Abstract 抽象的
Endoplasmic reticulum (ER)-mitochondria regions are specialized subdomains called also mitochondria-associated membranes (MAMs). MAMs allow regulation of lipid synthesis and represent hubs for ion and metabolite signaling. As these two organelles can module both the amplitude and the spatiotemporal patterns of calcium (Ca2+) signals, this particular interaction controls several Ca2+-dependent pathways well known for their contribution to tumorigenesis, such as metabolism, survival, sensitivity to cell death, and metastasis. Mitochondria-mediated apoptosis arises from mitochondrial Ca2+ overload, permeabilization of the mitochondrial outer membrane, and the release of mitochondrial apoptotic factors into the cytosol. Decreases in Ca2+ signaling at the ER-mitochondria interface are being studied in depth as failure of apoptotic-dependent cell death is one of the predominant characteristics of cancer cells. However, some recent papers that linked MAMs Ca2+ crosstalk-related upregulation to tumor onset and progression have aroused the interest of the scientific community.
内质网 (ER) 线粒体区域是专门的子域,也称为线粒体相关膜 (MAM)。 MAM 可以调节脂质合成,并代表离子和代谢物信号传导的中心。由于这两个细胞器可以调节钙 (Ca 2+ ) 信号的幅度和时空模式,因此这种特殊的相互作用控制着数条 Ca 2+依赖性途径,这些途径因其对肿瘤发生的贡献而闻名,例如代谢、存活、细胞敏感性死亡、转移。线粒体介导的细胞凋亡由线粒体 Ca 2+超载、线粒体外膜的透化以及线粒体凋亡因子释放到细胞质中引起。由于细胞凋亡依赖性细胞死亡失败是癌细胞的主要特征之一,因此正在深入研究 ER-线粒体界面处 Ca 2+信号传导的减少。然而,最近的一些论文将 MAM Ca 2+串扰相关的上调与肿瘤的发生和进展联系起来,引起了科学界的兴趣。
In this review, we will describe how different MAMs-localized proteins modulate the effectiveness of Ca2+-dependent apoptotic stimuli by causing both increases and decreases in the ER-mitochondria interplay and, specifically, by modulating Ca2+ signaling.
在这篇综述中,我们将描述不同的 MAM 定位蛋白如何通过引起 ER-线粒体相互作用的增加和减少,特别是通过调节 Ca 2+信号传导来调节 Ca 2+依赖性细胞凋亡刺激的有效性。
Access provided by University of Toronto Collections Development Department.
Download chapter PDF
访问权限由多伦多大学馆藏开发部提供。下载章节 PDF
Similar content being viewed by others
其他人正在查看类似内容
Keywords 关键词
1 Introduction
1简介
Ca2+ is the third most abundant metal in nature, and it was adopted as a regulator in the early evolutionary stages in prokaryotes (Cai et al. 2015). Ca2+ ions play a crucial role in countless biological processes, and one of their most important contributions is undoubtedly represented by Ca2+ signaling, a complex network of extra- and intracellular messenger systems that mediates a wide range of pathways (Rimessi et al. 2020). The characterization of the complex network involving Ca2+ signaling has been in progress for approximately 140 years since the first experiments examining the contraction of isolated rat hearts (Ringer 1883). Since then, extensive progress has been made in understanding the numerous molecular pathways involved, although many aspects are still being debated and still need to be defined. Evolutionarily, cells have developed systems to constantly maintain Ca2+ concentrations at very low background levels to avoid the precipitation of phosphate salts, making this ion the logical choice for the exchange of signals (Carafoli and Krebs 2016). The crucial role of Ca2+ in cell biology results from the ability of cells to shape Ca2+ signals in the dimensions of space, time, and amplitude (Alonso et al. 2009).
Ca 2+是自然界中第三丰富的金属,它在原核生物的早期进化阶段被用作调节剂(Cai et al. 2015 )。 Ca 2+离子在无数的生物过程中发挥着至关重要的作用,其最重要的贡献之一无疑是 Ca 2+信号传导,这是一个介导多种途径的细胞外和细胞内信使系统的复杂网络(Rimessi 等) 2020 )。自第一次检查离体大鼠心脏收缩的实验以来,涉及 Ca 2+信号传导的复杂网络的表征已经进行了大约 140 年(Ringer 1883 )。从那时起,尽管许多方面仍在争论并且仍需要定义,但在理解所涉及的众多分子途径方面取得了广泛进展。经过进化,细胞已经开发出了能够将 Ca 2+浓度持续维持在极低背景水平的系统,以避免磷酸盐沉淀,从而使该离子成为信号交换的合理选择(Carafoli 和 Krebs 2016 )。 Ca 2+在细胞生物学中的关键作用源于细胞在空间、时间和幅度维度上形成 Ca 2+信号的能力(Alonso et al. 2009 )。
Ca2+ enters cells through an assortment of Ca2+-permeable channels that respond to different stimuli or acts as a second messenger, e.g., in the phosphoinositol signaling pathway, in which inositol trisphosphate (IP3) binds to Ca2+ channels on the endoplasmic reticulum (ER), transporting Ca2+ into the cytoplasm. Once in the cell, the effects of Ca2+ can be mediated by direct binding to its effectors, such as the phosphatase calcineurin, or indirectly by activating the ubiquitous Ca2+-binding protein calmodulin, leading to the regulation of target molecules such as the Ca2+/calmodulin-dependent kinases CaMKII and CaMKIV (Kerkhofs et al. 2017). Temporally and spatially defined Ca2+ changes in the cytoplasm or in well-defined organelles represent a highly versatile intracellular signal capable of regulating many different processes, including depolarization, hormonal secretion, contraction of smooth and striated muscles, and cellular replication and activation of cytoplasmic, mitochondrial, and nuclear enzymes (Giorgi et al. 2018a).
Ca 2+通过各种 Ca 2+渗透性通道进入细胞,这些通道响应不同的刺激或充当第二信使,例如在磷酸肌醇信号传导途径中,其中肌醇三磷酸 (IP3) 与细胞上的 Ca 2+通道结合。内质网(ER),将Ca 2+转运到细胞质中。一旦进入细胞,Ca 2+的作用可以通过直接结合其效应物(例如磷酸酶钙调神经磷酸酶)来介导,或通过激活普遍存在的 Ca 2+结合蛋白钙调蛋白来间接介导,从而调节靶分子,例如Ca 2+ /钙调蛋白依赖性激酶 CaMKII 和 CaMKIV (Kerkhofs et al. 2017 )。细胞质或明确细胞器中的时间和空间限定的 Ca 2+变化代表了高度通用的细胞内信号,能够调节许多不同的过程,包括去极化、激素分泌、平滑肌和横纹肌的收缩以及细胞复制和细胞质激活、线粒体和核酶(Giorgi 等人, 2018a )。
Proteins that participate in Ca2+ signaling are mostly ubiquitous, but their distribution is highly tissue-specific (Berridge et al. 2003). Cells that need rapid Ca2+ signals, such as myocytes, express many voltage-activated calcium channels to allow quick Ca2+ entry through the plasma membrane, which then, via ryanodine receptors (RyRs) on the sarcoplasmic reticulum, triggers further calcium release. However, nonexcitable cells display calcium oscillations that last for tens of seconds and preferentially use the phosphoinositol signaling pathway to control gene expression and metabolism (Cui et al. 2017).
参与Ca 2+信号传导的蛋白质大多普遍存在,但它们的分布具有高度的组织特异性(Berridge et al. 2003 )。需要快速 Ca 2+信号的细胞(例如肌细胞)表达许多电压激活的钙通道,以允许 Ca 2+快速穿过质膜进入,然后通过肌浆网上的兰尼碱受体 (RyR) 触发进一步的钙释放。然而,非兴奋性细胞会表现出持续数十秒的钙振荡,并优先使用磷酸肌醇信号通路来控制基因表达和代谢(Cui et al. 2017 )。
Therefore, a lack of Ca2+ ions can lead to various issues, and excess Ca2+ ions have harmful effects. Indeed, a sustained rise in intracellular Ca2+ is considered the initial step of irreversible cellular injury, mediated by the activation of the intracellular self-destructive lysosomal enzymes responsible for breakdown of subcellular organelle membranes and increases in oxidative stress and for the hyperactivation of phospholipases and endonucleases, which, through DNA damage, participate in apoptosis (Danese et al. 2017). Intracellular Ca2+ signals are controlled by Ca2+ influx through the plasma membrane (PM) and Ca2+ release from intracellular stores, mainly the ER and Golgi. Intracellular Ca2+ stores are constantly refilled while cytosolic Ca2+ is extruded from the cell by the plasma membrane Ca2+ ATPase (PMCA) pump, to maintain the optimal cytosolic Ca2+ concentration (Marchi et al. 2018).
因此,缺乏Ca 2+离子会导致各种问题,而过量的Ca 2+离子则会产生有害影响。事实上,细胞内 Ca 2+ 的持续升高被认为是不可逆细胞损伤的第一步,由细胞内自毁性溶酶体酶的激活介导,该酶负责亚细胞细胞器膜的分解、氧化应激的增加以及磷脂酶的过度激活和核酸内切酶,它们通过 DNA 损伤参与细胞凋亡 (Danese et al. 2017 )。细胞内 Ca 2+信号由通过质膜 (PM) 的 Ca 2+流入和细胞内储存(主要是内质网和高尔基体)释放的 Ca 2+控制。细胞内 Ca 2+储存不断补充,而胞质 Ca 2+则通过质膜 Ca 2+ ATPase (PMCA) 泵从细胞中挤出,以维持最佳胞质 Ca 2+浓度 (Marchi et al. 2018 )。
In the cell, one of the organelles in which changes in [Ca2+] are particularly important is the mitochondrion (Giorgi et al. 2018b), which decodes Ca2+ signals in very sensitive and specific inputs that regulate metabolism, energy production, autophagy, and apoptosis (Giorgi et al. 2018a).
在细胞中,[Ca 2+ ] 变化特别重要的细胞器之一是线粒体(Giorgi et al. 2018b ),它以非常敏感和特定的输入方式解码 Ca 2+信号,调节新陈代谢、能量产生、自噬和细胞凋亡(Giorgi et al. 2018a )。
Membrane juxtaposition of both the mitochondria and the ER leads to the highly specialized MAMs compartment, which can be defined as areas of close organelle apposition but that are biochemically distinct from pure mitochondria and pure ER (Morciano et al. 2018). These contact sites are part of abundant heterotypic contacts, which, especially in recent years, have been well characterized and which include the ER-plasma membrane, ER-Golgi, lipid droplets–peroxisomes, mitochondria-lipid droplets, mitochondria–vacuoles/endosomes/lysosomes, ER-lipid droplets, mitochondria-plasma membrane, mitochondria–peroxisomes, ER-lipid droplets, and mitochondrial inner and outer membranes (Eisenberg-Bord et al. 2016).
线粒体和内质网的膜并置导致了高度专业化的 MAM 区室,它可以被定义为紧密细胞器并置的区域,但在生化上与纯线粒体和纯内质网不同 (Morciano et al. 2018 )。这些接触位点是丰富的异型接触的一部分,特别是近年来,这些接触已得到很好的表征,包括内质网质膜、内质网高尔基体、脂滴-过氧化物酶体、线粒体-脂滴、线粒体-液泡/内体/溶酶体、ER-脂滴、线粒体-质膜、线粒体-过氧化物酶体、ER-脂滴和线粒体内膜和外膜 (Eisenberg-Bord等2016 )。
To witness the strong tethering between the ER and mitochondria, an isolated MAM fraction contains membrane fragments of the outer mitochondrial membrane, the ER, and some plasma membrane proteins (Poston et al. 2013). Tomography analysis has revealed the morphology of these ER-mitochondria-connecting tethers (Csordas et al. 2006). The maintenance of this delicate structural juxtaposition results strategic for the regulation of a huge number of biological processes, essentially through Ca2+ exchange. Poston et al. reported that there are around 1,000 molecular components of the MAMs fraction (Poston et al. 2013) and their study led to an elucidation of the multiple roles played by this particular subcellular compartment. In particular, MAMs co-regulate and influence Ca2+ signaling/dynamics, synthesis/transport of lipids and lipid intermediates, autophagy, apoptosis, and energy metabolism.
为了见证 ER 和线粒体之间的牢固束缚,分离的 MAM 组分包含线粒体外膜、ER 和一些质膜蛋白的膜片段(Poston 等人, 2013 )。断层扫描分析揭示了这些内质网线粒体连接链的形态(Csordas 等人, 2006 )。这种微妙的结构并置的维持对大量生物过程的调节具有战略意义,主要是通过 Ca 2+交换。波斯顿等人。据报道,MAM 部分约有 1,000 种分子成分(Poston 等人, 2013 ),他们的研究阐明了这种特定亚细胞区室所发挥的多重作用。特别是,MAM 共同调节和影响 Ca 2+信号/动力学、脂质和脂质中间体的合成/运输、自噬、细胞凋亡和能量代谢。
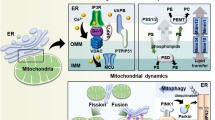
Noteworthy is the fact that MAM structures are sensitive to physiological cell conditions and this reflects in a transient and highly variable MAM composition. The length of ER-mitochondria tethers is a determining factor, critical for an efficient Ca2+ transfer, and an ER-mitochondria physical distance modulation is a condition found in different pathophysiological situations. About that, these two organelles’ interplay is also involved in mitochondrial shape and size, and MAM-regulated mitochondrial fusion/fission process undoubtedly covers a crucial role in governing mitochondrial dynamics. Dynamin-related protein 1 (Drp1) is responsible for mitochondrial fission; following its activation, Drp1 translocates from the cytosol to the mitochondria and oligomerizes and constricts this organelle until its division is achieved. Focusing on mitochondrial fusion, mitofusin 1 (Mfn1) and mitofusin 2 (Mfn2) are responsible for the outer membrane fusion, while optic atrophy 1 (Opa1) mediates mitochondrial inner membrane fusion (Ponte et al. 2020).
值得注意的是,MAM 结构对生理细胞条件敏感,这反映在瞬态且高度可变的 MAM 组成中。 ER-线粒体系链的长度是一个决定因素,对于有效的Ca 2+转移至关重要,并且ER-线粒体物理距离调制是在不同病理生理情况下发现的条件。关于这一点,这两种细胞器的相互作用也涉及线粒体的形状和大小,MAM 调节的线粒体融合/裂变过程无疑在控制线粒体动力学方面发挥着至关重要的作用。动力相关蛋白 1 (Drp1) 负责线粒体裂变;激活后,Drp1 从细胞质转移到线粒体,并使该细胞器寡聚化并收缩,直至实现分裂。重点关注线粒体融合,线粒体融合蛋白 1 (Mfn1) 和线粒体融合蛋白 2 (Mfn2) 负责外膜融合,而视神经萎缩 1 (Opa1) 介导线粒体内膜融合 (Ponte et al. 2020 )。
MAMs are enriched in channels involved in calcium transfer, allowing perfect and synergistic signaling between the ER and mitochondria. Moreover, MAMs target many proteins with oncogenic/oncosuppressive functions that modulate cell signaling pathways involved in physiopathological processes (Danese et al. 2017).
MAM 富含参与钙转移的通道,从而在 ER 和线粒体之间实现完美且协同的信号传导。此外,MAM 靶向许多具有致癌/抑癌功能的蛋白质,调节参与病理生理过程的细胞信号传导途径 (Danese et al. 2017 )。
As Ca2+ signaling-governed processes (such as energy production, metabolism, autophagy, and apoptosis) are dysregulated in cancer cells and play a key role in Ca2+ transfer and signaling in MAMs, the perturbation of these Ca2+ transport systems at the ER and the mitochondria in relation to tumor onset and progression has become a very hot topic, especially in recent times. In fact, the recent characterization of the many oncogenes and tumor suppressors residing at the MAMs has led many research groups to elucidate how these proteins mediate their functions by altering ER-mitochondrial Ca2+ transfer, thereby promoting or preventing cancer cell survival. Increases or decreases in calcium exchange through the MAMs interface can either exert protumorigenic effects (such as promoting metastatic transformations) or antitumorigenic effects (such as restoring sensitivity to apoptosis) in a cancer type- and cancer state-specific manner (Kerkhofs et al. 2018).
由于 Ca 2+信号传导控制的过程(例如能量产生、代谢、自噬和细胞凋亡)在癌细胞中失调,并且在 MAM 中的 Ca 2+转移和信号传导中发挥关键作用,因此这些 Ca 2+运输系统的扰动内质网和线粒体与肿瘤发生和进展的关系已成为一个非常热门的话题,特别是在最近。事实上,最近对 MAM 中许多癌基因和肿瘤抑制因子的表征已促使许多研究小组阐明这些蛋白质如何通过改变 ER 线粒体 Ca 2+转移来介导其功能,从而促进或阻止癌细胞存活。通过 MAM 界面增加或减少钙交换可以以癌症类型和癌症状态特异性的方式发挥促肿瘤作用(例如促进转移转化)或抗肿瘤作用(例如恢复对细胞凋亡的敏感性)(Kerkhofs 等人, 2018) )。
The aim of this review is to clarify how the perturbation of Ca2+ signaling at the ER-mitochondria interface can play a double-sided role in tumor pathology and progression. Modulation of calcium signaling at the MAMs, highly dynamic signaling hubs, could therefore represent a good therapeutic strategy in the future.
本综述的目的是阐明 ER-线粒体界面 Ca 2+信号传导的扰动如何在肿瘤病理学和进展中发挥双面作用。因此,调节 MAM(高度动态的信号中枢)的钙信号传导可能代表着未来良好的治疗策略。
2 MAM-Localized Ca2+ Signaling Modulators in Cancer: Channels and Receptors
2癌症中 MAM 定位的 Ca 2+信号调节剂:通道和受体
Ca2+ signaling represents an important tool that regulates many physiological cellular events from proliferation to cell death. Given the pivotal role it plays in such events, it is understandable why, over the past decades, remodeling of its shape has been demonstrated to be involved in the onset of many pathological conditions, such as tumor progression (Monteith et al. 2012; Prevarskaya et al. 2014; Marchi et al. 2020). Proteins involved in the maintenance of Ca2+ homeostasis consist of pumps, exchangers, and channels and have been described as part of the Ca2+ signaling “toolkit” (Berridge et al. 2003).
