Abstract 抽象
揭示单晶高温合金的初始氧化行为对于更好地了解涡轮叶片在使用条件下的氧化机制具有重要意义。当前研究的目的是观察单晶高温合金的初始氧化。仅在热暴露和热应力模式下进行了原位氧化实验。讨论了氧化皮成核和生长的机制。结果表明,γ/γ′ 相界面上的氧化物由 Al2O3 沉淀物组成,由 Al 原子或离子的外部扩散形成。负载应力增强了 Al 原子的扩散,导致高氧化速率。提出了一个对数模型,并与氧化过程很好地拟合。
Similar content viewed
其他人正在查看的相似内容
其他人正在查看的相似内容
Introduction 介绍
镍基单晶高温合金因其优异的蠕变和机械性能而被广泛用于涡轮叶片和现代航空发动机的其他热端部件1,2。为了提高涡轮机效率,提高涡轮机入口温度对高温合金的运行极限提出了挑战3。这种高温还导致旋转部件(涡轮盘和叶片)承受高离心应力4。实际上,除了高温和重应力外,氧化和热腐蚀不可避免地会降低涡轮叶片在使用状态下的性能5,6。尽管已经开发了热障涂层 (TBC) 技术来避免高温气体和涡轮叶片之间的直接接触7,并在一定程度上防止氧气与叶片之间的直接接触,但氧化仍然是不可避免的,尤其是当 TBC 发生剥落或开裂时。在许多情况下,氧化被描述为疲劳损伤8 的前体,也是薄壁高温合金9 蠕变性能较差的原因。因此,为了更好地了解氧化过程非常重要。
Though there are extensive researches focusing on oxidation behavior of superalloy during intermediate and high temperature10,11,12
尽管对高温合金在中高温下的氧化行为进行了广泛的研究10,11,12,但考虑到粘附氧化皮的快速形成,尚未清楚地观察到初始氧化过程。通过原位环境透射电子显微镜 (ETEM),Ding13 观察到仅在热暴露下发生初始氧化,并证明氧扩散路径更倾向于成为界面而不是基质通道14。然而,考虑到实际使用条件,在紧急情况下,涡轮叶片的温度可以达到1150°C左右甚至1200°C15,并且[001]热应力引起的载荷方向的变化约为400MPa16。到目前为止,结合热和应力的初始氧化的研究很少报道。
本文通过在 1150 °C 和 1150 °C/330 MPa 的扫描电子显微镜 (SEM) 中在 2 × 10−9 atm 的氧分压下进行原位实验,研究了镍基单晶高温合金在仅热暴露和热应力模式下氧化过程中的微观组织演变。透射电子显微镜 (TEM) 对氧化皮进行定性表征。讨论了氧化物成核和生长的机制。
Methods 方法
Materials 材料
[001] 取向镍基单晶高温合金棒的长度为170 mm,直径为15 mm,其化学成分为4.3 wt% Cr、9.1 wt% Co、8.0 wt% W、5.2 wt% Al、6.0 wt% Ta、2.5 wt% Re、1.5 wt% Mo、0.1 wt% Hf、0.5 wt% Nb等为Ni。标准热处理参照前文第17、18 条进行。然后,将合金棒沿[001]方向和垂直于[010]方向加工成原位试样,试样的形状如图1所示。1(a) 的,尺寸标记为图 1。1(b) 的。样品中间的狭窄区域用于 SEM 中的微观结构观察。将样品机械抛光至镜面,然后在 HNO3 (40%) + H3PO4 (12%) + H2SO4 (48%) 制成的溶液中蚀刻 10 秒。图 1(c) 显示了低倍率下的标本整体显微照片,γ微观结构如图 1(d) 所示 。它表明 γ' 长方体(有序 L12 相,平均立方体边长:450 nm)被较亮的薄γ通道(FCC 晶体结构,平均通道宽度:80 nm)隔开,并均匀分布在整个材料中。
Instrument 仪器
In-situ
合著者开发的原位拉伸试验设备由如图 2(b) 所示 的高温拉伸试验机和如图 2 所示的 SEM (TESCAN S8000) 组成。2(a) 项。镍基单晶高温合金试样固定在加载框架夹具上,位于加热器表面的正上方,如图 2(b) 所示。样品中心的加热区域直径为 8 mm,热电偶接触样品的背面。在 SEM 真空室中进行了 1150 °C 热暴露和 330 MPa 热暴露下的原位实验,腔室压力保持在 10−3 Pa,氧分压约为 2 × 10−9 个大气压,实验过程中进行了实时观察。
