝胶,用于将骨形态发生蛋白 2 和微血管碎片共同递送至血管化受损的复合骨肌肉损伤模型。生物材料学报。 93、210-221 (2019)。Ahmed, Z. et al. Decorin blocks scarring and cystic cavitation in acute and induces scar dissolution in chronic spinal cord wounds. Neurobiol. Dis. 64, 163–176 (2014).
艾哈迈德,Z.等人。核心蛋白聚糖可阻止急性疤痕形成和囊性空化,并诱导慢性脊髓伤口疤痕溶解。神经生物学。迪斯。 64、163-176 (2014)。Qiu, S. S., Dotor, J. & Hontanilla, B. Effect of P144® (Anti-TGF-β) in an “In Vivo” Human Hypertrophic Scar Model in Nude Mice. PLoS One 10, e0144489 (2015).
Qiu, SS, Dotor, J. 和 Hontanilla, B. P144®(抗 TGF-β)在裸鼠“体内”人类肥厚性疤痕模型中的作用。 PLoS One 10 ,e0144489 (2015)。Arce, C. et al. Anti-TGFβ (Transforming Growth Factor β) Therapy With Betaglycan-Derived P144 Peptide Gene Delivery Prevents the Formation of Aortic Aneurysm in a Mouse Model of Marfan Syndrome. Arterioscler Thromb. Vasc. Biol. 41, e440–e452 (2021).
阿尔塞,C.等人。使用 Betaglycan 衍生的 P144 肽基因传递进行抗 TGFβ(转化生长因子 β)治疗可预防马凡氏综合征小鼠模型中主动脉瘤的形成。动脉硬化血栓。瓦斯克。生物。 41 、e440–e452 (2021)。Li, L. et al. Postinfarction gene therapy with adenoviral vector expressing decorin mitigates cardiac remodeling and dysfunction. Am. J. Physiol. Heart Circ. Physiol. 297, H1504–H1513 (2009).
李,L.等人。使用表达核心蛋白聚糖的腺病毒载体进行梗塞后基因治疗可减轻心脏重塑和功能障碍。是。 J.生理学。心脏循环。生理学。 297 ,H1504–H1513(2009)。Yan, W. et al. Decorin gene delivery inhibits cardiac fibrosis in spontaneously hypertensive rats by modulation of transforming growth factor-beta/Smad and p38 mitogen-activated protein kinase signaling pathways. Hum. Gene Ther. 20, 1190–1200 (2009).
严,W.等人。核心蛋白聚糖基因递送通过调节转化生长因子-β/Smad 和 p38 丝裂原激活蛋白激酶信号通路来抑制自发性高血压大鼠的心脏纤维化。哼。吉恩·瑟尔. 20、1190-1200 (2009)。Hermida, N. et al. A synthetic peptide from transforming growth factor-beta1 type III receptor prevents myocardial fibrosis in spontaneously hypertensive rats. Cardiovasc Res 81, 601–609 (2009).
赫米达,N.等人。来自转化生长因子-β1 III 型受体的合成肽可预防自发性高血压大鼠的心肌纤维化。心血管研究81 , 601–609 (2009)。Nili, N. et al. Decorin inhibition of PDGF-stimulated vascular smooth muscle cell function: potential mechanism for inhibition of intimal hyperplasia after balloon angioplasty. Am. J. Pathol. 163, 869–878 (2003).
尼利,N.等人。核心蛋白聚糖抑制 PDGF 刺激的血管平滑肌细胞功能:球囊血管成形术后抑制内膜增生的潜在机制。是。 J.帕索尔. 163、869–878 (2003)。Recalde, S. et al. Transforming growth factor-β inhibition decreases diode laser-induced choroidal neovascularization development in rats: P17 and P144 peptides. Invest Ophthalmol. Vis. Sci. 52, 7090–7097 (2011).
Recalde,S.等人。转化生长因子-β 抑制可减少二极管激光诱导的大鼠脉络膜新生血管的发育:P17 和 P144 肽。投资眼科。维斯。科学。 52、7090–7097 (2011)。Aojula, A. et al. Diffusion tensor imaging with direct cytopathological validation: characterisation of decorin treatment in experimental juvenile communicating hydrocephalus. Fluids Barriers CNS 13, 9 (2016).
奥朱拉,A.等人。具有直接细胞病理学验证的扩散张量成像:实验性青少年交通性脑积水中核心蛋白聚糖治疗的特征。流体屏障 CNS 13 , 9 (2016)。Botfield, H. et al. Decorin prevents the development of juvenile communicating hydrocephalus. Brain 136, 2842–2858 (2013).
博特菲尔德,H.等人。核心蛋白聚糖可预防青少年交通性脑积水的发生。大脑136 , 2842–2858 (2013)。Murillo-Cuesta, S. et al. Transforming growth factor β1 inhibition protects from noise-induced hearing loss. Front Aging Neurosci. 7, 32 (2015).
Murillo-Cuesta,S. 等人。抑制转化生长因子 β1 可防止噪音引起的听力损失。前沿衰老神经科学。 7、32 (2015)。Border, W. A. et al. Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease. Nature 360, 361–364 (1992).
边境,WA 等。转化生长因子-β 的天然抑制剂可防止实验性肾病中的疤痕形成。自然360 , 361–364 (1992)。Juárez, P. et al. Soluble betaglycan reduces renal damage progression in db/db mice. Am. J. Physiol. Ren. Physiol. 292, F321–F329 (2007).
华雷斯,P.等人。可溶性β聚糖可减少 db/db 小鼠肾损伤的进展。是。 J.生理学。任。生理学。 292 ,F321-F329(2007)。Baltanás, A. et al. A synthetic peptide from transforming growth factor-β1 type III receptor inhibits NADPH oxidase and prevents oxidative stress in the kidney of spontaneously hypertensive rats. Antioxid. Redox Signal 19, 1607–1618 (2013).
巴尔塔纳斯,A.等人。来自转化生长因子-β 1 III 型受体的合成肽可抑制 NADPH 氧化酶并预防自发性高血压大鼠肾脏的氧化应激。抗氧化剂。氧化还原信号19,1607–1618 (2013)。Li, D. et al. TGF-β1 peptide-based inhibitor P144 ameliorates renal fibrosis after ischemia-reperfusion injury by modulating alternatively activated macrophages. Cell Prolif. 55, e13299 (2022).