Ca 2+信号传导是调节从增殖到细胞死亡的许多生理细胞事件的重要工具。鉴于它在此类事件中发挥的关键作用,可以理解为什么在过去的几十年中,其形状的重塑已被证明与许多病理状况的发生有关,例如肿瘤进展(Monteith et al. 2012 ; Prevarskaya等人, 2014 年;马尔奇等人, 2020 年)。参与维持 Ca 2+稳态的蛋白质由泵、交换器和通道组成,并被描述为 Ca 2+信号传导“工具包”的一部分(Berridge 等人, 2003 )。
In resting conditions, the free cytosolic Ca2+ concentration is much lower than that in most extracellular fluids, and an ion concentration gradient is generated. Thus, when Ca2+-permeable ion channels in the plasma membrane are open, Ca2+ flux into the cell increases (Carafoli 2002). However, as already mentioned, Ca2+ signaling can be generated by both external and internal cellular sources.
在静息条件下,游离胞质Ca 2+浓度远低于大多数细胞外液中的浓度,并产生离子浓度梯度。因此,当质膜中的Ca 2+可渗透离子通道打开时,进入细胞的Ca 2+通量增加(Carafoli 2002 )。然而,正如已经提到的,Ca 2+信号传导可以由外部和内部细胞源产生。
In the cell, the main ion reservoir from which Ca2+ can be transferred is the endoplasmic reticulum. On the one hand, the ER is the primary cell Ca2+ store; on the other hand, the main cellular Ca2+ signaling translators are the mitochondria.
在细胞中,Ca 2+可以从中转移的主要离子库是内质网。一方面,ER是原代细胞Ca 2+ 的储存;另一方面,主要的细胞Ca 2+信号传导转译者是线粒体。
Indeed, depletion of luminal ER Ca2+ levels is followed by a rapid increase in ion mitochondrial concentration. To ensure this interaction is effective, the ER and the mitochondria are juxtaposed on the MAMs at a short distance of approximately 10–25 nm (Csordas et al. 2006; Rizzuto et al. 1998; Marchi et al. 2014) in the smooth ER and at approximately 50 nm in the rough ER (Wang et al. 2015; Giacomello and Pellegrini 2016).
事实上,腔内 ER Ca 2+水平耗尽后,线粒体离子浓度迅速增加。为了确保这种相互作用有效,内质网和线粒体在平滑内质网中以大约 10-25 nm 的短距离并置在 MAM 上(Csordas 等人, 2006 年;Rizzuto 等人, 1998 年;Marchi 等人, 2014 年)粗内质网中的波长约为 50 nm(Wang 等人, 2015 年;Giacomello 和 Pellegrini ,2016 年)。
2.1 ER Side
2.1急诊室侧
Many ER-resident proteins involved in Ca2+ transfer have been found at the MAMs: the sarco-/endoplasmic reticulum Ca2+ ATPase (SERCA) and inositol 1,4,5-trisphosphate receptors (IP3R), among others. SERCAs are members of the P-type ATPase superfamily of primary active transporters (a large family of membrane-embedded pumps (Wang et al. 2015)) and can maintain the correct cytosolic and reticular Ca2+ concentrations.
在 MAM 中发现了许多参与 Ca 2+转移的内质网驻留蛋白:肌浆/内质网 Ca 2+ ATP 酶 (SERCA) 和肌醇 1,4,5-三磷酸受体 (IP3R) 等。 SERCA 是初级活性转运蛋白 P 型 ATP 酶超家族(膜嵌入泵大家族 (Wang et al. 2015 ))的成员,可以维持正确的胞质和网状 Ca 2+浓度。
The 110 kDa SERCA protein has 10 helix intramembrane domains involved in the interaction with two Ca2+ ions transferred to the ER lumen at the expense of adenosine triphosphate (ATP). The Ca2+ flux is coupled to the exchange of two to three protons moved to the cytoplasm (Palmgren and Nissen 2011). In addition to transmembrane domains, SERCA has three cytoplasmic regions: the nucleotide-binding domain (N), designed for ATP binding; the phosphorylation (P) domain, which hosts the amino acid residue phosphorylated by ATP; and the actuator (A) domain at the N-terminus, which controls enzyme dephosphorylation. During ATP hydrolysis, conformational changes in the protein domains occur, and as consequence, the intermembrane domains warp, enabling Ca2+ transport (Toyoshima et al. 2000; Moller et al. 2010).
110 kDa SERCA 蛋白具有 10 个螺旋膜内结构域,参与与转移到 ER 腔的两个 Ca 2+离子的相互作用,但需要消耗三磷酸腺苷 (ATP)。 Ca 2+通量与移动到细胞质的两到三个质子的交换耦合(Palmgren 和 Nissen 2011 )。除了跨膜结构域外,SERCA 还具有三个细胞质区域:核苷酸结合结构域 (N),设计用于 ATP 结合;磷酸化 (P) 结构域,包含被 ATP 磷酸化的氨基酸残基; N 末端的致动器 (A) 结构域控制酶的去磷酸化。在 ATP 水解过程中,蛋白质结构域发生构象变化,结果导致膜间结构域扭曲,从而实现 Ca 2+转运(Toyoshima 等人, 2000 ;Moller 等人, 2010 )。
To date, at least 12 isoforms of SERCA (SERCA1a-b, SERCA2a-d, SERCA3a-f) have been identified in vertebrates (Lipskaia et al. 2014), each characterized by tissue and developmental specificity. This diversity is because SERCAs are encoded by three different genes located on three chromosomes (ATP2A1, ATP2A2, and ATP2A3), each generating alternative splicing variants that differ mainly in the C-terminus of the protein.
迄今为止,已在脊椎动物中鉴定出至少 12 种 SERCA 亚型(SERCA1a-b、SERCA2a-d、SERCA3a-f)(Lipskaia 等, 2014 ),每种亚型均具有组织和发育特异性。这种多样性是因为 SERCA 由位于三个染色体上的三个不同基因(ATP2A1、ATP2A2 和 ATP2A3)编码,每个基因都会产生主要在蛋白质 C 末端不同的替代剪接变体。
The diversities in the coding sequencing of these proteins do not affect the protein tertiary structures, which are highly conserved among all isoforms, but instead lead to differences in Ca2+ affinity. Among all these proteins, ubiquitous SERCA2b is the isoform with the highest Ca2+ affinity and plays a crucial role in the regulation of ER Ca2+ uptake and Ca2+ homeostasis (Vandecaetsbeek et al. 2009). All SERCA isoforms are present along the entire ER membrane and are not particularly enriched in MAMs.
这些蛋白质编码序列的多样性不会影响蛋白质三级结构,这些结构在所有异构体中高度保守,但会导致Ca 2+亲和力的差异。在所有这些蛋白质中,普遍存在的 SERCA2b 是 Ca 2+亲和力最高的亚型,在调节 ER Ca 2+摄取和 Ca 2+稳态中发挥着至关重要的作用 (Vandecaetsbeek et al. 2009 )。所有 SERCA 亚型都存在于整个 ER 膜上,并且在 MAM 中并不是特别富集。
SERCA activity can be modulated by many proteins. Among them, the recently identified ER-luminal protein disulfide isomerase thioredoxin-related transmembrane protein 1 (TMX1) displays palmitoylation-dependent MAMs localization. TMX1 can directly interact with SERCA2b (Gutierrez and Simmen 2018; Lynes et al. 2012) and inhibit its activity, reducing Ca2+ transfer.
SERCA 活性可以通过许多蛋白质调节。其中,最近鉴定的内质网管腔蛋白二硫键异构酶硫氧还蛋白相关跨膜蛋白1(TMX1)显示出棕榈酰化依赖性MAMs定位。 TMX1 可以直接与 SERCA2b 相互作用(Gutierrez 和 Simmen 2018 ;Lynes 等人2012 )并抑制其活性,减少 Ca 2+转移。
If SERCA activity is lowered by TMX1, its activity is enhanced by the redox active form of the redox-sensitive selenoprotein N (SEPN1) (Gutierrez and Simmen 2018). MAMs result particularly enriched in redox regulatory proteins, and TMX1 and SEPN1 are among them (Krols et al. 2016; Marino et al. 2015).
如果 TMX1 降低 SERCA 活性,则氧化还原敏感硒蛋白 N (SEPN1) 的氧化还原活性形式会增强其活性 (Gutierrez 和 Simmen 2018 )。 MAM 特别富含氧化还原调节蛋白,TMX1 和 SEPN1 就是其中之一(Krols 等人, 2016 年;Marino 等人, 2015 年)。
Calnexin is a chaperone protein that localizes at the ER-mitochondrial contact sites in a palmitoylation-dependent manner (Lynes et al. 2012). The primary function of this protein is to interact with misfolded proteins to improve the folding efficiency of ER proteins (Lamriben et al. 2016). Upon palmitoylation, calnexin moves to the MAMs, where it interacts with SERCA2b, increasing Ca2+ transfer from the cytosol to the ER (Lynes et al. 2013). Interestingly, the modulation of SERCA2b activity by calnexin is counteracted by TMX1 in a way that may suggest competition for the same binding site (Krols et al. 2016; Raturi et al. 2016).
钙连接蛋白是一种伴侣蛋白,以棕榈酰化依赖性方式定位于内质网线粒体接触位点(Lynes et al. 2012 )。该蛋白的主要功能是与错误折叠的蛋白相互作用,以提高 ER 蛋白的折叠效率 (Lamriben et al. 2016 )。棕榈酰化后,钙联蛋白移动到 MAM,与 SERCA2b 相互作用,增加 Ca 2+从细胞质到内质网的转移 (Lynes et al. 2013 )。有趣的是,钙联蛋白对 SERCA2b 活性的调节被 TMX1 抵消,这可能表明对同一结合位点的竞争(Krols 等人, 2016 年;Raturi 等人, 2016 年)。
IP3Rs are large-conductance nonselective cation channels that together with the RyRs, which is mainly expressed in sarcoplasmic reticulum, are major structures through which Ca2+ exits the ER (Ashby and Tepikin 2001).
IP3Rs是大电导非选择性阳离子通道,与主要在肌浆网中表达的RyRs一起,是Ca 2+离开内质网的主要结构(Ashby and Tepikin 2001 )。
IP3R channels are homo- or heterotetramers composed of four subunits of approximately 300 kDa each. The molecular structure of the IP3R monomer, determined by cryogenic electron microscopy, consists of three structural domains: an N-terminal ligand-binding domain, containing both the IP3-binding core and the suppressor region, a central modulatory domain, and a Ca2+ channel region at the C-terminus containing six intramembrane helices. The C-tails interact directly with the N-terminal domains of the other subunits (Fan et al. 2015).
IP3R 通道是由四个亚基组成的同源或异源四聚体,每个亚基约 300 kDa。通过低温电子显微镜确定的 IP3R 单体的分子结构由三个结构域组成:N 端配体结合域(包含 IP3 结合核心和抑制区域)、中央调节域和 Ca 2 + C 末端的通道区域包含六个膜内螺旋。 C 尾直接与其他亚基的 N 端结构域相互作用(Fan 等人, 2015 )。
In vertebrates, there are three different isoforms of IP3R (IP3R1, IP3R2, and IP3R3) encoded by three genes (ITPPR1, ITPR2, and ITPR3, in humans). Despite the high homology in the amino acid sequences (60–80%), these isoforms have a different expression pattern, with IP3R1 mainly expressed in neuronal cells, IP3R2 in muscle and liver cells, and ubiquitous IP3R3 in most cultured cells (Mikoshiba 2007; Foskett et al. 2007). In addition, the different isoforms show differences in ligand-binding sensitivity and regulation by Ca2+ and ATP (Newton et al. 1994; Miyakawa et al. 1999; Tu et al. 2005; Khan et al. 2006; Betzenhauser et al. 2008; Wagner 2nd et al. 2008; Vervloessem et al. 2015).
在脊椎动物中,IP3R 存在三种不同的亚型(IP3R1、IP3R2 和 IP3R3),由三个基因(在人类中为 ITPPR1、ITPR2 和 ITPR3)编码。尽管氨基酸序列具有高度同源性(60-80%),但这些亚型具有不同的表达模式,IP3R1主要在神经元细胞中表达,IP3R2在肌肉和肝细胞中表达,而IP3R3在大多数培养细胞中普遍存在(Mikoshiba 2007 ;福斯克特等人, 2007 年。此外,不同的亚型在配体结合敏感性以及Ca 2+和ATP 调节方面表现出差异(Newton 等人, 1994 年;Miyakawa 等人, 1999 年;Tu 等人, 2005 年;Khan 等人, 2006 年;Betzenhauser 等人,2006 年)。 2008 ;瓦格纳 2nd 等人, 2015年)。
IP3Rs are enriched at MAMs levels, where they also exert a structural role, being in close proximity with the mitochondrial voltage-dependent anion channel 1 (VDAC1) and by interacting with the chaperone glucose-regulated protein GRP75 which acts as a tether between the two proteins and organelles (Bernard-Marissal et al. 2018). It has also been recently highlighted that IP3R isoforms differently regulate ER-mitochondrial contacts and local calcium transfer, proving that IP3Rs structural role in MAM compartment is crucial (Bartok et al. 2019).
IP3R 在 MAM 水平上富集,它们也发挥结构作用,与线粒体电压依赖性阴离子通道 1 (VDAC1) 紧密相连,并通过与伴侣葡萄糖调节蛋白 GRP75 相互作用,GRP75 充当两者之间的系绳蛋白质和细胞器(Bernard-Marissal 等人, 2018 )。最近还强调,IP3R 亚型以不同方式调节 ER 线粒体接触和局部钙转移,证明 IP3R 在 MAM 区室中的结构作用至关重要 (Bartok et al. 2019 )。
The activity of IP3R receptors is regulated primarily by inositol trisphosphate (IP3), released at the plasma membrane after the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) by phospholipase C (PLC).
IP3R 受体的活性主要受肌醇三磷酸 (IP3) 调节,在磷脂酶 C (PLC) 水解磷脂酰肌醇 4,5-二磷酸 (PIP2) 后,IP3 在质膜上释放。
However, IP3Rs can also be modulated by ATP, post-translational modification (Mak and Foskett 2015; Bansaghi et al. 2014; Yule et al. 2010; Prole and Taylor 2016; Ivanova et al. 2014; Ramos-Franco et al. 1998), and Ca2+ ions, which act both from the luminal ER side, increasing the sensitivity to its ligand, and from the cytoplasmatic sides from which Ca2+ plays a dual role as an activator at low concentrations and an inhibitor if its concentration is higher than 300 nM (Table 1).
然而,IP3R 也可以通过 ATP、翻译后修饰进行调节(Mak 和 Foskett 2015 ;Bansaghi 等人2014 ;Yule 等人2010 ;Prole 和 Taylor 2016 ;Ivanova 等人2014 ;Ramos-Franco 等人1998) ) 和 Ca 2+离子,它们都从管腔起作用ER 侧,增加对其配体的敏感性,并且来自细胞质侧,Ca 2+在低浓度下发挥双重作用,作为激活剂,如果其浓度高于300 nM,则作为抑制剂(表1 )。
As noted earlier, there is a juxtaposition between the two MAM-forming organelles, and Ca2+ release from the ER is followed by uptake at the mitochondrial interface.
如前所述,两个 MAM 形成细胞器之间并置,Ca 2+从内质网释放后被线粒体界面吸收。
2.2 Mitochondrial Side
2.2线粒体侧
After being released from the ER, Ca2+ ions can first cross the outer mitochondrial membrane through VDAC and, once in the mitochondrial intramembrane space, enter the matrix through the mitochondrial Ca2+ uniporter (MCU).
从内质网释放后,Ca 2+离子首先可以通过 VDAC 穿过线粒体外膜,一旦进入线粒体膜内空间,就通过线粒体 Ca 2+单向转运蛋白 (MCU) 进入基质。
VDAC is a 30-kDa protein existing in all eukaryotic cells in three different isoforms: VDAC1 and VDAC2 are expressed in most mammals, and VDAC3 is the isoform with the lowest expression (De Pinto et al. 2010; Huang et al. 2014; Maldonado et al. 2013). VDAC is the most abundant outer mitochondrial membrane protein, and due to its permeability not only to anions but also to respiratory substrates, ATP, reactive oxygen species (ROS), and cytochrome C can be considered master regulators of mitochondrial bioenergetics (Shoshan-Barmatz et al. 2010; Weisthal et al. 2014). The permeability of this channel is highly impacted by its two conformational states, opened and closed, since in the closed state, the channel is permeable only to small ions but not to anionic metabolites (Shoshan-Barmatz et al. 2010; Gincel et al. 2000; Schein et al. 1976). The switch between the opened and closed states is regulated by many factors, including Bcl2 family members (Tsujimoto and Shimizu 2000), Ca2+ ions (Bathori et al. 2006), and voltage. Indeed, high mitochondrial voltages induce VDAC to close (Gincel et al. 2000) in a N-terminus-mediated manner (Abu-Hamad et al. 2009).