采用 OXFORD X-Max 能量色散谱 (EDS) 对 FEI Titan G2 TEM 中实验前后的元素分布进行了表征,并通过 FEI Helios NanoLab FIB 从样品中提取样品。
The pattern stress
加热和应力加载的模式
加热和应力加载的模式
在本文中,通过在 0 ~ 8.3 V 范围内调节输入电压,通过电加热实现加热过程,通过单轴拉伸实现应力加载过程,设备的详细信息已在我们之前的研究 19 中报道,图19。3 显示了实验过程中不同时间的温度、位移和加载应力。开始时,温度通过增加电压而升高,温度曲线的转折点是由电压的突然增加引起的。加热 140 min 后温度达到 1150 °C。然后,它保持在 1150 °C,这段时间可以称为热暴露。热暴露 100 分钟后,应力加载了 1 μm/s 的拉伸位移,如图 1 中的红色和蓝色虚线所示。3. 虽然在加热前预加载了约 50 N 的力,但考虑到热膨胀的影响,应力加载过程并没有立即增加。当应力达到 330 MPa 时,停止位移以保持应力稳定。原位观测时间位于 160 ~ 340 min 左右。最后,试样在炉中冷却至室温,并在取出试样之前卸下应力。
Result 结果
Microstructure evolution
图 4 显示了高温合金在 1150 °C 热暴露条件下不同时间的一系列微观组织。热暴露 20 min 后,样品的微观结构与初始相同。热暴露 40 分钟后,小沉淀物出现在 γ′/γ 相的起始界面上,并且喜欢沿 [001] 方向聚集,如图 4(c) 中的 红色箭头所示。样品氧化 80 分钟后,小沉淀物也出现并沿 [100] 方向定位,如图 4(d) 中的 蓝色箭头所示。随着热暴露时间的增加,沉淀物在界面上缓慢生长。此外,γ' 相上的沉淀物显示出成核和生长的趋势,如图 4(e,f) 中 清楚地显示。
热暴露氧化 120 min 后,应力沿 [001] 方向加载到同一试样上,并逐渐增加到 330 MPa。虽然应力剂量不会立即增加,但试样的位移(或更准确地说是应变)随着时间的推移而增加,如图 3 所示 。当应力加载时,沉淀物似乎增长得更快。沉淀物在 135 min 的热暴露和 15 min 的应力加载后进展良好,它们已经完全占据了原始界面,如图 5(b) 所示 。图 5(c) 中观察到的沉淀物的增长更为明显,它表明沉淀物的增长方向是向外的,这使得该区域在图 5 中标记为虚线。4(a) 变小。然后,沉淀物在 175 分钟后生长趋于缓慢,如图 1 所示。5(d-f)。
SEM 图像显示了在 1150 °C 热暴露期间 (a) 120 min、(b) 135 min、(c) 150 min、(d) 175 min、(e) 190 min 和 (f) 200 min 期间应力加载下的微观结构演变。
Element
图 6(a) 显示了实验前样品表面的 HAADF STEM 概览图像,可以清楚地看到典型的立方体 γ/γ' 微观结构,它们的界面很明显。图 6(b–f) 显示了以 EDS 为特征的样品的代表性元素分布图,Ni3Al γ' 相显示 Al 和 Ni 的浓度。γ相主要包含 Ni(与 γ′ 相相比含量较低)、Cr、Co 和 Re,这些元素均匀地分离在 γ 通道中。
氧化实验后,用 EDS 绘制了图 7(a-f) 中 风冷样品的元素分布,Al 和 O 元素的分布几乎相同,表明沉淀物主要是 Al2O3,这也存在于 Weiser20 报道γ通道中。在本文中,更准确地说,清楚地观察到两相的原始界面上或称为γ相的侧面上的成核。元素 Cr、Co 和 Re 向 γ' 相的扩散略有发生。虽然 γ 相覆盖着 Al2O3 氧化层,但 Al 在氧化物-合金界面旁边被耗尽。然后 Ni 在图 4(e) 所示的 Al 耗尽区富集,这种现象类似于 Wagner 对 Cu-Pt 和 Cu-Pd 合金的实验21。实际上,γ/γ' 相的原始界面、γ相的侧面和氧化物-合金界面是相同的界面。
氧化实验后,风冷样品的元素分布如图 1 所示。EDS 的 7(a–f) 、Al 和 O 元素显示出几乎相同的分布,表明沉淀物主要是 Al2O3,这也存在于 Weiser20 报道的γ通道中。在本文中,更准确地说,清楚地观察到两相的原始界面上或称为γ相的侧面上的成核。元素 Cr、Co 和 Re 向 γ' 相的扩散略有发生。虽然 γ 相覆盖着 Al2O3 氧化层,但 Al 在氧化物-合金界面旁边被耗尽。然后 Ni 在 Al 耗尽区富集,如图 1 所示。4(e) 的,这种现象类似于瓦格纳对 Cu-Pt 和 Cu-Pd 合金的实验21。实际上,γ/γ' 相的原始界面、γ相的侧面和氧化物-合金界面是相同的界面。