李,D.等人。基于 TGF-β1 肽的抑制剂 P144 通过调节交替激活的巨噬细胞来改善缺血再灌注损伤后的肾纤维化。细胞增殖。 55 、e13299 (2022)。Zhang, Y., McCormick, L. L. & Gilliam, A. C. Latency-associated peptide prevents skin fibrosis in murine sclerodermatous graft-versus-host disease, a model for human scleroderma. J. Invest Dermatol 121, 713–719 (2003).
张,Y.,麦考密克,LL 和吉列姆,AC 潜伏相关肽可预防小鼠硬皮病移植物抗宿主病(人类硬皮病模型)的皮肤纤维化。 J. Invest Dermatol 121 , 713–719 (2003)。Jang, Y. O. et al. Effect of Function-Enhanced Mesenchymal Stem Cells Infected With Decorin-Expressing Adenovirus on Hepatic Fibrosis. Stem Cells Transl. Med 5, 1247–1256 (2016).
张,YO 等人。表达核心蛋白聚糖的腺病毒感染的功能增强间充质干细胞对肝纤维化的影响。干细胞翻译。医学5,1247–1256 (2016)。Kolb, M., Margetts, P. J., Sime, P. J. & Gauldie, J. Proteoglycans decorin and biglycan differentially modulate TGF-beta-mediated fibrotic responses in the lung. Am. J. Physiol. Lung Cell Mol. Physiol. 280, L1327–L1334 (2001).
Kolb,M.,Margetts,PJ,Sime,PJ 和Gauldie,J。蛋白多糖核心蛋白聚糖和双聚糖可差异调节肺中 TGF-β 介导的纤维化反应。是。 J.生理学。肺细胞分子。生理学。 280 ,L1327–L1334(2001)。Ezquerro, I. J. et al. A synthetic peptide from transforming growth factor beta type III receptor inhibits liver fibrogenesis in rats with carbon tetrachloride liver injury. Cytokine 22, 12–20 (2003).
埃兹奎罗,IJ 等人。来自转化生长因子β III 型受体的合成肽可抑制四氯化碳肝损伤大鼠的肝纤维化。细胞因子22 , 12–20 (2003)。Hirsch, C. S., Ellner, J. J., Blinkhorn, R. & Toossi, Z. In vitro restoration of T cell responses in tuberculosis and augmentation of monocyte effector function against Mycobacterium tuberculosis by natural inhibitors of transforming growth factor beta. Proc. Natl Acad. Sci. USA 94, 3926–3931 (1997).
Hirsch,CS,Ellner,JJ,Blinkhorn,R. 和 Toossi,Z。通过转化生长因子 β 的天然抑制剂,体外恢复结核病中的 T 细胞反应,并增强单核细胞对结核分枝杆菌的效应功能。过程。国家科学院。科学。美国94,3926-3931 (1997)。Bandyopadhyay, A. et al. Antitumor activity of a recombinant soluble betaglycan in human breast cancer xenograft. Cancer Res 62, 4690–4695 (2002).
Bandyopadhyay,A.等人。重组可溶性β聚糖在人乳腺癌异种移植物中的抗肿瘤活性。癌症研究62 , 4690–4695 (2002)。Liu, Z. et al. An Oncolytic Adenovirus Encoding Decorin and Granulocyte Macrophage Colony Stimulating Factor Inhibits Tumor Growth in a Colorectal Tumor Model by Targeting Pro-Tumorigenic Signals and via Immune Activation. Hum. Gene Ther. 28, 667–680 (2017).
刘,Z.等人。编码核心蛋白聚糖和粒细胞巨噬细胞集落刺激因子的溶瘤腺病毒通过靶向促肿瘤信号和免疫激活来抑制结直肠肿瘤模型中的肿瘤生长。哼。吉恩·瑟尔. 28、667-680 (2017)。Zhang, W. et al. Efficacy of an Oncolytic Adenovirus Driven by a Chimeric Promoter and Armed with Decorin Against Renal Cell Carcinoma. Hum. Gene Ther. 31, 651–663 (2020).
张,W.等人。由嵌合启动子驱动并配备核心蛋白聚糖的溶瘤腺病毒对抗肾细胞癌的功效。哼。吉恩·瑟尔. 31、651-663 (2020)。Yang, Y. et al. Systemic Delivery of an Oncolytic Adenovirus Expressing Decorin for the Treatment of Breast Cancer Bone Metastases. Hum. Gene Ther. 26, 813–825 (2015).
杨,Y.等人。表达核心蛋白聚糖的溶瘤腺病毒的全身递送用于治疗乳腺癌骨转移。哼。吉恩·瑟尔. 26、813-825 (2015)。Narayan, V. et al. PSMA-targeting TGFbeta-insensitive armored CAR T cells in metastatic castration-resistant prostate cancer: a phase 1 trial. Nat. Med 28, 724–734 (2022).
纳拉扬,V.等人。 PSMA 靶向 TGFbeta 不敏感的装甲 CAR T 细胞治疗转移性去势抵抗性前列腺癌:一项 1 期试验。纳特。医学28 , 724–734 (2022)。Wang, F. L. et al. TGF-beta insensitive dendritic cells: an efficient vaccine for murine prostate cancer. Cancer Immunol. Immunother. 56, 1785–1793 (2007).
王,FL 等人。 TGF-β 不敏感树突状细胞:一种有效的小鼠前列腺癌疫苗。癌症免疫学。免疫疗法。 56,1785-1793 (2007)。Tian, F. et al. Vaccination with transforming growth factor-beta insensitive dendritic cells suppresses pulmonary metastases of renal carcinoma in mice. Cancer Lett. 271, 333–341 (2008).
田,F.等人。接种转化生长因子-β不敏感树突状细胞可抑制小鼠肾癌的肺转移。癌症快报。 271、333-341 (2008)。Reid, R. R. et al. Reduction of hypertrophic scar via retroviral delivery of a dominant negative TGF-beta receptor II. J. Plast. Reconstr. Aesthet. Surg. 60, 64–72 (2007). discussion 73-64.
里德,RR 等人。通过逆转录病毒传递显性失活 TGF-β 受体 II 减少增生性疤痕。 J.普拉斯特。重建。审美。外科医生。 60、64-72 (2007)。讨论73-64。Santini, V. et al. Phase II Study of the ALK5 Inhibitor Galunisertib in Very Low-, Low-, and Intermediate-Risk Myelodysplastic Syndromes. Clin. Cancer Res 25, 6976–6985 (2019).