VDAC 是一种 30-kDa 的蛋白质,以三种不同的亚型存在于所有真核细胞中:VDAC1 和 VDAC2 在大多数哺乳动物中表达,VDAC3 是表达最低的亚型(De Pinto 等人, 2010 ;Huang 等人, 2014 ;Maldonado)等人, 2013 )。 VDAC 是最丰富的线粒体外膜蛋白,由于其不仅对阴离子具有渗透性,而且对呼吸底物、ATP、活性氧 (ROS) 和细胞色素 C 也具有渗透性,因此可被认为是线粒体生物能学的主要调节因子(Shoshan-Barmatz 等)韦斯塔尔等人, 2014 年)。该通道的渗透性受到其两种构象状态(打开和关闭)的高度影响,因为在关闭状态下,该通道仅可渗透小离子,但不可渗透阴离子代谢物(Shoshan-Barmatz 等人, 2010 年;Gincel 等人,2010 年)。 2000 ;沙因等, 1976 。打开和关闭状态之间的切换受到许多因素的调节,包括Bcl2家族成员(Tsujimoto和Shimizu 2000 )、Ca 2+离子(Bathori et al. 2006 )和电压。事实上,高线粒体电压会诱导 VDAC 以 N 末端介导的方式关闭 (Gincel et al. 2000 ) (Abu-Hamad et al. 2009 )。
Among VDAC channels, the most frequently expressed and consequently studied isoform is VDAC1 (Messina et al. 2012), which has been shown to be targeted to the MAMs (Hajnoczky et al. 2002; Shoshan-Barmatz and Gincel 2003; Colombini 2012) and to regulate the Ca2+ flux through the mitochondria outer membrane (Rapizzi et al. 2002). If regulation of mitochondrial Ca2+ signaling is not a unique feature of VDAC1, the ability to transmit proapoptotic stimuli to the mitochondria seems to be an exclusive characteristic of this isoform (De Stefani et al. 2012).
在 VDAC 通道中,最常表达并因此被研究的亚型是 VDAC1(Messina 等人, 2012 ),它已被证明是针对 MAM(Hajnoczky 等人, 2002 ;Shoshan-Barmatz 和 Gincel ,2003 ;Colombini ,2012 )和调节通过线粒体外膜的 Ca 2+通量(Rapizzi 等人, 2002 )。如果线粒体 Ca 2+信号传导的调节不是 VDAC1 的独特特征,那么将促凋亡刺激传递至线粒体的能力似乎是该亚型的独有特征 (De Stefani et al. 2012 )。
To reach the mitochondrial matrix and regulate all the previously mentioned processes, Ca2+ entering the outer mitochondrial membrane has to permeate the inner mitochondrial membrane that, unlike the outer membrane, is not permeable to ions. The accumulation of Ca2+ inside the mitochondrial matrix follows an electrogenic gradient and is driven by the low Ca2+ affinity uniporter complex MCU. Due to the low Ca2+ affinity of this MCU complex, the rapid mitochondrial ion accumulation is difficult to explain without considering the presence of close contacts between the ER and the mitochondria, which create microdomains with a high Ca2+ concentration (Rizzuto et al. 1998).
为了到达线粒体基质并调节所有前面提到的过程,进入线粒体外膜的 Ca 2+必须渗透线粒体内膜,与外膜不同,线粒体内膜不可渗透离子。 Ca 2+在线粒体基质内的积累遵循生电梯度,并由低 Ca 2+亲和力单向转运蛋白复合物 MCU 驱动。由于该 MCU 复合物的 Ca 2+亲和力较低,如果不考虑 ER 和线粒体之间存在的紧密接触(这会产生具有高 Ca 2+浓度的微域),则很难解释线粒体离子的快速积累(Rizzuto 等人) 1998 )。
MCU is a complex of approximately 480 kDa composed of the channel-forming subunits MCUa and MCUb, organized mainly in pentamers. MCUa and MCUb have opposite effects on Ca2+ ion transfer (allowing and inhibiting permeation, respectively), and their relative quantities in the complex regulate the Ca2+ transport capability of MCU itself. In addition to the channel-forming subunits, mitochondrial calcium uptake 1 and 2 (MICU1 and MICU2) and the essential MICU regulator (EMRE) are part of the uniporter complex and play a pivotal role in regulating the integrity of the complex itself (De Stefani et al. 2015; Oxenoid et al. 2016; Raffaello et al. 2013; Sancak et al. 2013). MCU complexes were enriched in MAMs, positioned more to the mitochondrial periphery, indicating high accessibility to cytoplasm-derived Ca2+ inputs (Marchi et al. 2017).
MCU 是大约 480 kDa 的复合体,由通道形成亚基 MCUa 和 MCUb 组成,主要以五聚体形式组织。 MCUa和MCUb对Ca 2+离子传输具有相反的作用(分别允许和抑制渗透),并且它们在复合物中的相对量调节MCU本身的Ca 2+传输能力。除了通道形成亚基外,线粒体钙摄取 1 和 2(MICU1 和 MICU2)以及必需的 MICU 调节器 (EMRE) 也是单向转运蛋白复合物的一部分,在调节复合物本身的完整性方面发挥着关键作用 (De Stefani等人, 2015 ;拉斐尔等人, 2013 ; MCU 复合物在 MAM 中富集,更靠近线粒体外围,表明细胞质来源的 Ca 2+输入具有较高的可及性 (Marchi et al. 2017 )。
Among the mitochondrial Ca2+ uptake family of regulator proteins MICU1 and MICU2, the best characterized is MICU1, which functions as a gatekeeper that can sense the Ca2+ levels of the intermembrane space. Indeed, at low concentrations, the gate is closed, but as soon as the Ca2+ levels pass the [Ca2+] threshold of 700 nM for MICU1-MICU2 heterodimers and 300 nM for MICU1 homodimers, the Ca2+-binding EF hands of MICU1 bind the ion and undergo a conformational change that opens the channel (Csordas et al. 2013; Mallilankaraman et al. 2012a; Perocchi et al. 2010; Petrungaro et al. 2015; Park et al. 2020) (Table 1).
在线粒体Ca 2+摄取调节蛋白MICU1 和MICU2 家族中,特征最明显的是MICU1,它充当可以感知膜间隙Ca 2+水平的看门人。事实上,在低浓度下,门关闭,但一旦 Ca 2+水平超过 MICU1-MICU2 异二聚体的 700 nM 和 MICU1 同二聚体的 300 nM [Ca 2+ ] 阈值,Ca 2+结合 EF MICU1 的手结合离子并经历打开通道的构象变化(Csordas 等人, 2013 年;Mallilankaraman 等人, 2012a ;Perocchi 等人, 2010 年;Petrungaro 等人, 2015 年;Park 等人, 2020 年)(表1 ) 。
表 1 MAM 中发现的与癌症发生和进展有关的 Ca 2+信号调节剂总结
3 Decrease in ER-Mitochondria Ca2+ Crosstalk
3 ER-线粒体 Ca 2+串扰减少
3.1 Dysfunctional ER-Ca2+ Release
3.1 ER-Ca 2+释放功能失调
As described in the introductory section, in recent years, increasing evidence has shown that organelles communicate with each other through Ca2+ signaling. In particular, at the MAMs level, interorganellar Ca2+ signaling is profoundly spatiotemporally regulated. Interestingly, in the tumor setting, an alteration of Ca2+ signaling has been shown to affect malignant transformation and tumor progression through the control of cell death programs and metabolism (Rimessi et al. 2020; Monteith et al. 2007).
如引言部分所述,近年来,越来越多的证据表明细胞器通过 Ca 2+信号传导相互通信。特别是,在 MAM 水平上,细胞间 Ca 2+信号传导受到深刻的时空调节。有趣的是,在肿瘤环境中,Ca 2+信号传导的改变已被证明可以通过控制细胞死亡程序和代谢来影响恶性转化和肿瘤进展(Rimessi 等人, 2020 ;Monteith 等人, 2007 )。
In this context, the ER not only plays a decisive role in Ca2+ signaling but also guarantees a control system for correct protein folding and stress sensing. Alterations in ER homeostasis, including substantial Ca2+ depletion, are associated with the pathophysiology of many diseases, including cancer (Mekahli et al. 2011).
在这种情况下,ER 不仅在 Ca 2+信号传导中发挥决定性作用,而且还保证了正确的蛋白质折叠和应激传感的控制系统。 ER稳态的改变,包括大量Ca 2+消耗,与包括癌症在内的许多疾病的病理生理学相关(Mekahli et al. 2011 )。
The normal Ca2+ exchange between the ER and the mitochondria requires adequate filling of the ER Ca2+ stores. Thus, decreasing the ER Ca2+ levels will compromise ER-mitochondrial Ca2+ transfer. As a consequence, changes in the ER Ca2+ store content affect the Ca2+ efflux from the ER to the mitochondria and ultimately cell survival (Ivanova et al. 2017).
ER 和线粒体之间的正常 Ca 2+交换需要充分填充 ER Ca 2+储备。因此,降低 ER Ca 2+水平将损害 ER 线粒体 Ca 2+转移。因此,内质网 Ca 2+储存含量的变化会影响从内质网到线粒体的 Ca 2+流出,并最终影响细胞存活 (Ivanova et al. 2017 )。
The maintenance of physiological low levels of mitochondrial Ca2+ uptake by IP3R is crucial to preserve cellular bioenergetics in normal and cancer cells by enabling the dehydrogenase activation of the tricarboxylic acid (TCA) cycle, strong ATP production and metabolic intermediates for the generation of building blocks, allowing the cells to enter the cell cycle and proliferate. In breast cancer cells but not in normal cells, Ca2+ release suppression mediated by the inhibition of IP3R activity caused cell death through a deregulated autophagic mechanism (Singh et al. 2017a) and mitotic disruption, as reported by Cárdenas C. et al. (2016).
IP3R 维持线粒体 Ca 2+摄取的生理低水平对于保护正常细胞和癌细胞中的细胞生物能至关重要,因为它能够激活三羧酸 (TCA) 循环的脱氢酶、强大的 ATP 产生和代谢中间体以产生构建阻断,使细胞进入细胞周期并增殖。据 Cárdenas C. 等人报道,在乳腺癌细胞中(而非正常细胞中),IP3R 活性抑制介导的 Ca 2+释放抑制通过自噬机制失调(Singh 等人, 2017a )和有丝分裂破坏导致细胞死亡。 ( 2016 )。
Regarding type 3 IP3R, the depletion of IP3R3 or its pharmacological blocking increased the level of the autophagic marker microtubule-associated protein 1A/1B-light chain 3 (LC3)-II through the upregulation of autophagic protein 5 (Atg5) and ROS generation, leading to the blockage of tumor growth in a mouse model of breast cancer (Singh et al. 2017a). This finding is correlated with the high expression of IP3R3 in human malignant tissues and high concentrations of metabolites in the serum of patients (Singh et al. 2017b).
对于 3 型 IP3R,IP3R3 的消耗或其药理学阻断通过上调自噬蛋白 5 (Atg5) 和 ROS 生成增加了自噬标记物微管相关蛋白 1A/1B-轻链 3 (LC3)-II 的水平,导致乳腺癌小鼠模型中肿瘤生长受阻(Singh et al. 2017a )。这一发现与人类恶性组织中 IP3R3 的高表达以及患者血清中代谢物的高浓度相关(Singh et al. 2017b )。
Moreover, it has been reported that the inhibition of IP3R with caffeine, a nonspecific inhibitor of these receptors, leads to a decreased migration of glioblastoma cells and a substantially increased mean survival in a mouse glioblastoma xenograft model (Kang et al. 2010). In the Caco-2 colon cancer cell line, IP3R3 silencing, or nonspecific pharmacological inhibition by 2-APB in gastric cancer cells, induced apoptosis, while overexpression protected cells from staurosporine-induced apoptotic death (Shibao et al. 2010).
此外,据报道,用咖啡因(这些受体的非特异性抑制剂)抑制IP3R会导致小鼠胶质母细胞瘤异种移植模型中胶质母细胞瘤细胞迁移的减少和平均存活率的显着增加(Kang等人, 2010 )。在 Caco-2 结肠癌细胞系中,IP3R3 沉默或胃癌细胞中 2-APB 的非特异性药理抑制可诱导细胞凋亡,而过表达可保护细胞免遭星形孢菌素诱导的细胞凋亡性死亡(Shibao 等人, 2010 )。
Interestingly, various MAM-located oncosuppressors and oncogenes have been reported to interact with IP3Rs, including the oncogene protein kinase B (PKB), also known as Akt, promyelocytic leukemia protein (PML), BRCA1 associated protein 1 (BAP1), phosphatase and tensin homolog deleted on chromosome 10 (PTEN) and B-cell lymphoma 2 (Bcl-2) family proteins, modifying the Ca2+ release patterns and cell fate (Bononi et al. 2017; Akl and Bultynck 2013; Missiroli et al. 2017; Kuchay et al. 2017; Giorgi et al. 2010). Although the aforementioned proteins are all present at the ER-mitochondria interface, only PTEN and PML are particularly enriched on MAMs (Missiroli et al. 2016; Bononi et al. 2013).
有趣的是,据报道,多种位于 MAM 的抑癌基因和癌基因可与 IP3R 相互作用,包括癌基因蛋白激酶 B (PKB),也称为 Akt、早幼粒细胞白血病蛋白 (PML)、BRCA1 相关蛋白 1 (BAP1)、磷酸酶和张力蛋白10 号染色体上的同源缺失 (PTEN) 和 B 细胞淋巴瘤 2 (Bcl-2) 家族蛋白,修饰 Ca 2+释放模式和细胞命运(Bononi 等人, 2017 年;Akl 和 Bultynck, 2013 年;Missiroli 等人, 2017 年;Kuchay 等人, 2017 年;Giorgi 等人, 2010 年)。尽管上述蛋白质都存在于 ER-线粒体界面,但只有 PTEN 和 PML 在 MAM 上特别富集(Missiroli 等人, 2016 年;Bononi 等人, 2013 年)。
Akt, as well as protein kinase C (PKC) isozymes (Pinton et al. 2004), is a key player in regulating multiple signaling pathways through calcium signaling tuning, such as cell metabolism, cell proliferation, and survival (Szado et al. 2008). Notably, in human breast cancers, the phosphoinositide 3-kinase (PI3K)/Akt/mTOR pathway is frequently dysregulated (Gonzalez-Angulo et al. 2011; Stemke-Hale et al. 2008).
Akt 以及蛋白激酶 C (PKC) 同工酶 (Pinton et al. 2004 ) 是通过钙信号调节调节多种信号传导途径的关键角色,例如细胞代谢、细胞增殖和存活 (Szado et al. 2008) )。值得注意的是,在人类乳腺癌中,磷酸肌醇 3-激酶 (PI3K)/Akt/mTOR 通路经常失调(Gonzalez-Angulo 等人, 2011 ;Stemke-Hale 等人, 2008 )。
On the ER side, IP3R Akt-mediated phosphorylation results in a decreased magnitude of Ca2+ release and, as a result, reduced mitochondrial Ca2+ uptake. Moreover, this decrease in Ca2+ flux protected glioblastoma cell lines from the effects of apoptotic stimuli (Szado et al. 2008).
在 ER 一侧,IP3R Akt 介导的磷酸化导致 Ca 2+释放量减少,从而减少线粒体 Ca 2+摄取。此外,Ca 2+流量的减少保护胶质母细胞瘤细胞系免受细胞凋亡刺激的影响(Szado et al.2008 )。
In 2012, our group demonstrated that Akt specifically phosphorylates type 3 IP3R, leading to diminished mitochondrial Ca2+ influx and, consequently, protecting cells from apoptosis (Marchi et al. 2012).
2012 年,我们的小组证明 Akt 特异性磷酸化 3 型 IP3R,导致线粒体 Ca 2+流入减少,从而保护细胞免于凋亡(Marchi 等人, 2012 )。
PML tumor suppressor protein has been implicated in diverse cellular processes ranging from tumor suppression to defense against virus infection (Bernardi and Pandolfi 2007; Everett and Chelbi-Alix 2007; Hsu and Kao 2018; Pinton et al. 2011). An extranuclear fraction of PML has been demonstrated to be targeted to the MAMs in a p53-dependent manner (Missiroli et al. 2016) and to form a multicomplex with type 3 IP3R, the serine threonine kinase Akt and protein phosphatase 2A (PP2A) (Giorgi et al. 2010).
PML肿瘤抑制蛋白参与多种细胞过程,从肿瘤抑制到防御病毒感染(Bernardi和Pandolfi 2007 ;Everett和Chelbi-Alix 2007 ;Hsu和Kao 2018 ;Pinton等2011 )。 PML 的核外部分已被证明以 p53 依赖性方式靶向 MAM(Missiroli 等人, 2016 ),并与 3 型 IP3R、丝氨酸苏氨酸激酶 Akt 和蛋白磷酸酶 2A (PP2A) 形成多重复合物(乔治等人, 2010 )。
It has been shown that PML regulates the phosphorylation of IP3R by controlling the activity of Akt through the recruitment of the PP2A phosphatase at the MAMs interface. Hence, PML can coordinate Ca2+ mobilization into the mitochondria, which then triggers the cell death program. Conversely, in the absence of PML, PP2A does not assemble with IP3R and Akt, resulting in a higher activation of Akt (phospho-Akt). Once activated, Akt can hyperphosphorylate IP3R, thereby suppressing ER Ca2+ release to the mitochondria (Giorgi et al. 2011).
研究表明,PML 通过在 MAM 界面招募 PP2A 磷酸酶来控制 Akt 的活性,从而调节 IP3R 的磷酸化。因此,PML 可以协调 Ca 2+动员到线粒体中,然后触发细胞死亡程序。相反,在没有 PML 的情况下,PP2A 不会与 IP3R 和 Akt 组装,从而导致 Akt(磷酸化 Akt)的更高活化。一旦激活,Akt 可使 IP3R 过度磷酸化,从而抑制 ER Ca 2+释放至线粒体(Giorgi 等人, 2011 )。
BAP1 is a member of the ubiquitin C-terminal hydrolase (UCH) subfamily of deubiquitylating enzymes and has tumor suppressor activity, which has been mainly correlated with its nuclear localization (Lee et al. 2014; Ismail et al. 2014). When BAP1 localizes to the ER, it binds, deubiquitylates, and stabilizes the activity of the IP3R3 channel, modulating Ca2+ release from the ER to the cytosol and then to the mitochondria, promoting apoptosis. In BAP1+/− carriers, the reduced level of BAP1 resulted in a diminished IP3R3 quote with a subsequent Ca2+ transfer decrease from the ER to the mitochondria. This event caused a reduced propensity of BAP1+/− cells to undergo apoptosis following DNA damage induced by asbestos or UV light (Bononi et al. 2017).