为了量化氧化后不同位置的成分,从样品表面采集了几次 EDS 线扫描,并穿过 γ 和 γ' 相,如图 7(b) 和图 7(b) 和图 7 中的水平红色虚线所示。 5. 合金氧化物表面在图 5 中标记为蓝色虚线。8 根据 Al 和 O 元素含量高分别由扩散和吸附引起。γ通道的宽度约为 70 nm,接近图 1(d) 中描述的平均宽度 80 nm。此外,Ta、W、Co、Cr 甚至 Re 在氧化物标度中表现出轻微的扩散,这是由浓度梯度引起的。此外,一些化合物氧化物可能会在氧化皮的新表面附近形成,但可以忽略不计。新表面由图 8 中的黄色虚线显示。可以看出,侧向氧化皮的厚度可以达到近 90 nm。该值略高于讨论部分中分析的值,因为氧化更有可能在冷却期间进行,并且还选择了不同的区域。
图 9(a-f) 显示了氧化后样品深度方向上 O、Al、Cr、Co、Ni 和 Re 的元素分布。可见在底部界面上 γ/γ′ 相也发生氧化。图 9(a) 中由白色虚线环绕的严重氧化区与图 9(a) 中的相似性。7(b) 中,这表明氧化发生在 γ/γ′ 相的侧面和底部界面上。它也可以从图 5(c-f) 中原始侧界面形态的变化中推断出来。底部形成的氧化皮厚度约为 50 nm。Cr、Co、Ni 和 Re 元素的扩散似乎是每周的深度方向。
图 10 显示了在深度方向上穿过 γ 和 γ' 相的元素分布,扫描线如图 9(b) 中的 红色虚线所示。可以清楚地看到,扩散只发生在最靠近表面的区域。在 γ/γ' 相的界面上有一个相当薄的氧化层,它与图 1 中的严重氧化区域完全对应。9(a) 及其值约为 30 nm。
为了更准确地分析形成的氧化皮,进行了详细的 TEM 实验。图 11(a) 是氧化皮和高温合金的 HAADF-STEM 图像,应该注意的是,包括 Cr、Co 和 Re 在内的其他元素都在基体高温合金中,而不是氧化皮,这些元素没有显示在图 11(b) 中。O 和 Al 的比率(图 1 中红色框标记的区域)。11(b))EDS 计算为 1.44,这与 Al2O3 的元素比例非常接近。此外,高分辨率 STEM 图像 (图 11c) 进一步支持了这些发现。图 1 中标记的蓝色虚线框区的晶面间距。计算出 11c,值为 2.3 Å,对应于 α-Al2O322 的 [110] 晶面间距。红色虚线箱区的晶面间距为 1.77 Å,对应于 Ni 的 [200] 晶面间距(fcc 晶体结构)。
Discussion 讨论
Oxide scale mechanism
进入氧后,O2 分子在 γ 相的表面解离并扩散,该过程也报道在 Cu-Au 合金的表面23。与 Ni 元素相比,Al 对 O 表现出更大的亲和力,然后 Al 原子向合金-氧界面扩散,并且也可能通过阳离子空位传输24。一旦 Al2O3 沉淀成核γ 相的原始表面,如图 4(b-d) 所示 。Al 离子可以通过氧化皮的位错和 Al2O3 晶粒之间的边界进行传输,氧化皮以向外传输的形式增长25,这样的过程如图 12(a) 所示 。
当应力加载时,γ相的位错会增加并聚集在 γ 相和 γ' 相的界面上,从而导致空位增加26,27。位错形成的空位和通道将增强 Al 原子的扩散,如图 12(b) 所示 。此外,在加载应力作用下,Al2O3 晶粒的位错和氧化皮中晶界上的缺陷可能会增加,沿这些缺陷的 Al 离子传输也会增强,这导致氧化皮的增长呈加速趋势,如图 5(a–f) 所示 。
Oxide scale
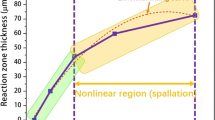
为了量化氧化程度,用红色虚线勾勒出氧化物-氧界面,如图 4(a) 所示。在本文中,选择了四个区域,每个区域在不同时间通过软件图像 J 测量,如图 13(a) 所示 。在热暴露期间,会发现轻微移动,如图 13(a) 中的 黑色轮廓和蓝色轮廓所示。一旦应力加载,氧化物的增长就会变得相当大,如图 13(a) 所示 为绿色和红色轮廓。由于形状几乎是正方形的,因此与整个形状相比,可以忽略略微锯齿形的轮廓。因此,不同时期的氧化物厚度可以描述为