桑蒂尼,V.等人。 ALK5 抑制剂 Galunisertib 在极低、低和中风险骨髓增生异常综合征中的 II 期研究。临床。癌症研究25 , 6976–6985 (2019)。Nadal, E. et al. A phase Ib/II study of galunisertib in combination with nivolumab in solid tumors and non-small cell lung cancer. BMC Cancer 23, 708 (2023).
纳达尔,E.等人。 galunisertib 与 nivolumab 联合治疗实体瘤和非小细胞肺癌的 Ib/II 期研究。 BMC 癌症23 , 708 (2023)。Kelley, R. K. et al. A Phase 2 Study of Galunisertib (TGF-beta1 Receptor Type I Inhibitor) and Sorafenib in Patients With Advanced Hepatocellular Carcinoma. Clin. Transl. Gastroenterol. 10, e00056 (2019).
凯利,RK 等人。 Galunisertib(TGF-β1 受体 I 型抑制剂)和索拉非尼治疗晚期肝细胞癌患者的 2 期研究。临床。译。胃肠道。 10 、e00056 (2019)。Faivre, S. et al. Novel transforming growth factor beta receptor I kinase inhibitor galunisertib (LY2157299) in advanced hepatocellular carcinoma. Liver Int 39, 1468–1477 (2019).
Faivre,S.等人。新型转化生长因子 β 受体 I 激酶抑制剂 galunisertib (LY2157299) 用于治疗晚期肝细胞癌。肝脏国际39,1468–1477 (2019)。Yamazaki, T. et al. Galunisertib plus neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer: a single-arm, phase 2 trial. Lancet Oncol. 23, 1189–1200 (2022).
山崎,T.等人。 Galunisertib 联合新辅助放化疗治疗局部晚期直肠癌:一项单组 2 期试验。柳叶刀 Oncol。 23,1189-1200 (2022)。Melisi, D. et al. Galunisertib plus gemcitabine vs. gemcitabine for first-line treatment of patients with unresectable pancreatic cancer. Br. J. Cancer 119, 1208–1214 (2018).
梅利西,D.等人。 Galunisertib 加吉西他滨与吉西他滨一线治疗不可切除的胰腺癌患者的比较。 Br。癌症杂志119 , 1208–1214 (2018)。Wick, A. et al. Phase 1b/2a study of galunisertib, a small molecule inhibitor of transforming growth factor-beta receptor I, in combination with standard temozolomide-based radiochemotherapy in patients with newly diagnosed malignant glioma. Invest N. Drugs 38, 1570–1579 (2020).
威克,A.等人。 galunisertib(一种转化生长因子-β受体 I 的小分子抑制剂)与基于替莫唑胺的标准放化疗联合治疗新诊断的恶性神经胶质瘤患者的 1b/2a 期研究。投资 N. 药物38,1570–1579 (2020)。Brandes, A. A. et al. A Phase II randomized study of galunisertib monotherapy or galunisertib plus lomustine compared with lomustine monotherapy in patients with recurrent glioblastoma. Neuro Oncol. 18, 1146–1156 (2016).
布兰德斯,AA 等。一项针对复发性胶质母细胞瘤患者进行 galunisertib 单药治疗或 galunisertib 加洛莫司汀与洛莫司汀单药治疗比较的 II 期随机研究。神经肿瘤。 18、1146-1156 (2016)。Kovacs, R. J. et al. Cardiac Safety of TGF-beta Receptor I Kinase Inhibitor LY2157299 Monohydrate in Cancer Patients in a First-in-Human Dose Study. Cardiovasc Toxicol. 15, 309–323 (2015).
科瓦奇,RJ 等人。首次人体剂量研究中 TGF-β 受体 I 激酶抑制剂 LY2157299 一水合物对癌症患者的心脏安全性。心血管毒素。 15、309-323 (2015)。Tolcher, A. W. et al. A phase 1 study of anti-TGFbeta receptor type-II monoclonal antibody LY3022859 in patients with advanced solid tumors. Cancer Chemother. Pharm. 79, 673–680 (2017).
托尔彻,AW 等人。抗 TGFbeta 受体 II 型单克隆抗体 LY3022859 在晚期实体瘤患者中的 1 期研究。癌症化疗者。医药。 79、673–680 (2017)。Suzuki, E. et al. A novel small-molecule inhibitor of transforming growth factor beta type I receptor kinase (SM16) inhibits murine mesothelioma tumor growth in vivo and prevents tumor recurrence after surgical resection. Cancer Res 67, 2351–2359 (2007).
铃木,E.等人。一种新型转化生长因子βI型受体激酶(SM16)小分子抑制剂可抑制小鼠间皮瘤体内肿瘤生长,并防止手术切除后肿瘤复发。癌症研究67 , 2351–2359 (2007)。Uhl, M. et al. SD-208, a novel transforming growth factor beta receptor I kinase inhibitor, inhibits growth and invasiveness and enhances immunogenicity of murine and human glioma cells in vitro and in vivo. Cancer Res 64, 7954–7961 (2004).
乌尔,M.等人。 SD-208 是一种新型转化生长因子 β 受体 I 激酶抑制剂,可抑制小鼠和人类神经胶质瘤细胞的生长和侵袭性,并增强体外和体内的免疫原性。癌症研究64 , 7954–7961 (2004)。Tanaka, H. et al. Transforming growth factor β signaling inhibitor, SB-431542, induces maturation of dendritic cells and enhances anti-tumor activity. Oncol. Rep. 24, 1637–1643 (2010).
田中,H.等人。转化生长因子 β 信号传导抑制剂 SB-431542 可诱导树突状细胞成熟并增强抗肿瘤活性。安科尔。报告24,1637–1643 (2010)。Halder, S. K., Beauchamp, R. D. & Datta, P. K. A specific inhibitor of TGF-beta receptor kinase, SB-431542, as a potent antitumor agent for human cancers. Neoplasia 7, 509–521 (2005).
Halder, SK, Beauchamp, RD & Datta, PK TGF-β 受体激酶的特异性抑制剂 SB-431542,作为人类癌症的有效抗肿瘤剂。肿瘤7 , 509–521 (2005)。Lee, J. E. et al. Vactosertib, TGF-β receptor I inhibitor, augments the sensitization of the anti-cancer activity of gemcitabine in pancreatic cancer. Biomed. Pharmacother. 162, 114716 (2023).