BAP1 是去泛素化酶的泛素 C 末端水解酶 (UCH) 亚家族的成员,具有肿瘤抑制活性,这主要与其核定位相关 (Lee et al. 2014 ;Ismail et al. 2014 )。当 BAP1 定位于 ER 时,它会结合、去泛素化并稳定 IP3R3 通道的活性,调节 Ca 2+从 ER 释放到细胞质,然后释放到线粒体,从而促进细胞凋亡。在 BAP1 +/-携带者中,BAP1 水平降低导致 IP3R3 引用减少,随后从 ER 到线粒体的 Ca 2+转移减少。这一事件导致 BAP1 +/-细胞在石棉或紫外线诱导 DNA 损伤后发生凋亡的可能性降低 (Bononi et al. 2017 )。
PTEN is another Ca2+-related tumor suppressor that has been shown to be mutated or suppressed in many tumors (Salmena et al. 2008). Bononi et al. demonstrated that a fraction of cellular PTEN is localized at the MAMs, where it interacts with IP3R3, antagonizing its Akt-mediated phosphorylation and enhancing Ca2+ transfer from the ER to mitochondria. In this way, it reestablishes cellular sensitivity to Ca2+-mediated proapoptotic stimuli. Conversely, PTEN knockdown reduced the Ca2+ release from the ER and decreased mitochondrial Ca2+ transients, thus preventing cell death activation (Bononi et al. 2013). Moreover, a novel role for PTEN has been proposed; it can compete with F-box and leucine-rich repeat protein 2 (FBXL2), an E3-ubiquitin ligase F-box protein, for binding to IP3R3 to prevent its degradation. It has been demonstrated that FBXL2 degradation of IP3R3 is enhanced in cancer cells in which PTEN expression is lowered, thereby resulting in the inhibition of apoptosis (Kuchay et al. 2017).
PTEN 是另一种 Ca 2+相关肿瘤抑制因子,已被证明在许多肿瘤中发生突变或抑制(Salmena 等人, 2008 )。博诺尼等人。证明细胞 PTEN 的一部分位于 MAM,在那里它与 IP3R3 相互作用,拮抗其 Akt 介导的磷酸化并增强 Ca 2+从 ER 到线粒体的转移。通过这种方式,它重新建立了细胞对 Ca 2+介导的促凋亡刺激的敏感性。相反,PTEN 敲低减少了 ER 的 Ca 2+释放,并减少了线粒体 Ca 2+瞬变,从而防止了细胞死亡激活(Bononi 等人, 2013 )。此外,还提出了 PTEN 的新作用;它可以与 F-box 和富含亮氨酸的重复蛋白 2 (FBXL2)(一种 E3-泛素连接酶 F-box 蛋白)竞争与 IP3R3 的结合,以防止其降解。已证明,在 PTEN 表达降低的癌细胞中,FBXL2 对 IP3R3 的降解增强,从而抑制细胞凋亡 (Kuchay et al. 2017 )。
The Bcl-2 family of anti- and proapoptotic proteins is predominantly localized to the mitochondria, ER, and MAMs, and their activities strongly reflect their intracellular localization (Morciano et al. 2018). Bcl-2 is a proto-oncogene known for its involvement in inhibition of apoptosis through its interaction with the proapoptotic proteins BCL2 associated X protein (Bax) and Bcl-2 homologous antagonist/killer (Bak) (Rimessi et al. 2020). Indeed, at the ER, Bcl-2 prevents excessive Ca2+ flux by directly targeting all three IP3R receptor isoforms, which would lead to mitochondrial Ca2+ overload and opening of the permeability transition pore (mPTP) (Chen et al. 2015; Bonora et al. 2017). Dysregulation of Bcl-2 expression has been highlighted in various cancers, including hematopoietic, lung, breast, and prostate tumors (Morciano et al. 2018).
Bcl-2 家族的抗凋亡和促凋亡蛋白主要定位于线粒体、ER 和 MAM,它们的活性强烈反映了它们的细胞内定位 (Morciano et al. 2018 )。 Bcl-2 是一种原癌基因,因其与促凋亡蛋白 BCL2 相关 X 蛋白 (Bax) 和 Bcl-2 同源拮抗剂/杀伤剂 (Bak) 相互作用而参与抑制细胞凋亡 (Rimessi et al. 2020 )。事实上,在 ER 中,Bcl-2 通过直接靶向所有三种 IP3R 受体亚型来防止过度的 Ca 2+通量,这将导致线粒体 Ca 2+过载和通透性转换孔 (mPTP) 打开 (Chen et al. 2015 ;博诺拉等人, 2017 年。 Bcl-2 表达失调在多种癌症中都得到了强调,包括造血肿瘤、肺癌、乳腺癌和前列腺肿瘤 (Morciano et al. 2018 )。
Bcl-XL is another antiapoptotic member of the same family that is frequently overexpressed in many tumors, such as multiple myeloma, melanoma, glioblastoma, prostate cancer, colorectal cancer, non-small cell lung cancer, and pancreatic cancers (Trisciuoglio et al. 2017; Scherr et al. 2016; Zhang et al. 2014; Yoshimine et al. 2013). This protein is localized at the MAMs (Monaco et al. 2015), where it directly binds the IP3R channels, regulating IP3R-related Ca2+ release. Bcl-XL caused a strong sensitization of IP3R, promoting prosurvival Ca2+ oscillations (White et al. 2005).
Bcl-XL 是同一家族的另一个抗凋亡成员,在许多肿瘤中经常过表达,例如多发性骨髓瘤、黑色素瘤、胶质母细胞瘤、前列腺癌、结直肠癌、非小细胞肺癌和胰腺癌 (Trisciuoglio et al. 2017)谢尔等人, 2016 ;张等人,2013 ;该蛋白定位于 MAM(Monaco et al. 2015 ),直接结合 IP3R 通道,调节 IP3R 相关的 Ca 2+释放。 Bcl-XL 引起 IP3R 强烈敏化,促进促存活 Ca 2+振荡(White et al. 2005 )。
Among the antiapoptotic proteins of the Bcl-2 family, myeloid cell leukemia 1 (Mcl-1) also lowers the calcium ER store content by stimulating IP3Rs outside of the MAMs, thereby increasing Ca2+ leakage from the ER, resulting in a decline in the basal ER Ca2+ levels (Eckenrode et al. 2010). In the presence of low [IP3], in Mcl-1-expressing cells, store depletion becomes more prominent, indicating that the sensitivity of IP3-dependent Ca2+ release is enhanced by Mcl-1. Mcl-1-mediated IP3R sensitization also contributes to low-level IP3R-mediated Ca2+ signaling from the ER to the mitochondria and thereby stimulates mitochondrial bioenergetics (Bittremieux et al. 2016).
在 Bcl-2 家族的抗凋亡蛋白中,骨髓细胞白血病 1 (Mcl-1) 还通过刺激 MAM 外部的 IP3R 来降低 ER 储存的钙含量,从而增加 ER 中的 Ca 2+渗漏,从而导致基础 ER Ca 2+水平(Eckenrode 等人, 2010 )。在低 [IP3] 存在的情况下,在表达 Mcl-1 的细胞中,储备耗尽变得更加突出,表明 Mcl-1 增强了 IP3 依赖性 Ca 2+释放的敏感性。 Mcl-1 介导的 IP3R 敏化还有助于从 ER 到线粒体的低水平 IP3R 介导的 Ca 2+信号传导,从而刺激线粒体生物能(Bittremieux et al. 2016 )。
At the MAMs, oncogenic H-Ras also affects Ca2+ transfer to the mitochondria to promote evasion from the apoptotic cascade (Rimessi et al. 2014). In colorectal cancer cells, oncogenic K-Ras modified the expression of IP3Rs, weakening the Ca2+ release from the ER to allow cells to escape Ca2+-mediated apoptosis (Pierro et al. 2014). Indeed, Ras-driven mitochondrial dysfunction causes metabolic and redox changes that favor tumorigenesis (Hu et al. 2012). Hence, proper maintenance of IP3R3 protein levels is crucial for preventing oncogenesis by strengthening tumor-suppressive ER-mitochondrial Ca2+ transfer.
在 MAM 中,致癌 H-Ras 还会影响 Ca 2+向线粒体的转移,以促进细胞凋亡级联的逃避 (Rimessi et al. 2014 )。在结直肠癌细胞中,致癌 K-Ras 改变了 IP3R 的表达,削弱了 ER 的 Ca 2+释放,使细胞能够逃避 Ca 2+介导的细胞凋亡 (Pierro et al. 2014 )。事实上,Ras 驱动的线粒体功能障碍会导致有利于肿瘤发生的代谢和氧化还原变化(Hu et al. 2012 )。因此,适当维持 IP3R3 蛋白水平对于通过加强肿瘤抑制性 ER-线粒体 Ca 2+转移来预防肿瘤发生至关重要。
Furthermore, MAMs are a molecular platform for the regulation of many oxidoreductases. In this context, endoplasmic reticulum oxidoreductin 1-α (ERO1-α) activity is broadly investigated for its enrichment at ER-mitochondria contact sites (Anelli et al. 2012) and its high expression in different tumor types (Kakihana et al. 2012). This oxidase impacts ER-Ca2+ storage and IP3-dependent fluxes. During ER stress, ERO1-α oxidizes type 1 IP3R, promoting the release of Ca2+ from the ER (Anelli et al. 2012). Furthermore, endoplasmic reticulum resident protein 44 (ERp44) (an ER luminal chaperone protein) binds to IP3R1 and inhibits its channel activity under reducing conditions, resulting in the blockade of Ca2+ transfer to the mitochondria (Higo et al. 2005). Oxidation of IP3R1 by ERO1-α causes the dissociation of ERp44, thus leading to the activation of Ca2+ release via IP3R1 (Li et al. 2009). ERO1-α silencing has been demonstrated to profoundly affect mitochondrial Ca2+ uptake, likely modifying MCU activity. Thus, ERO1-α links redox and Ca2+ homeostasis in MAMs (Anelli et al. 2012).
此外,MAM 是调节许多氧化还原酶的分子平台。在这种情况下,内质网氧化还原素 1-α (ERO1-α) 活性因其在 ER-线粒体接触位点的富集(Anelli et al. 2012 )及其在不同肿瘤类型中的高表达而被广泛研究(Kakihana et al. 2012 ) 。该氧化酶影响 ER-Ca 2+储存和 IP3 依赖性通量。在内质网应激期间,ERO1-α 氧化 1 型 IP3R,促进内质网释放 Ca 2+ (Anelli et al. 2012 )。此外,内质网驻留蛋白 44 (ERp44)(一种 ER 腔伴侣蛋白)与 IP3R1 结合并在还原条件下抑制其通道活性,导致 Ca 2+转移至线粒体的阻断 (Higo et al. 2005 )。 IP3R1被ERO1-α氧化导致ERp44解离,从而导致通过IP3R1激活Ca 2+释放(Li et al.2009 )。 ERO1-α 沉默已被证明会深刻影响线粒体 Ca 2+吸收,可能会改变 MCU 活性。因此,ERO1-α 将 MAM 中的氧化还原和 Ca 2+稳态联系起来(Anelli 等人, 2012 )。
Recently, the oncogenic transcription factor signal transducer and activator of transcription 3 (STAT3), which mediates the signaling of cytokines, growth factors, and oncogenes (Yu et al. 2014), has been shown to localize only to MAMs (Su et al. 2020). At this location, it modulates ER-mitochondria Ca2+ release by interacting with the IP3R3 channel and promoting its degradation, resulting in greater cellular resistance to apoptotic stimuli (Avalle et al. 2019). In breast cancer cell lines, silencing STAT3 enhances the ER Ca2+ release and sensitivity to apoptosis following oxidative stress, correlating with increased IP3R3 levels. This evidence suggests that STAT3-mediated IP3R3 downregulation in the ER crucially contributes to its antiapoptotic functions via Ca2+ flux modulation.
最近,致癌转录因子信号转导子和转录激活子 3 (STAT3) 介导细胞因子、生长因子和癌基因的信号传导 (Yu et al. 2014 ),已被证明仅定位于 MAM (Su et al. 2014)。 2020 )。在此位置,它通过与 IP3R3 通道相互作用并促进其降解来调节 ER 线粒体 Ca 2+释放,从而增强细胞对凋亡刺激的抵抗力 (Avalle et al. 2019 )。在乳腺癌细胞系中,沉默 STAT3 可增强 ER Ca 2+释放和氧化应激后对细胞凋亡的敏感性,与 IP3R3 水平升高相关。该证据表明,ER 中 STAT3 介导的 IP3R3 下调通过 Ca 2+通量调节对其抗凋亡功能做出了至关重要的贡献。
Together with the IP3R receptors, RyRs and SERCA are the major Ca2+ players in the ER (Berridge 2012). In general, RyRs regulate melanocyte and T cell proliferation (Hakamata et al. 1994; Kang et al. 2000) and astrocyte migration (Matyash et al. 2002). Ryanodine receptor type 2 (RyR2), a member of the RyR family, controls the Ca2+ release from the sarcoplasmic reticulum into the cytosol (Ding et al. 2017). Different studies have confirmed the association of RyR2 with several cancer types, including melanoma (Carpi et al. 2018), breast cancer (Lu et al. 2017), lymphoma (McCarthy et al. 2003), and prostate cancer (Mariot et al. 2000). Recently, it has been reported that RyR2 is downregulated in thyroid carcinoma tissues and that low expression levels of RyR2 are closely associated with poor prognosis in thyroid carcinoma patients (Xu et al. 2019).
与 IP3R 受体一起,RyR 和 SERCA 是 ER 中主要的 Ca 2+参与者 (Berridge 2012 )。一般来说,RyR 调节黑素细胞和 T 细胞增殖(Hakamata 等人, 1994 ;Kang 等人, 2000 )和星形胶质细胞迁移(Matyash 等人, 2002 )。 Ryanodine 受体 2 型 (RyR2) 是 RyR 家族的成员,控制 Ca 2+从肌浆网释放到细胞质中 (Ding et al. 2017 )。不同的研究已证实 RyR2 与多种癌症类型的关联,包括黑色素瘤 (Carpi et al. 2018 )、乳腺癌 (Lu et al. 2017 )、淋巴瘤 (McCarthy et al. 2003 ) 和前列腺癌 (Mariot et al. 2017)。co;2-m" aria-label="参考 2000" data-test="引用-ref" data-track-label="链接" data-track-action="参考锚点" data-track="click">最近有报道称,RyR2在甲状腺癌组织中表达下调,且RyR2的低表达水平与甲状腺癌患者的不良预后密切相关(Xu et al. 2019 )。
Over the past years, the tumor suppressor p53 has been shown to be altered in many human cancer tissues, including colon, breast, lung, brain, bladder, pancreatic, stomach, and esophageal cancer (Vogelstein et al. 2000). Some of p53 fraction is located at the MAMs, where it directly binds to the SERCA pump, changing its oxidative state and thus leading to an increased Ca2+ load, followed by an enhanced flux to the mitochondria. Consequently, during apoptotic stimulation, more Ca2+ can be released from the ER into the mitochondria, enhancing mitochondrial Ca2+ overload, opening of the mitochondrial mPTP, release of caspase cofactors, and ultimately induction of the intrinsic apoptosis pathway (Morciano et al. 2018). Dysregulation of p53-dependent Ca2+ homeostasis led to reduced ER Ca2+ release, resulting in a low responsiveness to apoptotic stimulation (Giorgi et al. 2015).
在过去的几年中,肿瘤抑制因子p53已被证明在许多人类癌症组织中发生改变,包括结肠癌、乳腺癌、肺癌、脑癌、膀胱癌、胰腺癌、胃癌和食道癌(Vogelstein等人, 2000 )。 p53 部分位于 MAM 处,直接与 SERCA 泵结合,改变其氧化状态,从而导致 Ca 2+负载增加,随后流向线粒体的流量增加。因此,在细胞凋亡刺激过程中,更多的 Ca 2+可以从 ER 释放到线粒体中,增强线粒体 Ca 2+超载,打开线粒体 mPTP,释放 caspase 辅因子,并最终诱导内在细胞凋亡途径 (Morciano 等人) 2018 )。 p53 依赖性 Ca 2+稳态的失调导致 ER Ca 2+释放减少,导致对细胞凋亡刺激的反应性较低 (Giorgi et al. 2015 )。
We must also note the phosphofurin acid cluster sorting 2 protein (PACS-2) and PKR-like ER kinase (PERK). PACS-2 is a multifunctional protein involved in retrograde ER-Golgi trafficking of multiple proteins (Youker et al. 2009). Although it is unclear whether a direct interaction of PACS-2 at the MAMs occurs, it was demonstrated that depletion of PACS-2 reduces mitochondrial-ER contact sites and mediates apoptosis (Simmen et al. 2005). PACS-2 was also demonstrated to be a fundamental player in rapamycin complex 2 (mTORC2)-dependent regulation of MAMs integrity (Betz et al. 2013). PERK is a protein kinase that, together with inositol-requiring enzyme 1 (IRE1) and transcription factor 6 (ATF6), acts as an ER stress sensor from the ER membrane, controlling UPR functioning. The function of this protein in the MAMs is independent of its role as an ER stress sensor and transcriptional regulator of redox homeostasis. Indeed, PERK maintains, through its cytoplasmic domains, the juxtaposition of the ER and the mitochondria, acting as a structural tether and permitting the transmission of ROS-mediated signals (Verfaillie et al. 2012).