Where d(t) is the thickness of oxide, St and S0 are the area of surrounded by oxide at t moment and initial time. Lt and L0
其中 d(t) 是氧化物的厚度,St 和 S0 是在 t 时刻和初始时间被氧化物包围的面积。Lt 和 L0 分别是周围区域在 t 时刻和初始时间的边长。
不同时间侧向氧化层的厚度如图 13(b) 中的 散点图所示。在初始氧化的早期阶段,金属的氧化以恒定的速率进行,这遵循“线性速率定律”,这个时期得到了很好的验证,特别是对于区域 3 的厚度在 180 min 到 260 min 期间增加。在该阶段,氧的化学吸附是 Pettit28 研究的速率控制步骤,Neil29 已经描述了这一步骤。然而,在本文中,由于 SEM 室中的高真空导致氧分子与合金表面碰撞的可能性很低,因此热暴露期间的速率非常小。一旦加载应力,氧化速率就会异常增加,这可能是由合金和氧化皮中 Al 原子或离子的扩散增强引发的。此外,对数模型 (d(t) = A + kln(t + B)) 仍然与应力加载热暴露过程中的氧化过程拟合良好,这表明应力不会使氧化皮在很大程度上变形,扩散仍然是速率控制步骤。
表 1 比较了四个区域的拟合参数值。可以理解,kln 的值随着面积的增加而增加,这意味着面积越大或边越长,出现氧化现象越多。这主要是由于γ相较长侧的位错密度较高,而位错的高密度增强了Al原子的扩散过程。
表 1 热暴露和应力负载期间的氧化速率常数。
Conclusions 结论
在仅 1150 °C 的温度下观察到镍基单晶高温合金上的氧化物成核和生长和热应力模式(1150 °C 和 /330 MPa)。在 γ/γ' 相界面上生长的氧化皮由 α-Al2O3 沉淀物组成。负载应力增强了 Al 原子在 γ 相中的扩散和 Al 离子在氧化皮中的扩散,并导致高氧化速率。对数模型与应力加载热暴露期间的氧化过程拟合良好
在仅 1150 °C 的温度下观察到镍基单晶高温合金上的氧化物成核和生长和热应力模式(1150 °C 和 /330 MPa)。在 γ/γ' 相界面上生长的氧化皮由 α-Al2O3 沉淀物组成。负载应力增强了 Al 原子在 γ 相中的扩散和 Al 离子在氧化皮中的扩散,并导致高氧化速率。对数模型与应力加载热暴露期间的氧化过程拟合良好
References 引用
Zhang, L. et al. study of slip-controlled short-crack growth in single-crystal nickel superalloy. Mater. Sci. Eng., A. 742, 564–572 (2019).
Zhang, L. 等人。单晶镍高温合金中滑移控制短裂纹扩展的研究。材料科学工程,A.742, 564–572 (2019 年)。Joyce, M., Wu, X. & Reed, P. The Mater Lett. 58, 99–103 (2004).effect fatigue
乔伊斯,M.,吴,X. & 里德,P.环境和取向对 CMSX-4 镍基单晶在 650 °C 下疲劳裂纹扩展行为的影响。58, 99–103 (2004 年)。Foss, B., Hardy, M., Child, D., McPhail, D. & Shollock, B. Oxidation of a commercial nickel-based superalloy under static loading. JOM. 66, 2516–2524 (2014).
Yuri, M., Masada, J., Tsukagoshi, K., Ito, E. & Hada, S. Development of 1600 C-class high-efficiency gas turbine for power Mitsubishi Heavy Ind. Tech. Rev. 50, 1–10 (2013).generation applying Khajavi, M. & Shariat, M. Failure of first stage gas turbine blades. Eng. Fail. Anal. 11, 589–597 (2004).