李,JE 等人。 Vactosertib 是 TGF-β 受体 I 抑制剂,可增强吉西他滨在胰腺癌中的抗癌活性的敏感性。生物医学。药剂师。 162、114716 (2023)。Zhang, P. et al. The programmed site-specific delivery of LY3200882 and PD-L1 siRNA boosts immunotherapy for triple-negative breast cancer by remodeling tumor microenvironment. Biomaterials 284, 121518 (2022).
张,P.等人。 LY3200882 和 PD-L1 siRNA 的程序化位点特异性递送通过重塑肿瘤微环境来增强三阴性乳腺癌的免疫治疗。生物材料284 , 121518 (2022)。Chen, J. et al. TGF-β Signaling Activation Confers Anlotinib Resistance in Gastric Cancer. Pharm. Res 40, 689–699 (2023).
陈,J.等人。 TGF-β 信号传导激活导致胃癌对安罗替尼产生耐药性。医药。第 40 号决议,689–699(2023 年)。Fu, K. et al. SM16, an orally active TGF-beta type I receptor inhibitor prevents myofibroblast induction and vascular fibrosis in the rat carotid injury model. Arterioscler Thromb. Vasc. Biol. 28, 665–671 (2008).
傅,K.等人。 SM16 是一种口服活性 TGF-β I 型受体抑制剂,可预防大鼠颈动脉损伤模型中的肌成纤维细胞诱导和血管纤维化。动脉硬化血栓。瓦斯克。生物。 28、665-671 (2008)。Engebretsen, K. V. et al. Attenuated development of cardiac fibrosis in left ventricular pressure overload by SM16, an orally active inhibitor of ALK5. J. Mol. Cell Cardiol. 76, 148–157 (2014).
Engebretsen,KV 等人。 SM16(一种口服活性 ALK5 抑制剂)可减轻左心室压力超负荷时心脏纤维化的发展。 J.莫尔。细胞心脏。 76、148-157 (2014)。Atis, M. et al. Targeting the blood-brain barrier disruption in hypertension by ALK5/TGF-Β type I receptor inhibitor SB-431542 and dynamin inhibitor dynasore. Brain Res 1794, 148071 (2022).
阿蒂斯,M.等人。通过 ALK5/TGF-β I 型受体抑制剂 SB-431542 和动力抑制剂 dynasore,针对高血压中的血脑屏障破坏。脑研究1794 , 148071 (2022)。Wu, D. et al. TGF-β1-PML SUMOylation-peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Pin1) form a positive feedback loop to regulate cardiac fibrosis. J. Cell Physiol. 234, 6263–6273 (2019).
吴,D.等人。 TGF-β1-PML SUMOylation-peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Pin1) 形成正反馈环来调节心脏纤维化。 J.细胞生理学。 234、6263–6273 (2019)。Ha, K. B. et al. EW-7197 Attenuates the Progression of Diabetic Nephropathy in db/db Mice through Suppression of Fibrogenesis and Inflammation. Endocrinol. Metab. (Seoul.) 37, 96–111 (2022).
哈,KB等人。 EW-7197 通过抑制纤维生成和炎症来减缓 db/db 小鼠糖尿病肾病的进展。内分泌。元数据。 (首尔) 37,96–111 (2022)。Nassar, K. et al. A TGF-β receptor 1 inhibitor for prevention of proliferative vitreoretinopathy. Exp. Eye Res 123, 72–86 (2014).
纳萨尔,K.等人。一种 TGF-β 受体 1 抑制剂,用于预防增殖性玻璃体视网膜病变。过期。眼科研究123 , 72–86 (2014)。Maeda, S., Hayashi, M., Komiya, S., Imamura, T. & Miyazono, K. Endogenous TGF-beta signaling suppresses maturation of osteoblastic mesenchymal cells. Embo j. 23, 552–563 (2004).
Maeda, S.、Hayashi, M.、Komiya, S.、Imamura, T. 和 Miyazono, K. 内源性 TGF-β 信号传导抑制成骨细胞间充质细胞的成熟。恩博 J. 23、552-563 (2004)。Lee, A. J. et al. Sustained Delivery of SB-431542, a Type I Transforming Growth Factor Beta-1 Receptor Inhibitor, to Prevent Arthrofibrosis. Tissue Eng. Part A 27, 1411–1421 (2021).
李,AJ 等人。持续递送 SB-431542(一种 I 型转化生长因子 Beta-1 受体抑制剂)以预防关节纤维化。组织工程。 A 部分27,1411–1421 (2021)。Anscher, M. S. et al. Small molecular inhibitor of transforming growth factor-beta protects against development of radiation-induced lung injury. Int J. Radiat. Oncol. Biol. Phys. 71, 829–837 (2008).
安舍尔,MS 等人。转化生长因子-β 的小分子抑制剂可防止辐射引起的肺损伤。国际 J.辐射。安科尔。生物。物理。 71、829–837 (2008)。Park, S. A. et al. EW-7197 inhibits hepatic, renal, and pulmonary fibrosis by blocking TGF-β/Smad and ROS signaling. Cell Mol. Life Sci. 72, 2023–2039 (2015).
帕克,SA 等。 EW-7197 通过阻断 TGF-β/Smad 和 ROS 信号传导来抑制肝、肾和肺纤维化。细胞分子。生命科学。 72、2023-2039 (2015)。Alyoussef, A. Blocking TGF-β type 1 receptor partially reversed skin tissue damage in experimentally induced atopic dermatitis in mice. Cytokine 106, 45–53 (2018).
Alyoussef, A. 阻断 TGF-β 1 型受体可部分逆转实验诱导的小鼠特应性皮炎的皮肤组织损伤。细胞因子106,45-53 (2018)。Binabaj, M. M. et al. EW-7197 prevents ulcerative colitis-associated fibrosis and inflammation. J. Cell Physiol. 234, 11654–11661 (2019).
Binabaj,MM 等人。 EW-7197 可预防溃疡性结肠炎相关的纤维化和炎症。 J.细胞生理学。 234、11654–11661 (2019)。Ko, H. K. et al. The role of transforming growth factor-β2 in cigarette smoke-induced lung inflammation and injury. Life Sci. 320, 121539 (2023).
Ko,HK 等人。转化生长因子-β2 在香烟烟雾引起的肺部炎症和损伤中的作用。生命科学。 320、121539 (2023)。Waghabi, M. C. et al. Pharmacological inhibition of transforming growth factor beta signaling decreases infection and prevents heart damage in acute Chagas’ disease. Antimicrob. Agents Chemother. 53, 4694–4701 (2009).