我们还必须注意磷酸呋喃酸簇分选 2 蛋白 (PACS-2) 和 PKR 样 ER 激酶 (PERK)。 PACS-2 是一种多功能蛋白,参与多种蛋白的逆行 ER-高尔基体运输(Youker et al. 2009 )。尽管尚不清楚 PACS-2 在 MAM 上是否发生直接相互作用,但已证明 PACS-2 的耗尽会减少线粒体 - ER 接触位点并介导细胞凋亡(Simmen 等人, 2005 )。 PACS-2 还被证明是雷帕霉素复合物 2 (mTORC2) 依赖性 MAM 完整性调节的重要参与者 (Betz et al. 2013 )。 PERK 是一种蛋白激酶,与肌醇需求酶 1 (IRE1) 和转录因子 6 (ATF6) 一起充当 ER 膜的 ER 应激传感器,控制 UPR 功能。该蛋白在 MAM 中的功能与其作为 ER 应激传感器和氧化还原稳态转录调节因子的作用无关。事实上,PERK 通过其细胞质结构域维持 ER 和线粒体的并置,充当结构系链并允许 ROS 介导的信号传输(Verfaillie 等人, 2012 )。
In conclusion, changes in the ER Ca2+-store content would perturb Ca2+ transfer from the ER to the mitochondria and ultimately influence cell death or survival. A reduction in intracellular store Ca2+ release is certainly the main mechanism adopted by cancer cells to escape mitochondria-mediated apoptosis (Fig. 1).
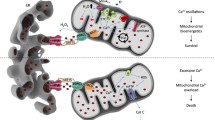
总之,内质网 Ca 2+储存量的变化会干扰 Ca 2+从内质网到线粒体的转移,并最终影响细胞死亡或存活。细胞内储存Ca 2+释放的减少无疑是癌细胞逃避线粒体介导的细胞凋亡的主要机制(图1 )。
Downregulation of MAMs Ca2+ crosstalk in cancer: graphical representation of the calcium signaling regulators involved in a cancer-related decreased Ca2+ crosstalk state. See text for further details. Ca2+, calcium; ER, endoplasmic reticulum
癌症中 MAM Ca 2+串扰的下调:参与癌症相关 Ca 2+串扰状态降低的钙信号调节因子的图形表示。请参阅文本了解更多详细信息。 Ca 2+ ,钙; ER-- 内质网
3.2 Perturbed Mitochondrial Ca2+ Uptake
3.2线粒体 Ca 2+摄取受到干扰
Cancer-derived modifications in cellular physiology could be related to impairment of the Ca2+ signaling network, which is frequently associated with the dysregulation of several Ca2+ channels and pumps (Prevarskaya et al. 2014; Hanahan and Weinberg 2000).
细胞生理学中癌症衍生的改变可能与 Ca 2+信号网络的损伤有关,而 Ca 2+ 信号网络的损伤通常与多个 Ca 2+通道和泵的失调有关(Prevarskaya 等人, 2014 ;Hanahan 和 Weinberg ,2000 )。
In addition to limiting the excessive release of Ca2+ from the ER, cancer cells can effectively prevent mitochondrial Ca2+ overload by limiting mitochondrial Ca2+ uptake.
除了限制内质网过度释放Ca 2+外,癌细胞还可以通过限制线粒体Ca 2+摄取来有效防止线粒体Ca 2+过载。
Among the proteins responsible for limitation of mitochondrial calcium influx are Bcl-2 and Bcl-XL, the antiapoptotic Bcl-2-family proteins discussed in the previous paragraph; Bcl-2 and Bcl-XL are partially localized at the mitochondrial outer membrane and, similar to other antiapoptotic proteins, are frequently upregulated in cancer; these proteins can regulate mitochondrial Ca2+ uptake through VDAC1 (Shoshan-Barmatz et al. 2010).
负责限制线粒体钙流入的蛋白质包括 Bcl-2 和 Bcl-XL,它们是上一段讨论的抗凋亡 Bcl-2 家族蛋白质; Bcl-2 和 Bcl-XL 部分位于线粒体外膜,与其他抗凋亡蛋白类似,在癌症中经常上调;这些蛋白质可以通过 VDAC1 调节线粒体 Ca 2+摄取 (Shoshan-Barmatz et al. 2010 )。
Considering that VDAC1 is involved in death and cell survival, it is not surprising that this channel could be a target for Bcl-2 family proteins (De Stefani et al. 2012). These proteins target the N-terminal region of VDAC1 (Abu-Hamad et al. 2009; Arbel and Shoshan-Barmatz 2010), and it has been demonstrated that only the Bcl-XL BH4 domain is essential to bind VDAC1 and inhibit cell death (Monaco et al. 2015). Several studies demonstrated that the interaction between Bcl-XL and VDAC1 suppresses proapoptotic Ca2+ uptake, preventing the dissipation of the mitochondrial potential and the release of cytochrome c and apoptosis-inducing factor (AIF) through the outer membrane.
考虑到 VDAC1 参与细胞死亡和存活,因此该通道可能成为 Bcl-2 家族蛋白的靶标也就不足为奇了 (De Stefani et al. 2012 )。这些蛋白靶向 VDAC1 的 N 末端区域(Abu-Hamad 等, 2009 ;Arbel 和 Shoshan-Barmatz ,2010 ),并且已证明,只有 Bcl-XL BH4 结构域对于结合 VDAC1 和抑制细胞死亡至关重要(摩纳哥等, 2015 )。多项研究表明,Bcl-XL 和 VDAC1 之间的相互作用可抑制促凋亡 Ca 2+摄取,从而防止线粒体电位的耗散以及细胞色素 c 和凋亡诱导因子 (AIF) 通过外膜的释放。
Indeed, studies concerning mitochondrial Ca2+ uptake that compare Bcl-XL-overexpressing versus Bcl-XL-deficient cells have demonstrated that this protein may be involved in MAMs microdomain reorganization and results in an alteration of the capacity of mitochondrial Ca2+ uptake, proving that Bcl-XL inhibits VDAC1 (Monaco et al. 2015; Bittremieux et al. 2016; Shimizu et al. 2000; Li et al. 2008).
事实上,有关线粒体 Ca 2+摄取的研究比较了 Bcl-XL 过表达细胞与 Bcl-XL 缺陷细胞,结果表明该蛋白可能参与 MAM 微域重组并导致线粒体 Ca 2+摄取能力的改变。证明 Bcl-XL 抑制 VDAC1(Monaco et al. 2015 ;Bittremieux et al. 2016 ;Shimizu et al. 2000 ;Li et al. 2008 )。
Nevertheless, VDAC1 in hepatocarcinoma tissues can be downregulated by the small noncoding RNA miR-7, influencing tumor proliferation and metastasis (Chaudhuri et al. 2016a; Bargaje et al. 2012). Chaudhuri et al. showed that in human neuroblastoma cells and in mouse primary cortical neurons, miR-7 can reduce VDAC1 expression, with consequent inhibition of mitochondrial Ca2+ uptake, membrane depolarization, mitochondrial fragmentation, cytochrome c release, and ROS production, promoting cancer cell survival (Chaudhuri et al. 2016a).
然而,肝癌组织中的VDAC1可以被小非编码RNA miR-7下调,影响肿瘤增殖和转移(Chaudhuri et al. 2016a ;Bargaje et al. 2012 )。乔杜里等人。研究表明,在人神经母细胞瘤细胞和小鼠原代皮质神经元中,miR-7 可以降低 VDAC1 表达,从而抑制线粒体 Ca 2+摄取、膜去极化、线粒体碎片、细胞色素 c 释放和 ROS 产生,促进癌细胞存活。乔胡里等人, 2016a )。
MCU allows calcium ion permeation into the mitochondrial matrix, and its overexpression leads to an increase in mitochondrial Ca2+ entry and ROS production, influencing the migration, invasion, and size of different tumor types (Yu et al. 2017; Tang et al. 2015; Wang et al. 2007). However, a reduction in MCU expression decreases mitochondrial Ca2+ uptake, the opening of the mPTP and the release of proapoptotic factors, thus having a protective effect on apoptosis (Marchi et al. 2019b; Sebag et al. 2018; Oropeza-Almazan et al. 2017; Yuan et al. 2017; Liao et al. 2015; Qiu et al. 2013; Penston and Wormsley 1986).
MCU允许钙离子渗透到线粒体基质中,其过度表达导致线粒体Ca 2+进入和ROS产生增加,影响不同肿瘤类型的迁移、侵袭和大小(Yu et al. 2017 ;Tang et al. 2017 ) 2015 ;王等人, 2007 )。然而,MCU表达的减少会减少线粒体Ca 2+ 的摄取、mPTP的开放和促凋亡因子的释放,从而对细胞凋亡具有保护作用(Marchi et al. 2019b ; Sebag et al. 2018 ; Oropeza-Almazan et al. 2018 ; Oropeza-Almazan et al.袁等人,2017 ;邱等人, 2013 ;
Marchi et al. showed that, through MCU downregulation, the miR-25 MCU-targeting microRNA could perturb Ca2+ homeostasis, reducing the concentration of mitochondrial Ca2+ levels in HeLa cells. However, high levels of miR-25 have been observed both in prostate and colon cancer. The miR-25-dependent reduction in mitochondrial Ca2+ uptake correlates with resistance to proapoptotic stimuli and can be reversed by anti-miR-25 overexpression. Treatment with anti-miR-25 can restore the MCU expression levels and reverse the pathophysiology, thus suggesting a novel therapeutic target for prostate and colon cancer (Marchi et al. 2013).
马尔基等人。结果表明,通过 MCU 下调,靶向 miR-25 MCU 的 microRNA 可以扰乱 Ca 2+稳态,降低 HeLa 细胞中线粒体 Ca 2+水平的浓度。然而,在前列腺癌和结肠癌中均观察到高水平的 miR-25。线粒体 Ca 2+摄取的 miR-25 依赖性减少与促凋亡刺激的抵抗相关,并且可以通过抗 miR-25 过表达来逆转。抗 miR-25 治疗可以恢复 MCU 表达水平并逆转病理生理学,从而提出前列腺癌和结肠癌的新治疗靶点 (Marchi et al. 2013 )。
One gene that is frequently deleted in many human cancers, principally in those caused by environmental carcinogens, is fragile histidine triad (FHIT). Consequently, its product, the Fhit protein, is absent or reduced in most cancers (Huebner and Croce 2003). The Fhit protein is localized in the mitochondria and the cytosol and acts as a tumor suppressor, increasing susceptibility to apoptosis (Siprashvili et al. 1997). Reintroduction of Fhit to the highly carcinogen-susceptible Fhit−/− mouse model reduced tumor sizes by activating apoptotic cell death (Zanesi et al. 2005). The Fhit protein generates ROS and enhances mitochondrial Ca2+ uptake by increasing mitochondrial Ca2+ hotspots. Therefore, Fhit acts as a tumor suppressor by modulating MCU opening and enhancing the susceptibility of cells to apoptosis, thus potentiating the effect of apoptotic agents (Rimessi et al. 2009).
在许多人类癌症(主要是由环境致癌物引起的癌症)中经常被删除的一种基因是脆性组氨酸三联体(FHIT)。因此,其产物 Fhit 蛋白在大多数癌症中不存在或减少(Huebner 和 Croce 2003 )。 Fhit 蛋白位于线粒体和细胞质中,充当肿瘤抑制因子,增加细胞凋亡的易感性(Siprashvili 等人, 1997 )。将 Fhit 重新引入高度致癌物敏感的 Fhit -/−小鼠模型中,可通过激活细胞凋亡来减小肿瘤大小(Zanesi 等人, 2005 )。 Fhit 蛋白产生 ROS 并通过增加线粒体 Ca 2+热点来增强线粒体 Ca 2+吸收。因此,Fhit通过调节MCU开放并增强细胞对细胞凋亡的敏感性来充当肿瘤抑制剂,从而增强细胞凋亡剂的作用(Rimessi等人, 2009 )。
Transient receptor potential cation channel subfamily C member 3 (TRPC3) belongs to a group of nonselective cation channels that are involved in different cellular mechanisms. TRPC3 channels can influence the mitochondrial membrane potential following their up- and downregulation. The activation of Ca2+-sensitive downstream pathways occurs through the influx of calcium from transient receptor potential channels (TRP channels), which act as apoptotic regulators (Wang et al. 2019; Takahashi et al. 2018; Raphael et al. 2014; Monet et al. 2010). However, Shengjie Feng et al. have shown that a fraction of the TRPC3 protein is localized to the mitochondria and mediates mitochondrial Ca2+ uptake when the cytosolic calcium concentration is elevated. Since, as we previously noted, mitochondrial membrane potential seems to be affected by TRPC3 channels and because mitochondrial Ca2+ uptake is not abolished when MCU expression is downregulated (De Stefani et al. 2011), TRPC3 might be another channel that allows the entry of calcium into the mitochondria, in addition to MCU (Kirichok et al. 2004). In particular, resistance to apoptosis and the proliferation of some tumors could be related to its downregulation, which results in reduced mitochondrial calcium uptake (Feng et al. 2013).
瞬时受体电位阳离子通道亚家族 C 成员 3 (TRPC3) 属于一组参与不同细胞机制的非选择性阳离子通道。 TRPC3 通道的上调和下调可以影响线粒体膜电位。 Ca 2+敏感下游通路的激活是通过钙从瞬时受体电位通道(TRP 通道)流入而发生的,瞬时受体电位通道充当细胞凋亡调节剂(Wang 等人, 2019 年;Takahashi 等人, 2018 年;Raphael 等人, 2014 年;莫奈等人, 2010 )。然而,冯胜杰等人。已经表明,TRPC3 蛋白的一部分定位于线粒体,并在细胞质钙浓度升高时介导线粒体 Ca 2+摄取。正如我们之前指出的,由于线粒体膜电位似乎受到 TRPC3 通道的影响,并且当 MCU 表达下调时,线粒体 Ca 2+摄取不会被消除(De Stefani 等人, 2011 ),TRPC3 可能是允许进入的另一个通道除了 MCU 之外,还包括钙进入线粒体(Kirichok 等人, 2004 )。特别是,一些肿瘤对细胞凋亡和增殖的抵抗可能与其下调有关,从而导致线粒体钙摄取减少(Feng et al. 2013 )。
Fetal and adult testis-expressed 1 protein (FATE1) is a 21-kDa protein that belongs to the cancer-testis antigen proteins that are mainly expressed in the testis under physiological conditions and are upregulated in different cancer types (Dong et al. 2003; Whitehurst 2014; Simpson et al. 2005). This molecule, being a member of the mitochondrial fission factor (Miff) protein family, shares some structural similarities with Mff (Gandre-Babbe and van der Bliek 2008). The oncoprotein FATE1, which is located on the mitochondrial outer membrane preferentially in the MAMs compartment, is implicated in the regulation of Ca2+-dependent apoptosis in cancer cells, acting as an anti-tether agent through the modulation of the distance between the ER and the mitochondria (Doghman-Bouguerra et al. 2016), being a direct connection between its increased expression and MAMs morphology in adrenocortical carcinoma (AAC) patients with a poor prognosis (Doghman-Bouguerra et al. 2016). Overexpression of FATE1 in adenoid cystic carcinoma (ACC) was related to a decrease in mitochondrial Ca2+ uptake that confers resistance to proapoptotic stimuli and chemotherapeutic drugs (Doghman-Bouguerra et al. 2016).
胎儿和成人睾丸表达1蛋白(FATE1)是一种21-kDa蛋白,属于癌症睾丸抗原蛋白,在生理条件下主要在睾丸中表达,并且在不同癌症类型中表达上调(Dong et al. 2003 ;怀特赫斯特2014 ;辛普森等人2005 )。该分子是线粒体裂变因子 (Miff) 蛋白家族的成员,与 Mff 有一些结构相似性 (Gandre-Babbe 和 van der Bliek 2008 )。癌蛋白 FATE1 优先位于 MAM 区室中的线粒体外膜上,参与癌细胞中 Ca 2+依赖性细胞凋亡的调节,通过调节 ER 之间的距离充当抗束缚剂。和线粒体 (Doghman-Bouguerra et al. 2016 ),在预后不良的肾上腺皮质癌 (AAC) 患者中,其表达增加与 MAM 形态之间有直接联系(Doghman-Bouguerra 等人, 2016 年)。 FATE1 在腺样囊性癌 (ACC) 中的过度表达与线粒体 Ca 2+摄取减少有关,从而赋予对促凋亡刺激和化疗药物的抵抗力 (Doghman-Bouguerra et al. 2016 )。
In most human cancer types, including head and neck squamous cell carcinoma (HNSCC), high levels of enhancer of zeste homolog 2 (EZH2) have been detected. EZH2 is the enzymatic subunit of the PRC2 complex (polycomb repressive complex 2), which methylates lysine 9 and lysine 27 of histone H3, and is fundamental for transcriptional repression (Kim and Roberts 2016; Schuettengruber et al. 2007; Boyer et al. 2006). EZH2 acts as an oncogene, and its high expression levels are associated with tumor cell proliferation and migration (Zhou et al. 2015a; Ning et al. 2015). Furthermore, it has been shown that inhibition of EZH2 in HNSCC cells in vitro and in vivo induces loss of mitochondrial membrane potential (ΔΨm) with consequent activation of cell death pathways. Inhibition of EZH2 involves accumulation of Ca2+ into the mitochondria, induced by inactivation of MICU1 (Zhou et al. 2015b; Cosentino and Garcia-Saez 2014) (Fig. 1).