Bhagi, L. K., Gupta, P. & Rastogi, V. A brief Proceedings STME-2013 Smart Technologies for Mechanical Engineering, Delhi, 25–26 (2013).review Hardwicke, C. U. & Lau, Y.-C. J. Therm. Spray Technol. 22, 564–576 (2013).Advances generation review Zhang, J., Harada, H., Koizumi, Y. & Kobayashi, T. Crack Scripta Mater. 61, 1105–1108 (2009).appearance fatigue Bensch, M., Konrad, C., Fleischmann, E., Rae, C. & Glatzel, U. Mater. Sci. Eng., A. 577, 179–188 (2013).Influence Sato, A., Chiu, Y.-L. & Reed, R. Oxidation of nickel-based single-crystal superalloys for industrial gas turbine applications. Acta Mater. 59, 225–240 (2011).
Evangelou, A., Soady, K., Lockyer, S., Gao, N. & Reed, P. Oxidation behaviour of single crystal nickel-based superalloys: intermediate temperature Mater. Sci. Technol. 34, 1679–1692 (2018).effects Li, M. et al. Oxidation behavior of a single-crystal Ni-base superalloy in air. I: at 800 and 900 C. Oxid Met. 59, 591–605 (2003).
Ding, Q. et al. In-situ
environmental TEM study of γ′-γ phase transformation J. Alloy Compd. 651, 255–258 (2015).induced Edmonds, I., Evans, H., Jones, C. & Broomfield, R. Intermediate temperature internal oxidation in fourth Oxid Met. 69, 95–108 (2008).generation Viguier, B., Touratier, F. & Andrieu, E. High-temperature creep of single-crystal nickel-based superalloy: microstructural changes and Philos. Mag. 91, 4427–4446 (2011).effects Dye, D., Ma, A. & Reed, R. C. Numerical modelling of creep deformation in a CMSX-4 single crystal superalloy turbine blade. Superalloys. 911, e919 (2008).
Xiong, X. et al. Tensile behavior of nickel-base single-crystal superalloy DD6. Mater. Sci. Eng., A. 636, 608–612 (2015).
Jichun, X., Jiarong, L. & Shizhong, L. Surface recrystallization in nickel base single crystal superalloy DD6. Chin. J. Aeronaut. 23, 478–485 (2010).
Ma, J. et al. Investigation of In Situ
1150 degrees C High Temperature Deformation Behavior and Fracture Acta Metall Sin. 55, 987–996 (2019).Mechanism Generation Weiser, M., Eggeler, Y. M., Spiecker, E. & Virtanen, S. Early stages of Corros Sci. 135, 78–86 (2018).scale Wagner, C. Internal oxidation of Cu-Pd and Cu-Pt alloys. Corros Sci. 8, 889–893 (1968).
Ishizawa, N. et al. A structural investigation of α-Al2O3 at 2170 K. Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem. 36, 228–230 (1980).
Wang, L., Zhou, G.-W., Eastman, J. A. & Yang, J. C. Initial oxidation kinetics and energetics of Cu0. 5Au0. 5 (0 0 1) film in situ ultrahigh vacuum transmission electron microscopy.investigated Surf Choi, S., Cho, H., Kim, Y. & Lee, D. High-temperature oxidation behavior of pure Ni 3 Al. Oxid Met. 46, 51–72 (1996).
Heuer, A. et al
. On the growth of Al2O3 Acta Mater. 61, 6670–6683 (2013).scales Shishvan, S. S., McMeeking, R. M., Pollock, T. M. & Deshpande, V. S. Discrete dislocation plasticity analysis of the Acta Mater. 135, 188–200 (2017).effect Gao, S., Fivel, M., Ma, A. & Hartmaier, A. 3D discrete dislocation dynamics study of creep behavior in Ni-base single crystal superalloys by a J. Mech. Phys. Solids. 102, 209–223 (2017).combined Pettit, F. & Wagner, J. Jr. Transition from the linear to the parabolic Acta Metall Mater. 12, 35–40 (1964).rate Birks, N., Meier, G. H. & Pettit, F. S. Introduction to the high temperature oxidation of metals. (Cambridge University Press, 2006).
Acknowledgements
Author information
Authors and Affiliations
Contributions
Corresponding author
Ethics declarations
Competing
Additional
Publisher’s note
Rights and permissions
About this article
Cite
Ma, J., Jiang, W., Wang, J. et al.
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-59968-3
Subjects
This article is cited by
-
Oxidation behavior of Ni-based superalloy GH4738 under tensile stress
Rare Metals (2024)
-
In situ EBSD study of formation and propagation of deformation bands in a single-crystal superalloy during tensile deformation
Journal of Materials Science (2023)
-
Oxidation behavior of a nickel-based single crystal superalloy at 1100 °C under different oxygen concentration
Journal of Materials Science (2022)