瓦加比,MC 等人。转化生长因子β信号传导的药理学抑制可减少急性恰加斯病的感染并预防心脏损伤。抗菌剂。特工化疗。 53、4694-4701 (2009)。Waghabi, M. C. et al. SB-431542, a transforming growth factor beta inhibitor, impairs Trypanosoma cruzi infection in cardiomyocytes and parasite cycle completion. Antimicrob. Agents Chemother. 51, 2905–2910 (2007).
瓦加比,MC 等人。 SB-431542 是一种转化生长因子 β 抑制剂,可损害心肌细胞中的克氏锥虫感染和寄生虫周期的完成。抗菌剂。特工化疗。 51、2905-2910 (2007)。Mezger, M. C. et al. Inhibitors of Activin Receptor-like Kinase 5 Interfere with SARS-CoV-2 S-Protein Processing and Spike-Mediated Cell Fusion via Attenuation of Furin Expression. Viruses 14, 1308 (2022).
梅兹格,MC 等人。激活素受体样激酶 5 抑制剂通过减弱弗林蛋白酶表达来干扰 SARS-CoV-2 S 蛋白加工和刺突介导的细胞融合。病毒14 , 1308 (2022)。Xiao, Y. Q., Liu, K., Shen, J. F., Xu, G. T. & Ye, W. SB-431542 inhibition of scar formation after filtration surgery and its potential mechanism. Invest Ophthalmol. Vis. Sci. 50, 1698–1706 (2009).
肖,YQ,刘,K.,沉,JF,徐,GT&Ye,W.SB-431542抑制滤过手术后疤痕形成及其潜在机制。投资眼科。维斯。科学。 50,1698-1706 (2009)。Hasegawa, T., Nakao, A., Sumiyoshi, K., Tsuchihashi, H. & Ogawa, H. SB-431542 inhibits TGF-beta-induced contraction of collagen gel by normal and keloid fibroblasts. J. Dermatol Sci. 39, 33–38 (2005).
Hasekawa, T.、Nakao, A.、Sumiyoshi, K.、Tschihashi, H. 和 Okawa, H. SB-431542 抑制正常和瘢痕疙瘩成纤维细胞 TGF-β 诱导的胶原凝胶收缩。 J. Dermatol Sci。 39、33-38 (2005)。Soleimani, A. et al. Novel oral transforming growth factor-β signaling inhibitor potently inhibits postsurgical adhesion band formation. J. Cell Physiol. 235, 1349–1357 (2020).
苏莱马尼,A.等人。新型口服转化生长因子-β信号抑制剂可有效抑制术后粘连带的形成。 J.细胞生理学。 235、1349–1357 (2020)。Monteleone, G. et al. Phase I clinical trial of Smad7 knockdown using antisense oligonucleotide in patients with active Crohn’s disease. Mol. Ther. 20, 870–876 (2012).
蒙特莱昂,G.等人。在活动性克罗恩病患者中使用反义寡核苷酸敲低 Smad7 的 I 期临床试验。摩尔。瑟尔。 20、870-876 (2012)。Monteleone, G. et al. Mongersen, an oral SMAD7 antisense oligonucleotide, and Crohn’s disease. N. Engl. J. Med 372, 1104–1113 (2015).
蒙特莱昂,G.等人。 Mongersen,一种口服 SMAD7 反义寡核苷酸,与克罗恩病。 N. 英格兰。医学杂志372 , 1104–1113 (2015)。Sands, B. E. et al. Mongersen (GED-0301) for Active Crohn’s Disease: Results of a Phase 3 Study. Am. J. Gastroenterol. 115, 738–745 (2020).
Sands,BE 等。 Mongersen (GED-0301) 治疗活动性克罗恩病:3 期研究结果。是。 J.胃肠病学。 115、738–745 (2020)。Huo, D., Bi, X. Y., Zeng, J. L., Dai, D. M. & Dong, X. L. Drugs targeting TGF-β/Notch interaction attenuate hypertrophic scar formation by optic atrophy 1-mediated mitochondrial fusion. Mol. Cell Biochem. https://doi.org/10.1007/s11010-023-04912-y (2023).
Huo, D., Bi, XY, Zeng, JL, Dai, DM & Dong, XL 针对 TGF-β/Notch 相互作用的药物可减轻视神经萎缩 1 介导的线粒体融合引起的肥厚性疤痕形成。摩尔。细胞生物化学。 https://doi.org/10.1007/s11010-023-04912-y(2023 )。Zhang, C. et al. Mitomycin C induces pulmonary vascular endothelial-to-mesenchymal transition and pulmonary veno-occlusive disease via Smad3-dependent pathway in rats. Br. J. Pharm. 178, 217–235 (2021).
张,C.等人。 Mitomycin C 通过 Smad3 依赖性途径诱导大鼠肺血管内皮向间质转化和肺静脉闭塞性疾病。 Br。 J.Pharm。 178 , 217–235 (2021)。Meng, J. et al. Treatment of Hypertensive Heart Disease by Targeting Smad3 Signaling in Mice. Mol. Ther. Methods Clin. Dev. 18, 791–802 (2020).
孟,J.等人。通过靶向小鼠 Smad3 信号传导治疗高血压心脏病。摩尔。瑟尔。方法临床。开发。 18 , 791–802 (2020)。Liu, S. et al. Suppression of TGFβR-Smad3 pathway alleviates the syrinx induced by syringomyelia. Cell Biosci. 13, 98 (2023).
刘,S.等人。抑制 TGFβR-Smad3 通路可减轻脊髓空洞症引起的空洞。细胞生物科学。 13 , 98 (2023)。Ji, X. et al. Specific Inhibitor of Smad3 (SIS3) Attenuates Fibrosis, Apoptosis, and Inflammation in Unilateral Ureteral Obstruction Kidneys by Inhibition of Transforming Growth Factor β (TGF-β)/Smad3 Signaling. Med Sci. Monit. 24, 1633–1641 (2018).
吉,X.等人。 Smad3 (SIS3) 特异性抑制剂通过抑制转化生长因子 β (TGF-β)/Smad3 信号传导来减轻单侧输尿管梗阻肾的纤维化、细胞凋亡和炎症。医学科学。监视。 24,1633-1641 (2018)。Zhang, Y., Meng, X. M., Huang, X. R. & Lan, H. Y. The preventive and therapeutic implication for renal fibrosis by targetting TGF-β/Smad3 signaling. Clin. Sci. (Lond.) 132, 1403–1415 (2018).