在大多数人类癌症类型中,包括头颈鳞状细胞癌 (HNSCC),已检测到高水平的 zeste 同源物增强子 2 (EZH2)。 EZH2 是 PRC2 复合物(多梳抑制复合物 2)的酶亚基,可甲基化组蛋白 H3 的赖氨酸 9 和赖氨酸 27,并且是转录抑制的基础(Kim 和 Roberts 2016 ;Schuettengruber 等人2007 ;Boyer 等人2006) )。 EZH2作为癌基因,其高表达水平与肿瘤细胞增殖和迁移相关(Zhou et al. 2015a ;Ning et al. 2015 )。此外,研究表明,体外和体内抑制 HNSCC 细胞中的 EZH2 会导致线粒体膜电位 (ΔΨ m ) 丧失,从而激活细胞死亡途径。 EZH2的抑制涉及由MICU1失活诱导的Ca 2+积累到线粒体中(Zhou等人2015b ;Cosentino和Garcia-Saez 2014 )(图1 )。
4 Upregulation of ER-Mitochondria Ca2+ Crosstalk
4 ER-线粒体 Ca 2+串扰的上调
4.1 New Insights into Ca2+ Signaling Perturbation in the MAMs
4.1对 MAM 中 Ca 2+信号传导扰动的新见解
The numerous molecular pathways described thus far all involve a decreased uptake of Ca2+ to the mitochondria, resulting from decreased ER release or mitochondrial defects. Historically, reports that have assessed the remodeling of MAMs Ca2+ signaling associated with tumorigenesis, invasion, and metastasis all led to the conclusion that cancer cells undergo minor mitochondria-dependent apoptosis because of decreases in the Ca2+ release from the ER. Recently, the characterization of new MAM-localized proteins and the finding of new mechanisms of action led the scientific community to consider that even an upregulation of Ca2+ signaling at the MAMs level could be harmful and drive tumor onset and progression. In the following paragraphs, we will describe how this condition, hitherto described as the cause of apoptotic cell death, can lead to the onset and development of tumor diseases.
迄今为止描述的众多分子途径都涉及线粒体对 Ca 2+的吸收减少,这是由于 ER 释放减少或线粒体缺陷所致。历史上,评估与肿瘤发生、侵袭和转移相关的 MAM Ca 2+信号重塑的报告都得出这样的结论:由于 ER 释放的 Ca 2+减少,癌细胞会经历轻微的线粒体依赖性细胞凋亡。最近,新的 MAM 定位蛋白的表征和新的作用机制的发现使科学界认为,即使 MAM 水平上的 Ca 2+信号传导上调也可能是有害的,并会促进肿瘤的发生和进展。在下面的段落中,我们将描述这种迄今为止被描述为细胞凋亡原因的情况如何导致肿瘤疾病的发生和发展。
4.2 Increased ER-Ca2+ Release
4.2 ER-Ca 2+释放增加
The endoplasmic reticulum is an organelle that contains a network of tubules and flattened sacs and is mainly known for its major role in the production, processing, and transport of proteins and lipids. The ER also represents the major intracellular store of Ca2+, an ion that is necessary on its lumen for second-messenger-induced Ca2+ release, the control of capacitative Ca2+ influx, and intra-ER chaperone activities such as polypeptide translocation, protein folding, and ER-associated degradation (Buck et al. 2007). In normal tissue cells, a sustained Ca2+ flux from the ER to the mitochondria can enhance the sensitivity of mitochondria to apoptotic stimuli; however, in some cases, an increase in Ca2+ ion leakage from the ER to the MAMs can promote tumor formation, especially in specific tissues and organs. For ER-mitochondria interorganellar Ca2+ signaling and, in particular, increased ER Ca2+ release, the recent revelation of the mechanisms by which IP3R3 upregulation drives oncogenesis via ER-mitochondrial Ca2+ crosstalk is particularly important. This statement is particularly strong because until last year, IP3R3 was well characterized as a Ca2+-related proapoptotic protein. In fact, the tumor suppressors BAP1 and PTEN have a stabilizing effect on IP3R3 in the ER, promoting susceptibility to cell death (Bononi et al. 2017; Kuchay et al. 2017), and in contrast, the oncogene K-RasG13D downregulates IP3R3, preventing the apoptotic death of cancer cells (Pierro et al. 2014). Three recent works by Guerra et al. (2019), Rezuchova et al. (2019), and Ueasilamongkol et al. (2020), for the first time, have deviated from the idea that IP3Rs only have an anti-oncogenic potential by driving proapoptotic Ca2+ signals to mitochondria but attributed an oncogenic potential to ER-mitochondria Ca2+ crosstalk. In an analysis of tumor tissues, the IP3R3-protein levels were elevated in hepatocellular carcinoma biopsies compared to healthy liver biopsies (Guerra et al. 2019), in clear cell renal cell carcinoma kidney biopsies compared to healthy regions (Rezuchova et al. 2019) and in cholangiocarcinoma cancer biopsies and cancer cell lines compared to normal tissues and normal cholangiocyte cell models (Ueasilamongkol et al. 2020). In all cases, only type 3 IP3Rs were found to be overexpressed in tumor tissues, with no changes or slight downregulation of type 1 and type 2. In particular, IP3R3 seems to be completely absent in normal human hepatocytes but is clearly present in biopsies from individuals with hepatitis B virus, hepatitis C virus (HCV), non-alcoholic fatty liver disease (NAFLD), and alcoholic liver disease (ALD), which are the four most common predisposing factors to the development of hepatocellular carcinoma (Guerra et al. 2019). This increase was more pronounced in the late stages of hepatocellular carcinoma.
内质网是一种细胞器,包含小管和扁平囊网络,主要因其在蛋白质和脂质的生产、加工和运输中的重要作用而闻名。 ER 还代表 Ca 2+的主要细胞内储存,Ca 2+ 是其管腔上第二信使诱导的 Ca 2+释放、控制电容性 Ca 2+流入和内质网内分子伴侣活性(如多肽)所必需的离子。易位、蛋白质折叠和 ER 相关降解(Buck 等人, 2007 )。在正常组织细胞中,从内质网到线粒体的持续Ca 2+通量可以增强线粒体对凋亡刺激的敏感性;然而,在某些情况下,Ca 2+离子从 ER 渗漏至 MAM 的增加可促进肿瘤形成,特别是在特定组织和器官中。对于 ER-线粒体细胞间 Ca 2+信号传导,特别是增加 ER Ca 2+释放,最近揭示的 IP3R3 上调通过 ER-线粒体 Ca 2+串扰驱动肿瘤发生的机制尤为重要。这种说法特别有力,因为直到去年,IP3R3 才被充分表征为 Ca 2+相关促凋亡蛋白。事实上,肿瘤抑制因子 BAP1 和 PTEN 对 ER 中的 IP3R3 具有稳定作用,促进细胞死亡的易感性 (Bononi et al. 2017 ; Kuchay et al. 2017 ),相反,癌基因 K-Ras G13D下调 IP3R3 ,防止癌细胞凋亡(Pierro et al. 2014 )。 Guerra 等人最近的三部作品。 ( 2019 ),Rezuchova 等人。 ( 2019 )和Ueasilamongkol等人。 ( 2020 ) 首次偏离了 IP3R 仅通过向线粒体驱动促凋亡 Ca 2+信号而具有抗癌潜力的观点,而是将致癌潜力归因于 ER-线粒体 Ca 2+串扰。在对肿瘤组织的分析中,与健康肝脏活检组织相比,肝细胞癌活检组织中的 IP3R3 蛋白水平升高(Guerra et al. 2019 ),与健康区域相比,透明细胞肾细胞癌肾活检组织中的 IP3R3 蛋白水平升高(Rezuchova et al. 2019 )以及胆管癌癌症活检和癌细胞系与正常组织和正常胆管细胞模型的比较(Ueasilamongkol et al. 2020 )。在所有情况下,仅发现 3 型 IP3R 在肿瘤组织中过度表达,1 型和 2 型没有变化或略有下调。特别是,IP3R3 似乎在正常人肝细胞中完全不存在,但在活检组织中明显存在。患有乙型肝炎病毒、丙型肝炎病毒(HCV)、非酒精性脂肪肝病(NAFLD)和酒精性肝病(ALD)的个体,这是发生肝细胞癌的四种最常见的诱发因素(Guerra等,2017)。 2019 )。这种增加在肝细胞癌晚期更为明显。
Notably, in cholangiocarcinoma cells, most IP3R3 is localized to the MAMs, while in normal cholangiocytes, it resides in the ER subapical pole. In these cells, MAM localization promotes basal respiration by increasing mitochondrial Ca2+ signaling, and thus, depletion of this channel in these cells is deleterious for nuclear and mitochondrial functionality (Ueasilamongkol et al. 2020). In HepG2 cells, IP3R3 upregulation promotes cell death, but its chronic overexpression can increase the resistance of these cells to cell death inducers, enhancing malignant cell survival (Guerra et al. 2019).
值得注意的是,在胆管癌细胞中,大多数 IP3R3 定位于 MAM,而在正常胆管细胞中,它位于 ER 根尖下极。在这些细胞中,MAM 定位通过增加线粒体 Ca 2+信号传导来促进基础呼吸,因此,这些细胞中该通道的耗尽对核和线粒体功能有害 (Ueasilamongkol et al. 2020 )。在 HepG2 细胞中,IP3R3 上调会促进细胞死亡,但其长期过度表达可以增加这些细胞对细胞死亡诱导剂的抵抗力,从而增强恶性细胞的存活率 (Guerra et al. 2019 )。
The common key in all these cases is the extreme adaptation ability that drives oncogenesis and malignant cell transformation. These cancer cells became addicted to high IP3R3 levels at the MAM compartment for their survival, to maintain sustained cell metabolism and to obtain malignant features such as increased motility, migration, and invasion.
所有这些情况的共同关键是驱动肿瘤发生和恶性细胞转化的极端适应能力。这些癌细胞对 MAM 区室中高水平的 IP3R3 上瘾,以求生存、维持持续的细胞代谢并获得恶性特征,例如运动、迁移和侵袭增加。
We want to include in this section the already mentioned ERO1-α, an extensively studied protein due to its ability to regulate many processes. ERO1-α is particularly enriched at the ER-mitochondria interface, controlling ER redox homeostasis and oxidative folding and regulating Ca2+ efflux from the ER and, consequently, mitochondrial Ca2+ accumulation (Anelli et al. 2012). ERO1-α is highly expressed in different tumor types and is associated with a poor prognosis in breast cancer (Kutomi et al. 2013). In fact, the expression of ERO1-α in triple-negative breast cancer cells is correlated with that of programmed cell death-ligand 1 (PD-L1), both at the protein and mRNA levels, via hypoxia-inducible factor 1-α (HIF-1α). Depletion of ERO1-α led to a significant reduction in PD-L1-mediated T-cell apoptosis, suggesting that ERO1-α has a key role in tumor-mediated immunosuppression (Tanaka et al. 2017).
我们希望在本节中包括已经提到的 ERO1-α,这是一种经过广泛研究的蛋白质,因为它能够调节许多过程。 ERO1-α 在 ER-线粒体界面特别富集,控制 ER 氧化还原稳态和氧化折叠,并调节 Ca 2+从 ER 流出,从而调节线粒体 Ca 2+积累 (Anelli et al. 2012 )。 ERO1-α 在不同的肿瘤类型中高表达,并且与乳腺癌的不良预后相关(Kutomi et al. 2013 )。事实上,三阴性乳腺癌细胞中 ERO1-α 的表达通过缺氧诱导因子 1-α 在蛋白质和 mRNA 水平上与程序性细胞死亡配体 1 (PD-L1) 的表达相关。 HIF-1α)。 ERO1-α 的耗竭导致 PD-L1 介导的 T 细胞凋亡显着减少,表明 ERO1-α 在肿瘤介导的免疫抑制中具有关键作用 (Tanaka et al. 2017 )。
Another MAMs Ca2+- and tumor-related protein that acts at the ER level is the receptor chaperone stress-activated chaperone sigma-1 receptor (Sig1R), which senses ER Ca2+ concentrations and regulates cell survival. This protein could be considered “borderline” in this section considering its mechanism of action; in fact, Sig1R is an ER-localized protein that favors the efflux of calcium ions from the endoplasmic reticulum and has been described as being overexpressed in breast cancer, especially in cancer cells with metastatic potential (Gueguinou et al. 2017). ER chaperones are important in maintaining proper intracellular Ca2+ levels, protein folding, and the unfolded protein response (UPR) under ER stress conditions (Bartoszewska and Collawn 2020).
另一种在 ER 水平发挥作用的 MAM Ca 2+和肿瘤相关蛋白是受体伴侣应激激活伴侣 sigma-1 受体 (Sig1R),它可感知 ER Ca 2+浓度并调节细胞存活。考虑到其作用机制,该蛋白质在本节中可以被视为“边缘”;事实上,Sig1R 是一种内质网定位蛋白,有利于钙离子从内质网流出,并且被描述为在乳腺癌中过度表达,尤其是在具有转移潜力的癌细胞中 (Gueguinou et al. 2017 )。 ER 伴侣对于在内质网应激条件下维持适当的细胞内 Ca 2+水平、蛋白质折叠和未折叠蛋白反应 (UPR) 非常重要 (Bartoszewska 和 Collawn 2020 )。
Two MAM-localized chaperones that belong to the heat shock 70 kDa (HSP70) protein family are of considerable importance in Ca2+ signaling: chaperone glucose-regulated protein GRP75 and glucose-regulated protein 78 (GRP78, also known as immunoglobulin heavy-chain-binding protein BiP) (Brocchieri et al. 2008; Wadhwa et al. 2002).
属于热休克 70 kDa (HSP70) 蛋白家族的两个 MAM 定位伴侣在 Ca 2+信号传导中具有相当重要的作用:伴侣葡萄糖调节蛋白 GRP75 和葡萄糖调节蛋白 78 (GRP78,也称为免疫球蛋白重链) -结合蛋白 BiP)(Brocchieri 等人, 2008 ;Wadhwa 等人, 2002 )。
GRP75 ensures the juxtaposition between IP3R and VDAC1 in the mitochondrial outer membrane (Szabadkai et al. 2006). Its localization is mainly mitochondrial, but it is also present at low levels in the cytoplasm, nucleus, ER, and Golgi apparatus (Ran et al. 2000; Wadhwa et al. 1995), where it exerts many different functions from the import of unfolded proteins into the mitochondrial matrix to modulation of exocytosis and endocytosis (Flachbartova and Kovacech 2013; Voos and Rottgers 2002; Schneider et al. 1996; Kronidou et al. 1994; Scherer et al. 1992). Sig1Rs are particularly enriched at the MAMs and in normal tissues form a complex with GRP78, another MAM-localized chaperone. GRP78 can bind to misfolded proteins and to unassembled complexes and modulates ER-associated degradation (ERAD), which regulates the UPR (Pfaffenbach and Lee 2011; Wang et al. 2009; Little et al. 1994). Its molecular structure displays two domains: the substrate-binding domain (SBD), involved in binding unfolded peptides, and the nucleotide-binding domain (NBD), which binds ATP to be hydrolyzed to obtain energy to prevent unfolded protein aggregation at the N-terminus (Luo et al. 2006; Lindquist and Craig 1988). GRP78, like almost all other chaperones, is useful for storing ER Ca2+ as a high-capacity Ca2+-binding protein under physiological conditions (Hendershot 2004).
GRP75 确保线粒体外膜中 IP3R 和 VDAC1 的并置(Szabadkai 等人, 2006 )。它的定位主要是线粒体,但它也以低水平存在于细胞质、细胞核、内质网和高尔基体中(Ran 等人, 2000 年;Wadhwa 等人, 1995 年),在其中发挥许多不同的功能。蛋白质进入线粒体基质以调节胞吐作用和内吞作用(Flachbartova 和 Kovacech 2013 ;Voos 和 Rottgers 2002 ;Schneider 等人1996 ;Kronidou 等人1994 ;Scherer 等人1992 )。 Sig1R 在 MAM 中特别富集,并且在正常组织中与 GRP78(另一种 MAM 定位分子伴侣)形成复合物。 GRP78 可以与错误折叠的蛋白质和未组装的复合物结合,并调节 ER 相关降解 (ERAD),从而调节 UPR (Pfaffenbach 和 Lee 2011 ;Wang 等人2009 ;Little 等人1994 )。其分子结构显示出两个结构域:底物结合结构域(SBD),参与结合未折叠肽;核苷酸结合结构域(NBD),结合ATP进行水解以获得能量,以防止未折叠蛋白在N-上聚集。终点站(Luo et al. 2006 ;Lindquist and Craig 1988 )。与几乎所有其他伴侣一样,GRP78 可用于在生理条件下将 ER Ca 2+存储为高容量 Ca 2+结合蛋白(Hendershot 2004 )。
Szabadkai et al. highlighted the mechanism by which Sig1R, dissociating from BiP, binds IP3R3 following the activation of IP3Rs. This event leads to IP3R3 stabilization at the MAMs and to an enhancement of IP3R3-mediated Ca2+ fluxes to the mitochondria (Szabadkai et al. 2006). Although BiP is an excellent target to consider for neuroprotective therapeutic strategies (Enogieru et al. 2019), it also influences how tumor cells survive, proliferate, and develop chemoresistance. During chronic ER stress conditions that involve prolonged ER Ca2+ depletion, Sig1R localization changes from the MAMs to the peripheral ER, reducing cellular damage and thus preventing cell death. Another mechanism of Ca2+ homeostasis perturbation implemented by Sig1R that has direct consequences on cell invasiveness in breast cancer has been described by Gueguinou et al. (2017). Sig1R favors the migration of cancer cells by forming a functional molecular platform with the calcium-activated K+ channels SK3 and ORAI calcium release-activated calcium modulator 1 (Orai1) (Gueguinou et al. 2017) (Fig. 2).
萨巴德凯等人。强调了 IP3R 激活后 Sig1R 从 BiP 解离并结合 IP3R3 的机制。该事件导致IP3R3在MAM处稳定并导致IP3R3介导的Ca 2+流向线粒体的增强(Szabadkai等人2006 )。尽管 BiP 是神经保护治疗策略的绝佳靶点(Enogieru et al. 2019 ),但它也会影响肿瘤细胞的生存、增殖和化疗耐药性。在涉及长期 ER Ca 2+耗竭的慢性 ER 应激条件下,Sig1R 定位从 MAM 变为外周 ER,减少细胞损伤,从而防止细胞死亡。 Gueguinou 等人描述了由 Sig1R 实现的 Ca 2+稳态扰动的另一种机制,该机制对乳腺癌细胞侵袭性具有直接影响。 ( 2017 )。 Sig1R通过与钙激活的K +通道SK3和ORAI钙释放激活的钙调节剂1(Orai1)形成功能性分子平台来促进癌细胞的迁移(Gueguinou等人, 2017 )(图2 )。
Upregulation of MAMs Ca2+ crosstalk in cancer: graphical representation of the calcium signaling regulators involved in a cancer-related increased Ca2+ crosstalk state. See text for further details. Ca2+, calcium; ER, endoplasmic reticulum
癌症中 MAM Ca 2+串扰的上调:涉及癌症相关 Ca 2+串扰增加状态的钙信号调节因子的图形表示。请参阅文本了解更多详细信息。 Ca 2+ ,钙; ER-- 内质网
4.3 Increased Mitochondrial Ca2+ Uptake
4.3增加线粒体 Ca 2+摄取
Before the identification of the molecular players forming the MCU complex, the role of mitochondrial Ca2+ in cancer progression was simply confined to receiving Ca2+ from the ER, thereby regulating the apoptotic response. Low ER Ca2+ release results in reduced mitochondrial [Ca2+], mPTP inhibition, and resistance to chemotherapeutic-induced cell death. Consistent with this view, many oncogenic factors act at the MAMs to limit ER-mitochondria Ca2+ transfer (see the “Downregulation of ER-mitochondria calcium crosstalk” section). However, many mitochondrial Ca2+ channels that are responsible for favoring Ca2+ accumulation, such as VDACs, are overexpressed, rather than reduced, in cancer (Mazure 2017). These observations suggest that an increased intrinsic capacity of the mitochondrial compartment to accumulate Ca2+ could contribute to sustained malignant progression, although, at least theoretically, it predisposes cells to Ca2+-induced cell death. The oncogenic mechanisms regulated by mitochondrial Ca2+ mainly rely on the association between Ca2+ and the formation of mitogenic ROS, as well as pure stimulation of mitochondrial metabolism. Ca2+ accumulation activates four mitochondrial dehydrogenases, which in turn stimulate the respiratory chain and hence ATP production (Denton 2009). Thus, as a consequence of increased metabolic activity, ROS are generated inside the matrix, but they fail to trigger cell death, probably due to the superior antioxidant defense that often distinguishes the malignant phenotype (Gorrini et al. 2013).
在确定形成 MCU 复合体的分子参与者之前,线粒体 Ca 2+在癌症进展中的作用仅局限于从 ER 接收 Ca 2+ ,从而调节细胞凋亡反应。低 ER Ca 2+释放导致线粒体 [Ca 2+ ] 减少、mPTP 抑制和对化疗诱导的细胞死亡的抵抗。与这一观点一致,许多致癌因素作用于 MAM,限制 ER-线粒体 Ca 2+转移(参见“ER-线粒体钙串扰下调”部分)。然而,许多负责 Ca 2+积累的线粒体 Ca 2+通道(例如 VDAC)在癌症中过度表达,而不是减少 (Mazure 2017 )。这些观察结果表明,线粒体区室积累Ca 2+的内在能力增加可能有助于持续的恶性进展,尽管至少在理论上,它使细胞易于发生Ca 2+诱导的细胞死亡。线粒体Ca 2+调控的致癌机制主要依赖于Ca 2+与促有丝分裂ROS形成的关联,以及对线粒体代谢的纯粹刺激。 Ca 2+积累会激活四种线粒体脱氢酶,进而刺激呼吸链,从而刺激 ATP 产生 (Denton 2009 )。因此,由于代谢活动增加,ROS在基质内产生,但它们未能触发细胞死亡,这可能是由于通常区分恶性表型的卓越抗氧化防御(Gorrini et al. 2013 )。
The correlation between augmented mitochondrial Ca2+ entry, ROS production, and cancer growth appears evident for tumors overexpressing the uniporter complex pore-forming subunit MCU. Indeed, increased levels of MCU have been reported in different tumors, including breast and hepatocellular carcinomas (Vultur et al. 2018). In breast cancer, MCU-dependent mitochondrial Ca2+ entry is associated with ROS overproduction and higher metastatic potential through a mechanism that involves the downstream activation of HIF1-α transcriptional activity (Tosatto et al. 2016). Consistent with these observations, upregulation of MCU in triple-negative breast cancer cells promoted metastasis in an in vivo mouse model by enhancing glycolysis, a series of neoplastic events that is counteracted by the tumor-suppressor activity of miRNA-340 (Yu et al. 2017). Moreover, receptor-interacting protein kinase 1 (RIPK1) binds MCU to promote Ca2+ entry and colorectal cancer progression through stimulation of mitochondrial bioenergetics (Zeng et al. 2018). In hepatocellular carcinomas, the Ca2+-ROS axis orchestrated by MCU resulted in activation of metalloproteinase-2 (MMP2) (Ren et al. 2017), a zinc-dependent endopeptidase associated with extracellular matrix degradation and metastasis (Shay et al. 2015).
对于过表达单向转运蛋白复合物成孔亚基 MCU 的肿瘤来说,增加的线粒体 Ca 2+进入、ROS 产生和癌症生长之间的相关性似乎很明显。事实上,据报道,不同肿瘤中的 MCU 水平有所增加,包括乳腺癌和肝细胞癌 (Vultur et al. 2018 )。在乳腺癌中,MCU 依赖性线粒体 Ca 2+进入通过涉及 HIF1-α 转录活性下游激活的机制与 ROS 过量产生和更高的转移潜力相关 (Tosatto et al. 2016 )。与这些观察结果一致,三阴性乳腺癌细胞中 MCU 的上调通过增强糖酵解促进体内小鼠模型的转移,糖酵解是一系列肿瘤事件,可被 miRNA-340 的肿瘤抑制活性抵消(Yu 等,2017)。 2017 )。此外,受体相互作用蛋白激酶 1 (RIPK1) 与 MCU 结合,通过刺激线粒体生物能促进 Ca 2+进入和结直肠癌进展 (Zeng et al. 2018 )。在肝细胞癌中,MCU 协调的 Ca 2+ -ROS 轴导致金属蛋白酶-2 (MMP2) 的激活 (Ren et al. 2017 ),这是一种与细胞外基质降解和转移相关的锌依赖性内肽酶 (Shay et al. 2015) )。
The link between Ca2+ and ROS overproduction is also relevant for the cancer-related functions of MICU1, the principal member of the MCU complex that regulates the gating of the channel (Kamer and Mootha 2015). Our group recently showed that MICU1 downregulation, as a result of higher AKT activity, could sustain cancer progression through Ca2+-dependent ROS generation (Marchi et al. 2019a). Indeed, loss of MICU1 disinhibits MCU, leading to Ca2+ permeation under resting (nonstimulated) conditions and increased mitochondrial ROS levels (Csordas et al. 2013), which could ultimately result in cell death (Mallilankaraman et al. 2012a; Liu et al. 2016). This finding implies that malignant cells showing low MICU1 levels predispose concomitant mechanisms to minimize the detrimental effects induced by ROS. Consistent with this view, MICU1 depletion in normal hepatocytes triggered extensive cell death, but upon pharmacological inhibition of mPTP opening, the loss of MICU1 conferred a strong proliferative advantage (Antony et al. 2016). Moreover, a combination of high mitochondrial Ca2+ entry through genetic manipulation of the MCU complex and mPTP closure exacerbated the tumorigenic potential of different cancer cells (Marchi et al. 2019b). Taken together, these observations suggest that variations in the composition of the MCU complex are a key event that cooperates with other oncogenic pathways to favor cancer growth.
Ca 2+和 ROS 过量产生之间的联系也与 MICU1 的癌症相关功能相关,MICU1 是调节通道门控的 MCU 复合体的主要成员 (Kamer 和 Mootha 2015 )。我们的小组最近表明,由于 AKT 活性较高,MICU1 下调可以通过 Ca 2+依赖性 ROS 生成维持癌症进展 (Marchi et al. 2019a )。事实上,MICU1 的缺失会抑制 MCU,导致静息(未刺激)条件下 Ca 2+渗透并增加线粒体 ROS 水平(Csordas 等人, 2013 ),这最终可能导致细胞死亡(Mallilankaraman 等人, 2012a ;Liu 等人) 2016 )。这一发现意味着,表现出低 MICU1 水平的恶性细胞容易产生伴随机制,以最大限度地减少 ROS 引起的有害影响。与这一观点一致,正常肝细胞中MICU1的缺失引发了广泛的细胞死亡,但在药理抑制mPTP开放后,MICU1的缺失赋予了强大的增殖优势(Antony et al. 2016 )。此外,通过对 MCU 复合体进行基因操作和 mPTP 关闭来实现高线粒体 Ca 2+进入,加剧了不同癌细胞的致瘤潜力(Marchi 等人, 2019b )。综上所述,这些观察结果表明,MCU 复合体组成的变化是与其他致癌途径协同促进癌症生长的关键事件。
Further evidence that supports this scenario derives from the protumorigenic role of MCU regulator 1 (MCUR1), which has been described as a matrix-located, positive regulator of the uniporter complex (Mallilankaraman et al. 2012b). In hepatocellular carcinomas, MCUR1 was strongly upregulated, and ROS production was augmented, leading to ROS-dependent degradation of p53 and consequent resistance to apoptosis (Ren et al. 2018). Notably, the cancer cell detoxification capacity was also increased due to activation of nuclear factor erythroid 2-related factor 2 (NRF2) (Jin et al. 2019), a master gene in the orchestration of the cellular antioxidant response (Cuadrado et al. 2019). Thus, MCUR1 can regulate two cancer hallmarks at once: Ca2+-mediated metastatic potential and resistance to apoptosis. However, the expression of MCUR1 correlates with the permeability transition and reduced cell survival (Chaudhuri et al. 2016b), indicating that MCUR1 oncogenic activities might be solely due to the concomitant inhibition of the functions of the mPTP through a superior mechanism. Nevertheless, it has been proposed that MCUR1 could act as a complex IV assembly factor rather than as an MCU interactor (Paupe et al. 2015). In this context, variations in mitochondrial Ca2+ uptake and ROS levels are side products of respiratory chain defects; therefore, the active role of Ca2+ in MCUR1-mediated oncogenesis should be completely reevaluated.
支持这种情况的进一步证据来自 MCU 调节器 1 (MCUR1) 的促肿瘤作用,它被描述为位于基质的单向转运蛋白复合物的正调节器 (Mallilankaraman et al. 2012b )。在肝细胞癌中,MCUR1 强烈上调,ROS 产生增加,导致 ROS 依赖性 p53 降解,从而抵抗细胞凋亡 (Ren et al. 2018 )。值得注意的是,由于核因子红细胞 2 相关因子 2 (NRF2) 的激活,癌细胞的解毒能力也有所增强 (Jin et al. 2019 ),这是协调细胞抗氧化反应的主基因 (Cuadrado et al. 2019 ) )。因此,MCUR1 可以同时调节两个癌症标志:Ca 2+介导的转移潜能和细胞凋亡抵抗。然而,MCUR1 的表达与通透性转变和细胞存活率降低相关(Chaudhuri et al. 2016b ),表明 MCUR1 致癌活性可能仅仅是由于通过一种优越的机制同时抑制 mPTP 的功能。尽管如此,有人提出 MCUR1 可以充当复杂的 IV 组装因子,而不是 MCU 相互作用子 (Paupe et al. 2015 )。在这种情况下,线粒体 Ca 2+摄取和 ROS 水平的变化是呼吸链缺陷的副产物;因此,Ca 2+在MCUR1介导的肿瘤发生中的积极作用应该完全重新评估。
Overall, these observations indicate that increased mitochondrial Ca2+ uptake acts with other oncogenic mechanisms (e.g., mPTP inhibition or activation of antioxidant systems) to sustain cancer growth and dissemination. The protumorigenic role of mitochondrial Ca2+ signaling involves other pathways in addition to ROS production and excess malignant cell bioenergetics, including the MCU-dependent control of cytosolic Ca2+ through store-operated Ca2+ entry (SOCE). The activity of the MCU complex sustains cytosolic Ca2+ fluxes through SOCE, which in turn regulates cytoskeletal dynamics and cellular migration (Prudent et al. 2016). Moreover, recent findings suggest that spontaneous mitochondrial Ca2+ oscillations through the MCU complex are essential for mitotic entry and cell cycle progression (Koval et al. 2019; Zhao et al. 2019), thus revealing another mechanism that could account for the aberrant proliferation of cancer cells with an altered composition of the MCU complex (Fig. 2).
总体而言,这些观察结果表明线粒体Ca 2+摄取增加与其他致癌机制(例如,mPTP 抑制或抗氧化系统激活)共同作用,以维持癌症生长和扩散。线粒体 Ca 2+信号传导的促肿瘤作用除了 ROS 产生和过量的恶性细胞生物能量外还涉及其他途径,包括通过钙池操纵的 Ca 2+进入 (SOCE) 对胞质 Ca 2+进行 MCU 依赖性控制。 MCU 复合体的活性通过 SOCE 维持细胞质 Ca 2+通量,进而调节细胞骨架动力学和细胞迁移 (Prudent et al. 2016 )。此外,最近的研究结果表明,通过 MCU 复合体的自发线粒体 Ca 2+振荡对于有丝分裂进入和细胞周期进展至关重要(Koval et al. 2019 ;Zhao et al. 2019 ),从而揭示了另一种可以解释异常增殖的机制MCU 复合体组成发生改变的癌细胞(图2 )。
5 Conclusions
5结论
The importance of the multiple and complex signaling pathways generated by the displacement of Ca2+ ions and, specifically, the Ca2+-dependent communication between structurally and functionally interconnected intracellular organelles has been increasingly highlighted and described, especially in recent years. Evidence of this phenomenon is the dramatic effects on cell health that derive from perturbation of the MAMs morphology and modification of the ER-mitochondria tethering distance. Moreover, alterations in the MAMs protein pool and functionality have been connected with several pathological conditions, including diabetes, neurodegeneration, infection, and antiviral response and cancer (Pinton 2018). Tumor cells, in fact, could modify the systems that maintain cellular Ca2+ homeostasis to promote their survival and metastasis. The crucial role of the regulation of spatiotemporal Ca2+ signaling in the MAMs in cancer is confirmed by evidence that different oncogenes and tumor suppressors reside at the ER-mitochondria interface.
由Ca 2+离子的置换产生的多种复杂信号传导途径的重要性,特别是结构和功能上相互连接的细胞内细胞器之间的Ca 2+依赖性通讯,已越来越被强调和描述,特别是近年来。这种现象的证据是 MAM 形态的扰动和 ER-线粒体束缚距离的改变对细胞健康的巨大影响。此外,MAM 蛋白库和功能的改变与多种病理状况有关,包括糖尿病、神经退行性变、感染、抗病毒反应和癌症 (Pinton 2018 )。事实上,肿瘤细胞可以修改维持细胞 Ca 2+稳态的系统,以促进其生存和转移。不同癌基因和肿瘤抑制因子位于 ER-线粒体界面的证据证实了癌症中 MAM 中时空 Ca 2+信号传导的调节的关键作用。
As shown previously, both an increase and a decrease of calcium ion exchange between these two organelles can, in a nonexclusive way, lead to the promotion or suppression of tumor behaviors in many tissues. This phenomenon is an indication of how the equilibrium that rules calcium homeostasis in this subcellular compartment is delicate, complex, and intimate. Specifically, although Ca2+ oscillations are essential at MAMs to feed mitochondrial metabolism, a persistent increase in mitochondrial [Ca2+] can lead to cell death. In this scenario, by limiting mitochondrial calcium uptake, many cancer cells develop resistance to death. On the other hand, it was also highlighted that an increased mitochondrial ability to accumulate Ca2+ supports malignant progression, by boosting mitochondrial metabolism and sustaining mitogenic ROS production. Thus, depending on the tumor context, MAM-localized Ca2+ signaling can exert different functions, also according to the different oncogenic paths involved.
如前所述,这两个细胞器之间钙离子交换的增加和减少都可以以非排他性的方式导致许多组织中肿瘤行为的促进或抑制。这种现象表明,在这个亚细胞区室中,控制钙稳态的平衡是如何微妙、复杂和密切的。具体来说,尽管 Ca 2+振荡对于 MAM 促进线粒体代谢至关重要,但线粒体 [Ca 2+ ] 的持续增加可能导致细胞死亡。在这种情况下,通过限制线粒体钙的吸收,许多癌细胞产生了对死亡的抵抗力。另一方面,还强调了线粒体积累 Ca 2+ 的能力增加,通过促进线粒体代谢和维持有丝分裂活性氧的产生,支持恶性进展。因此,根据肿瘤背景,MAM 定位的 Ca 2+信号传导可以发挥不同的功能,也根据所涉及的不同致癌路径。
Several questions have yet to be answered, many aspects remain to be clarified, and molecular pathways must be described to reach a good understanding of the complex mechanisms that stem from calcium signaling at the MAMs, knowledge that will be very useful in the development of novel therapeutic strategies for several tumors.