张Y.,孟XM,黄XR和Lan HY通过靶向TGF-β/Smad3信号传导对肾纤维化的预防和治疗意义。临床。科学。 (伦敦) 132,1403–1415 (2018)。Pan, W. et al. SIS3 suppresses osteoclastogenesis and ameliorates bone loss in ovariectomized mice by modulating Nox4-dependent reactive oxygen species. Biochem Pharm. 195, 114846 (2022).
潘,W.等人。 SIS3 通过调节 Nox4 依赖性活性氧来抑制卵巢切除小鼠的破骨细胞生成并改善骨质流失。生化制药。 195、114846 (2022)。Shou, J. et al. SIS3, a specific inhibitor of smad3, attenuates bleomycin-induced pulmonary fibrosis in mice. Biochem Biophys. Res Commun. 503, 757–762 (2018).
寿,J.等人。 SIS3 是 smad3 的一种特异性抑制剂,可减轻博莱霉素诱导的小鼠肺纤维化。生物化学生物物理学。资源通讯。 503、757–762 (2018)。Rudnik, M. et al. Elevated Fibronectin Levels in Profibrotic CD14(+) Monocytes and CD14(+) Macrophages in Systemic Sclerosis. Front Immunol. 12, 642891 (2021).
鲁德尼克,M.等人。系统性硬化症中促纤维化 CD14(+) 单核细胞和 CD14(+) 巨噬细胞中纤连蛋白水平升高。前免疫学。 12、642891 (2021)。Xiang, W. et al. Inhibition of SMAD3 effectively reduces ADAMTS-5 expression in the early stages of osteoarthritis. BMC Musculoskelet. Disord. 24, 130 (2023).
向,W.等人。抑制 SMAD3 可有效降低骨关节炎早期的 ADAMTS-5 表达。 BMC 肌肉骨骼。混乱。 24、130 (2023)。He, H. et al. Treatment for type 2 diabetes and diabetic nephropathy by targeting Smad3 signaling. Int J. Biol. Sci. 20, 200–217 (2024).
他,H.等人。通过靶向 Smad3 信号传导治疗 2 型糖尿病和糖尿病肾病。 Int J.Biol。科学。 20 , 200–217 (2024)。He, H. et al. Smad3 Mediates Diabetic Dyslipidemia and Fatty Liver in db/db Mice by Targeting PPARδ. Int J. Mol. Sci. 24, 11396 (2023).
他,H.等人。 Smad3 通过靶向 PPARδ 介导 db/db 小鼠的糖尿病血脂异常和脂肪肝。国际 J. 摩尔。科学。 24 , 11396 (2023)。Wu, C. P. et al. SIS3, a specific inhibitor of Smad3 reverses ABCB1- and ABCG2-mediated multidrug resistance in cancer cell lines. Cancer Lett. 433, 259–272 (2018).
吴CP等人。 SIS3 是 Smad3 的一种特异性抑制剂,可逆转癌细胞系中 ABCB1 和 ABCG2 介导的多药耐药性。癌症快报。 433、259–272 (2018)。Chihara, Y. et al. A small-molecule inhibitor of SMAD3 attenuates resistance to anti-HER2 drugs in HER2-positive breast cancer cells. Breast Cancer Res Treat. 166, 55–68 (2017).
Chihara,Y.等人。 SMAD3 的小分子抑制剂可减弱 HER2 阳性乳腺癌细胞对抗 HER2 药物的耐药性。乳腺癌研究治疗。 166 , 55–68 (2017)。Conidi, A., van den Berghe, V. & Huylebroeck, D. Aptamers and their potential to selectively target aspects of EGF, Wnt/beta-catenin and TGFbeta-smad family signaling. Int J. Mol. Sci. 14, 6690–6719 (2013).
Conidi, A.、van den Berghe, V. 和 Huylebroeck, D. 适体及其选择性靶向 EGF、Wnt/β-catenin 和 TGFbeta-smad 家族信号传导方面的潜力。国际 J. 摩尔。科学。 14、6690-6719 (2013)。Lim, S. K. & Hoffmann, F. M. Smad4 cooperates with lymphoid enhancer-binding factor 1/T cell-specific factor to increase c-myc expression in the absence of TGF-beta signaling. Proc. Natl Acad. Sci. USA 103, 18580–18585 (2006).
Lim, SK & Hoffmann, FM Smad4 与淋巴增强子结合因子 1/T 细胞特异性因子配合,在缺乏 TGF-beta 信号传导的情况下增加 c-myc 表达。过程。国家科学院。科学。美国103,18580–18585 (2006)。Zhao, B. M. & Hoffmann, F. M. Inhibition of transforming growth factor-beta1-induced signaling and epithelial-to-mesenchymal transition by the Smad-binding peptide aptamer Trx-SARA. Mol. Biol. Cell 17, 3819–3831 (2006).
赵,BM 和霍夫曼,FM 通过 Smad 结合肽适体 Trx-SARA 抑制转化生长因子-β1 诱导的信号传导和上皮间质转化。摩尔。生物。细胞17,3819–3831 (2006)。Huang, C. et al. Expression, purification, and functional characterization of recombinant PTD-SARA. Acta Biochim Biophys. Sin. (Shanghai) 43, 110–117 (2011).
黄,C.等人。重组 PTD-SARA 的表达、纯化和功能表征。生物化学学报。罪。 (上海) 43,110-117 (2011)。Ji, W. P. & Dong, Y. Targeting Yes-associated Protein with Evolved Peptide Aptamers to Disrupt TGF-beta Signaling Pathway: Therapeutic Implication for Bone Tumor. Mol. Inf. 34, 771–777 (2015).
Ji, WP 和 Dong, Y. 用进化的肽适体靶向 Yes 相关蛋白以破坏 TGF-β 信号通路:骨肿瘤的治疗意义。摩尔。信息。 34、771-777 (2015)。Cheifetz, S. et al. Heterodimeric transforming growth factor beta. Biological properties and interaction with three types of cell surface receptors. J. Biol. Chem. 263, 10783–10789 (1988).
切菲茨,S.等人。异二聚体转化生长因子β。生物学特性以及与三种类型细胞表面受体的相互作用。 J.Biol。化学。 263、10783–10789 (1988)。Goumans, M. J. et al. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. Embo j. 21, 1743–1753 (2002).
古曼斯,MJ 等人。通过两种不同的 TGF-β I 型受体平衡内皮细胞的激活状态。恩博 J. 21,1743-1753 (2002)。van den Bosch, M. H. et al. Canonical Wnt signaling skews TGF-β signaling in chondrocytes towards signaling via ALK1 and Smad 1/5/8. Cell Signal 26, 951–958 (2014).
范登博斯,MH 等人。典型的 Wnt 信号传导使软骨细胞中的 TGF-β 信号转变成通过 ALK1 和 Smad 1/5/8 的信号传导。细胞信号26 , 951–958 (2014)。Pannu, J., Nakerakanti, S., Smith, E., ten Dijke, P. & Trojanowska, M. Transforming growth factor-beta receptor type I-dependent fibrogenic gene program is mediated via activation of Smad1 and ERK1/2 pathways. J. Biol. Chem. 282, 10405–10413 (2007).
Pannu, J.、Nakerakanti, S.、Smith, E.、10 Dijke, P. 和 Trojanowska, M. 转化生长因子-β 受体 I 型依赖性纤维化基因程序通过 Smad1 和 ERK1/2 途径的激活介导。 J.Biol。化学。 282、10405–10413 (2007)。Bharathy, S., Xie, W., Yingling, J. M. & Reiss, M. Cancer-associated transforming growth factor beta type II receptor gene mutant causes activation of bone morphogenic protein-Smads and invasive phenotype. Cancer Res 68, 1656–1666 (2008).
Bharathy, S., Xie, W., Yingling, JM & Reiss, M. 癌症相关转化生长因子β II 型受体基因突变体导致骨形态发生蛋白 Smads 激活和侵袭表型。癌症研究68,1656–1666 (2008)。Daly, A. C., Randall, R. A. & Hill, C. S. Transforming growth factor beta-induced Smad1/5 phosphorylation in epithelial cells is mediated by novel receptor complexes and is essential for anchorage-independent growth. Mol. Cell Biol. 28, 6889–6902 (2008).
Daly,AC,Randall,RA 和 Hill,CS 上皮细胞中转化生长因子 β 诱导的 Smad1/5 磷酸化是由新型受体复合物介导的,对于锚定非依赖性生长至关重要。摩尔。细胞生物学。 28、6889-6902 (2008)。Hussein, Y. M., Mohamed, R. H., El-Shahawy, E. E. & Alzahrani, S. S. Interaction between TGF-β1 (869C/T) polymorphism and biochemical risk factor for prediction of disease progression in rheumatoid arthritis. Gene 536, 393–397 (2014).
Hussein, YM, Mohamed, RH, El-Shahawy, EE & Alzahrani, SS TGF-β1 (869C/T) 多态性与生化危险因素之间的相互作用,用于预测类风湿关节炎疾病进展。基因536 , 393–397 (2014)。Nakao, E. et al. Elevated Plasma Transforming Growth Factor β1 Levels Predict the Development of Hypertension in Normotensives: The 14-Year Follow-Up Study. Am. J. Hypertens. 30, 808–814 (2017).
Nakao,E.等人。血浆转化生长因子 β1 水平升高可预测血压正常者发生高血压:14 年随访研究。是。 J.高血压。 30、808-814 (2017)。Kanzler, S. et al. Prediction of progressive liver fibrosis in hepatitis C infection by serum and tissue levels of transforming growth factor-beta. J. Viral Hepat. 8, 430–437 (2001).
Kanzler,S.等人。通过转化生长因子-β的血清和组织水平预测丙型肝炎感染中进行性肝纤维化。 J.病毒性肝炎。 8、430-437 (2001)。Wei, Y., Tian, Q., Zhao, X. & Wang, X. Serum transforming growth factor beta 3 predicts future development of nonalcoholic fatty liver disease. Int J. Clin. Exp. Med 8, 4545–4550 (2015).
Wei,Y.,Tian,Q.,Zhao,X.和Wang,X。血清转化生长因子β3预测非酒精性脂肪肝病的未来发展。 Int J. Clin。过期。医学8 , 4545–4550 (2015)。Wu, C. Y., Li, L. & Zhang, L. H. Detection of serum MCP-1 and TGF-β1 in polymyositis/dermatomyositis patients and its significance. Eur. J. Med Res 24, 12 (2019).
吴CY,李丽,张丽华,多发性肌炎/皮肌炎患者血清MCP-1、TGF-β1的检测及其意义。欧元。医学研究杂志24 , 12 (2019)。Boix, F. et al. A high concentration of TGF-β correlates with opportunistic infection in liver and kidney transplantation. Hum. Immunol. 82, 414–421 (2021).
博瓦,F.等人。高浓度的 TGF-β 与肝肾移植中的机会性感染相关。哼。免疫学。 82、414-421 (2021)。Leppäpuska, I. M. et al. Low TGF-β1 in Wound Exudate Predicts Surgical Site Infection After Axillary Lymph Node Dissection. J. Surg. Res 267, 302–308 (2021).
Leppäpuska,IM 等人。伤口渗出液中的低TGF-β1可预测腋窝淋巴结清扫术后的手术部位感染。 J.外科医生。第 267 号决议、第 302–308 号决议(2021 年)。Capuano, A. et al. Hepatocyte growth factor and transforming growth factor beta1 ratio at baseline can predict early response to cyclophosphamide in systemic lupus erythematosus nephritis. Arthritis Rheum. 54, 3633–3639 (2006).
卡普阿诺,A.等人。基线时肝细胞生长因子和转化生长因子β1的比率可以预测系统性红斑狼疮肾炎对环磷酰胺的早期反应。关节炎大黄。 54、3633-3639 (2006)。Daïen, C. I. et al. TGF beta1 polymorphisms are candidate predictors of the clinical response to rituximab in rheumatoid arthritis. Jt. Bone Spine 79, 471–475 (2012).
Daïen,CI 等。 TGFβ1 多态性是类风湿性关节炎中利妥昔单抗临床反应的候选预测因子。 Jt。骨脊柱79 , 471–475 (2012)。Sambuelli, A. et al. Serum transforming growth factor-beta1 levels increase in response to successful anti-inflammatory therapy in ulcerative colitis. Aliment Pharm. Ther. 14, 1443–1449 (2000).
Sambuelli,A.等人。溃疡性结肠炎抗炎治疗成功后,血清转化生长因子-β1 水平升高。营养制药。瑟尔。 14,1443-1449 (2000)。Rodrigues-Junior, D. M. et al. Circulating extracellular vesicle-associated TGFβ3 modulates response to cytotoxic therapy in head and neck squamous cell carcinoma. Carcinogenesis 40, 1452–1461 (2019).
罗德里格斯·朱尼尔,DM 等人。循环细胞外囊泡相关的 TGFβ3 调节头颈鳞状细胞癌对细胞毒治疗的反应。癌变40,1452–1461 (2019)。Scarpa, M. et al. TGF-beta1 and IGF-1 production and recurrence of Crohn’s disease after ileo-colonic resection. J. Surg. Res 152, 26–34 (2009).
斯卡帕,M.等人。回结肠切除术后 TGF-β1 和 IGF-1 的产生以及克罗恩病的复发。 J.外科医生。第 152 号决议,26-34 (2009)。Scarpa, M. et al. TGF-beta1 and IGF-1 and anastomotic recurrence of Crohn’s disease after ileo-colonic resection. J. Gastrointest. Surg. 12, 1981–1990 (2008).
斯卡帕,M.等人。 TGF-β1 和 IGF-1 与回结肠切除术后克罗恩病的吻合口复发。 J.胃肠测试。外科医生。 12、1981-1990 (2008)。Memon, A. A. et al. Transforming growth factor (TGF)-β levels and unprovoked recurrent venous thromboembolism. J. Thromb. Thrombolysis 38, 348–354 (2014).
梅蒙,AA 等人。转化生长因子 (TGF)-β 水平和无端复发性静脉血栓栓塞。 J.血栓。溶栓38 , 348–354 (2014)。Mattey, D. L., Nixon, N., Dawes, P. T. & Kerr, J. Association of polymorphism in the transforming growth factor {beta}1 gene with disease outcome and mortality in rheumatoid arthritis. Ann. Rheum. Dis. 64, 1190–1194 (2005).
Mattey, DL、Nixon, N.、Dawes, PT 和 Kerr, J. 转化生长因子 {β}1 基因多态性与类风湿关节炎疾病结果和死亡率的关联。安.感冒。迪斯。 64、1190-1194 (2005)。Watanabe, Y. et al. Transforming Growth Factor-β1 as a Predictor for the Development of Hepatocellular Carcinoma: A Nested Case-Controlled Study. EBioMedicine 12, 68–71 (2016).
渡边,Y.等人。转化生长因子-β1 作为肝细胞癌发生的预测因子:一项巢式病例对照研究。 EBioMedicine 12 , 68–71 (2016)。Ikeguchi, M., Iwamoto, A., Taniguchi, K., Katano, K. & Hirooka, Y. The gene expression level of transforming growth factor-beta (TGF-beta) as a biological prognostic marker of hepatocellular carcinoma. J. Exp. Clin. Cancer Res 24, 415–421 (2005).
Ikeguchi, M.、Iwamoto, A.、Taniguchi, K.、Katano, K. 和 Hirooka, Y. 转化生长因子-β (TGF-β) 的基因表达水平作为肝细胞癌的生物预后标志物。 J.Exp。临床。癌症研究24 , 415–421 (2005)。Wu, X. et al. Development of a TGF-β signaling-related genes signature to predict clinical prognosis and immunotherapy responses in clear cell renal cell carcinoma. Front Oncol. 13, 1124080 (2023).
吴,X.等人。开发 TGF-β 信号相关基因特征来预测透明细胞肾细胞癌的临床预后和免疫治疗反应。前安科尔。 13、1124080 (2023)。
Acknowledgements 致谢
Not applicable. 不适用。
Funding 资金
This work was supported by the National Key R&D Program of China (2021YFF1201303), the Beijing Natural Science Foundation (BJNSF) (7242119), and the CAMS Innovation Fund for Medical Sciences (CIFMS) (2021-I2M-1-012).
该工作得到了国家重点研发计划(2021YFF1201303)、北京市自然科学基金(BJNSF)(7242119)和中国医学科学院医学科学创新基金(CIFMS)(2021-I2M-1-012)的支持。
Author information 作者信息
Authors and Affiliations
Contributions
J.H. supervised the project. J.H., C.L., and T.F. conceived the idea. Z.D., T.F., and C.L. drafted the manuscript. Z.D., C.X., H.T., and Y.Z. polished the language. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations 道德声明
Competing interests 利益竞争
The authors declare no competing interests.
作者声明没有竞争利益。
Ethics approval and consent to participate
道德批准并同意参与
Not applicable. 不适用。
Consent for publication 同意发表
Not applicable. 不适用。
Rights and permissions 权利和权限
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
开放获取本文根据知识共享署名 4.0 国际许可证获得许可,该许可证允许以任何媒介或格式使用、共享、改编、分发和复制,只要您对原作者和来源给予适当的认可,提供知识共享许可的链接,并指出是否进行了更改。本文中的图像或其他第三方材料包含在文章的知识共享许可中,除非材料的出处中另有说明。如果文章的知识共享许可中未包含材料,并且您的预期用途不受法律法规允许或超出了允许的用途,则您需要直接获得版权所有者的许可。要查看此许可证的副本,请访问http://creativecommons.org/licenses/by/4.0/ 。
About this article
Cite this article
Deng, Z., Fan, T., Xiao, C. et al. TGF-β signaling in health, disease and therapeutics. Sig Transduct Target Ther 9, 61 (2024). https://doi.org/10.1038/s41392-024-01764-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41392-024-01764-w
Subjects
This article is cited by
这篇文章被引用
-
Effect of 3-hydrazinylquinoxaline-2-thiol hydrogel on skin wound healing process in diabetic rats
3-肼基喹喔啉-2-硫醇水凝胶对糖尿病大鼠皮肤伤口愈合过程的影响Scientific Reports (2024)
科学报告(2024) -
Anti-synthetase and myelodysplastic syndromes with deep morphea: an example of shared immunopathogenesis? A case-based review
抗合成酶和骨髓增生异常综合征伴深部硬斑病:共同免疫发病机制的一个例子?基于案例的审查Rheumatology International (2024)
国际风湿病学(2024) -
Thunbergia’s Flowers Secondary Metabolites a Natural Armor Against Kidney Damage by Diclofenac
山楂花的次生代谢物是对抗双氯芬酸肾损伤的天然盔甲Cell Biochemistry and Biophysics (2024)
细胞生物化学与生物物理学(2024) -
TNF-α and RPLP0 drive the apoptosis of endothelial cells and increase susceptibility to high-altitude pulmonary edema
TNF-α和RPLP0驱动内皮细胞凋亡并增加高原肺水肿的易感性Apoptosis (2024)
细胞凋亡(2024)