有几个问题尚未得到解答,许多方面仍有待澄清,必须描述分子途径,以便更好地理解 MAM 钙信号传导的复杂机制,这些知识对于开发新型药物非常有用。几种肿瘤的治疗策略。
References 参考
Abu-Hamad S, Arbel N, Calo D, Arzoine L, Israelson A, Keinan N, Ben-Romano R, Friedman O, Shoshan-Barmatz V (2009) The VDAC1 N-terminus is essential both for apoptosis and the protective effect of anti-apoptotic proteins. J Cell Sci 122(Pt 11):1906–1916. https://doi.org/10.1242/jcs.040188IF: 3.3 Q3
Abu-Hamad S, Arbel N, Calo D, Arzoine L, Israelson A, Keinan N, Ben-Romano R, Friedman O, Shoshan-Barmatz V (2009) VDAC1 N 末端对于细胞凋亡和保护作用至关重要抗凋亡蛋白。 《细胞科学杂志》122(第 11 部分):1906–1916。 https://doi.org/10.1242/jcs.040188IF:3.3 第三季度Akl H, Bultynck G (2013) Altered Ca(2+) signaling in cancer cells: proto-oncogenes and tumor suppressors targeting IP3 receptors. Biochim Biophys Acta 1835(2):180–193. https://doi.org/10.1016/j.bbcan.2012.12.001IF: 9.7 Q1
Akl H, Bultynck G (2013) 改变癌细胞中的 Ca(2+) 信号传导:原癌基因和靶向 IP3 受体的肿瘤抑制因子。 Biochim Biophys Acta 1835(2):180–193。 https://doi.org/10.1016/j.bbcan.2012.12.001IF:9.7 第一季度Alonso MT, Manjarres IM, Garcia-Sancho J (2009) Modulation of calcium signalling by intracellular organelles seen with targeted aequorins. Acta Physiol (Oxf) 195(1):37–49. https://doi.org/10.1111/j.1748-1716.2008.01920.xIF: 5.6 Q1
Alonso MT、Manjarres IM、Garcia-Sancho J (2009) 通过靶向水母发光蛋白观察到的细胞内细胞器对钙信号的调节。生理学报 (Oxf) 195(1):37–49。 https://doi.org/10.1111/j.1748-1716.2008.01920.xIF:5.6 第一季度Anelli T, Bergamelli L, Margittai E, Rimessi A, Fagioli C, Malgaroli A, Pinton P, Ripamonti M, Rizzuto R, Sitia R (2012) Ero1alpha regulates Ca(2+) fluxes at the endoplasmic reticulum-mitochondria interface (MAM). Antioxid Redox Signal 16(10):1077–1087. https://doi.org/10.1089/ars.2011.4004IF: 5.9 Q1
Anelli T, Bergamelli L, Margittai E, Rimessi A, Fagioli C, Malgaroli A, Pinton P, Ripamonti M, Rizzuto R, Sitia R (2012) Ero1alpha 调节内质网-线粒体界面 (MAM) 的 Ca(2+) 通量。抗氧化剂氧化还原信号 16(10):1077–1087。 https://doi.org/10.1089/ars.2011.4004IF:5.9 第一季度Antony AN, Paillard M, Moffat C, Juskeviciute E, Correnti J, Bolon B, Rubin E, Csordas G, Seifert EL, Hoek JB, Hajnoczky G (2016) MICU1 regulation of mitochondrial Ca(2+) uptake dictates survival and tissue regeneration. Nat Commun 7:10955. https://doi.org/10.1038/ncomms10955IF: 14.7 Q1
Antony AN, Paillard M, Moffat C, Juskeviciute E, Correnti J, Bolon B, Rubin E, Csordas G, Seifert EL, Hoek JB, Hajnoczky G (2016) MICU1 对线粒体 Ca(2+) 摄取的调节决定存活和组织再生。 《自然通讯》7:10955。 https://doi.org/10.1038/ncomms10955IF:14.7 第一季度Arbel N, Shoshan-Barmatz V (2010) Voltage-dependent anion channel 1-based peptides interact with Bcl-2 to prevent antiapoptotic activity. J Biol Chem 285(9):6053–6062. https://doi.org/10.1074/jbc.M109.082990IF: 4.0 Q2
Arbel N, Shoshan-Barmatz V (2010) 基于电压依赖性阴离子通道 1 的肽与 Bcl-2 相互作用以防止抗凋亡活性。生物化学杂志 285(9):6053–6062。 https://doi.org/10.1074/jbc.M109.082990IF:4.0 第二季度Ashby MC, Tepikin AV (2001) ER calcium and the functions of intracellular organelles. Semin Cell Dev Biol 12(1):11–17. https://doi.org/10.1006/scdb.2000.0212IF: 6.2 Q1
Ashby MC, Tepikin AV (2001) ER 钙和细胞内细胞器的功能。精明细胞发育生物学 12(1):11–17。 https://doi.org/10.1006/scdb.2000.0212IF:6.2 第一季度Avalle L, Camporeale A, Morciano G, Caroccia N, Ghetti E, Orecchia V, Viavattene D, Giorgi C, Pinton P, Poli V (2019) STAT3 localizes to the ER, acting as a gatekeeper for ER-mitochondrion Ca(2+) fluxes and apoptotic responses. Cell Death Differ 26(5):932–942. https://doi.org/10.1038/s41418-018-0171-yIF: 13.7 Q1
Avalle L, Camporeale A, Morciano G, Caroccia N, Ghetti E, Orecchia V, Viavattene D, Giorgi C, Pinton P, Poli V (2019) STAT3 定位于 ER,充当 ER-线粒体 Ca(2+) 的看门人)通量和细胞凋亡反应。细胞死亡差异 26(5):932–942。 https://doi.org/10.1038/s41418-018-0171-yIF:13.7 第一季度Bansaghi S, Golenar T, Madesh M, Csordas G, RamachandraRao S, Sharma K, Yule DI, Joseph SK, Hajnoczky G (2014) Isoform- and species-specific control of inositol 1,4,5-trisphosphate (IP3) receptors by reactive oxygen species. J Biol Chem 289(12):8170–8181. https://doi.org/10.1074/jbc.M113.504159IF: 4.0 Q2
Bansaghi S、Golenar T、Madesh M、Csordas G、RamachandraRao S、Sharma K、Yule DI、Joseph SK、Hajnoczky G (2014) 通过异构体和物种特异性控制肌醇 1,4,5-三磷酸 (IP3) 受体活性氧。生物化学杂志 289(12):8170–8181。 https://doi.org/10.1074/jbc.M113.504159IF:4.0 第二季度Bargaje R, Gupta S, Sarkeshik A, Park R, Xu T, Sarkar M, Halimani M, Roy SS, Yates J, Pillai B (2012) Identification of novel targets for miR-29a using miRNA proteomics. PLoS One 7(8):e43243. https://doi.org/10.1371/journal.pone.0043243IF: 2.9 Q1
Bargaje R, Gupta S, Sarkeshik A, Park R, Xu T, Sarkar M, Halimani M, Roy SS, Yates J, Pillai B (2012) 使用 miRNA 蛋白质组学鉴定 miR-29a 的新靶标。 《公共科学图书馆一号》7(8):e43243。 https://doi.org/10.1371/journal.pone.0043243IF:2.9 第一季度Bartok A, Weaver D, Golenar T, Nichtova Z, Katona M, Bansaghi S, Alzayady KJ, Thomas VK, Ando H, Mikoshiba K, Joseph SK, Yule DI, Csordas G, Hajnoczky G (2019) IP3 receptor isoforms differently regulate ER-mitochondrial contacts and local calcium transfer. Nat Commun 10(1):3726. https://doi.org/10.1038/s41467-019-11646-3IF: 14.7 Q1
Bartok A, Weaver D, Golenar T, Nichtova Z, Katona M, Bansaghi S, Alzayady KJ, Thomas VK, Ando H, Mikoshiba K, Joseph SK, Yule DI, Csordas G, Hajnoczky G (2019) IP3 受体亚型以不同方式调节 ER -线粒体接触和局部钙转移。 《自然通讯》10(1):3726。 https://doi.org/10.1038/s41467-019-11646-3IF:14.7 第一季度Bartoszewska S, Collawn JF (2020) Unfolded protein response (UPR) integrated signaling networks determine cell fate during hypoxia. Cell Mol Biol Lett 25:18. https://doi.org/10.1186/s11658-020-00212-1IF: 9.2 Q1
Bartoszewska S, Collawn JF (2020) 未折叠蛋白反应 (UPR) 整合信号网络决定缺氧期间的细胞命运。细胞分子生物学快报 25:18。 https://doi.org/10.1186/s11658-020-00212-1IF:9.2 第一季度Bathori G, Csordas G, Garcia-Perez C, Davies E, Hajnoczky G (2006) Ca2+−dependent control of the permeability properties of the mitochondrial outer membrane and voltage-dependent anion-selective channel (VDAC). J Biol Chem 281(25):17347–17358. https://doi.org/10.1074/jbc.M600906200IF: 4.0 Q2
Bathori G、Csordas G、Garcia-Perez C、Davies E、Hajnoczky G (2006) Ca2+ 依赖性控制线粒体外膜和电压依赖性阴离子选择性通道 (VDAC) 的通透性特性。生物化学杂志 281(25):17347–17358。 https://doi.org/10.1074/jbc.M600906200IF:4.0 第二季度Bernardi R, Pandolfi PP (2007) Structure, dynamics and functions of promyelocytic leukaemia nuclear bodies. Nat Rev Mol Cell Biol 8(12):1006–1016. https://doi.org/10.1038/nrm2277IF: 81.3 Q1
Bernardi R, Pandolfi PP (2007) 早幼粒细胞白血病核体的结构、动力学和功能。自然修订分子细胞生物学 8(12):1006–1016。 https://doi.org/10.1038/nrm2277IF:81.3 第一季度Bernard-Marissal N, Chrast R, Schneider BL (2018) Endoplasmic reticulum and mitochondria in diseases of motor and sensory neurons: a broken relationship? Cell Death Dis 9(3):333. https://doi.org/10.1038/s41419-017-0125-1IF: 8.1 Q1
Bernard-Marissal N、Chrast R、Schneider BL (2018) 运动和感觉神经元疾病中的内质网和线粒体:关系破裂?细胞死亡 Dis 9(3):333。 https://doi.org/10.1038/s41419-017-0125-1IF:8.1 第一季度Berridge MJ (2012) Calcium signalling remodelling and disease. Biochem Soc Trans 40(2):297–309. https://doi.org/10.1042/BST20110766IF: 3.8 Q2
Berridge MJ, Bootman MD, Roderick HL (2003) Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 4(7):517–529. https://doi.org/10.1038/nrm1155IF: 81.3 Q1
Betz C, Stracka D, Prescianotto-Baschong C, Frieden M, Demaurex N, Hall MN (2013) Feature article: mTOR complex 2-Akt signaling at mitochondria-associated endoplasmic reticulum membranes (MAM) regulates mitochondrial physiology. Proc Natl Acad Sci U S A 110(31):12526–12534. https://doi.org/10.1073/pnas.1302455110IF: 9.4 Q1
Betzenhauser MJ, Wagner LE 2nd, Iwai M, Michikawa T, Mikoshiba K, Yule DI (2008) ATP modulation of Ca2+ release by type-2 and type-3 inositol (1, 4, 5)-triphosphate receptors. Differing ATP sensitivities and molecular determinants of action. J Biol Chem 283(31):21579–21587. https://doi.org/10.1074/jbc.M801680200IF: 4.0 Q2
Bittremieux M, Parys JB, Pinton P, Bultynck G (2016) ER functions of oncogenes and tumor suppressors: modulators of intracellular Ca(2+) signaling. Biochim Biophys Acta 1863(6 Pt B):1364–1378. https://doi.org/10.1016/j.bbamcr.2016.01.002IF: 4.6 Q1
Bittremieux M, La Rovere RM, Akl H, Martines C, Welkenhuyzen K, Dubron K, Baes M, Janssens A, Vandenberghe P, Laurenti L, Rietdorf K, Morciano G, Pinton P, Mikoshiba K, Bootman MD, Efremov DG, De Smedt H, Parys JB, Bultynck G (2019) Constitutive IP3 signaling underlies the sensitivity of B-cell cancers to the Bcl-2/IP3 receptor disruptor BIRD-2. Cell Death Differ 26(3):531–547. https://doi.org/10.1038/s41418-018-0142-3IF: 13.7 Q1
Bononi A, Bonora M, Marchi S, Missiroli S, Poletti F, Giorgi C, Pandolfi PP, Pinton P (2013) Identification of PTEN at the ER and MAMs and its regulation of Ca(2+) signaling and apoptosis in a protein phosphatase-dependent manner. Cell Death Differ 20(12):1631–1643. https://doi.org/10.1038/cdd.2013.77IF: 13.7 Q1
Bononi A, Giorgi C, Patergnani S, Larson D, Verbruggen K, Tanji M, Pellegrini L, Signorato V, Olivetto F, Pastorino S, Nasu M, Napolitano A, Gaudino G, Morris P, Sakamoto G, Ferris LK, Danese A, Raimondi A, Tacchetti C, Kuchay S, Pass HI, Affar EB, Yang H, Pinton P, Carbone M (2017) BAP1 regulates IP3R3-mediated Ca(2+) flux to mitochondria suppressing cell transformation. Nature 546(7659):549–553. https://doi.org/10.1038/nature22798IF: 50.5 Q1
Bonora M, Morganti C, Morciano G, Pedriali G, Lebiedzinska-Arciszewska M, Aquila G, Giorgi C, Rizzo P, Campo G, Ferrari R, Kroemer G, Wieckowski MR, Galluzzi L, Pinton P (2017) Mitochondrial permeability transition involves dissociation of F1FO ATP synthase dimers and C-ring conformation. EMBO Rep 18(7):1077–1089. https://doi.org/10.15252/embr.201643602IF: 6.5 Q1
Boyer LA, Plath K, Zeitlinger J, Brambrink T, Medeiros LA, Lee TI, Levine SS, Wernig M, Tajonar A, Ray MK, Bell GW, Otte AP, Vidal M, Gifford DK, Young RA, Jaenisch R (2006) Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature 441(7091):349–353. https://doi.org/10.1038/nature04733IF: 50.5 Q1
Brocchieri L, Conway de Macario E, Macario AJ (2008) hsp70 genes in the human genome: conservation and differentiation patterns predict a wide array of overlapping and specialized functions. BMC Evol Biol 8:19. https://doi.org/10.1186/1471-2148-8-19
Buck TM, Wright CM, Brodsky JL (2007) The activities and function of molecular chaperones in the endoplasmic reticulum. Semin Cell Dev Biol 18(6):751–761. https://doi.org/10.1016/j.semcdb.2007.09.001IF: 6.2 Q1
Cai X, Wang X, Patel S, Clapham DE (2015) Insights into the early evolution of animal calcium signaling machinery: a unicellular point of view. Cell Calcium 57(3):166–173. https://doi.org/10.1016/j.ceca.2014.11.007IF: 4.3 Q2
Campbell KJ, Dhayade S, Ferrari N, Sims AH, Johnson E, Mason SM, Dickson A, Ryan KM, Kalna G, Edwards J, Tait SWG, Blyth K (2018) MCL-1 is a prognostic indicator and drug target in breast cancer. Cell Death Dis 9(2):19. https://doi.org/10.1038/s41419-017-0035-2IF: 8.1 Q1
Carafoli E (2002) Calcium signaling: a tale for all seasons. Proc Natl Acad Sci U S A 99(3):1115–1122. https://doi.org/10.1073/pnas.032427999IF: 9.4 Q1
Carafoli E, Krebs J (2016) Why calcium? How calcium became the best communicator. J Biol Chem 291(40):20849–20857. https://doi.org/10.1074/jbc.R116.735894IF: 4.0 Q2
Cardenas C, Muller M, McNeal A, Lovy A, Jana F, Bustos G, Urra F, Smith N, Molgo J, Diehl JA, Ridky TW, Foskett JK (2016) Selective vulnerability of cancer cells by inhibition of Ca(2+) transfer from endoplasmic reticulum to mitochondria. Cell Rep 14(10):2313–2324. https://doi.org/10.1016/j.celrep.2016.02.030IF: 7.5 Q1
Carpi S, Polini B, Poli G, Alcantara Barata G, Fogli S, Romanini A, Tuccinardi T, Guella G, Frontini FP, Nieri P, Di Giuseppe G (2018) Anticancer activity of Euplotin C, isolated from the marine ciliate Euplotes crassus, against human melanoma cells. Mar Drugs 16(5). https://doi.org/10.3390/md16050166IF: 4.9 Q1
Chaudhuri AD, Choi DC, Kabaria S, Tran A, Junn E (2016a) MicroRNA-7 regulates the function of mitochondrial permeability transition pore by targeting VDAC1 expression. J Biol Chem 291(12):6483–6493. https://doi.org/10.1074/jbc.M115.691352IF: 4.0 Q2
Chaudhuri D, Artiga DJ, Abiria SA, Clapham DE (2016b) Mitochondrial calcium uniporter regulator 1 (MCUR1) regulates the calcium threshold for the mitochondrial permeability transition. Proc Natl Acad Sci U S A 113(13):E1872–E1880. https://doi.org/10.1073/pnas.1602264113IF: 9.4 Q1
Chen Q, Xu H, Xu A, Ross T, Bowler E, Hu Y, Lesnefsky EJ (2015) Inhibition of Bcl-2 sensitizes mitochondrial permeability transition pore (MPTP) opening in ischemia-damaged mitochondria. PLoS One 10(3):e0118834. https://doi.org/10.1371/journal.pone.0118834IF: 2.9 Q1
Chen G, Park D, Magis AT, Behera M, Ramalingam SS, Owonikoko TK, Sica GL, Ye K, Zhang C, Chen Z, Curran WJ, Deng X (2019) Mcl-1 interacts with Akt to promote lung cancer progression. Cancer Res 79(24):6126–6138. https://doi.org/10.1158/0008-5472.CAN-19-0950IF: 12.5 Q1
Colombini M (2012) VDAC structure, selectivity, and dynamics. Biochim Biophys Acta 1818(6):1457–1465. https://doi.org/10.1016/j.bbamem.2011.12.026IF: 2.8 Q2
Cosentino K, Garcia-Saez AJ (2014) Mitochondrial alterations in apoptosis. Chem Phys Lipids 181:62–75. https://doi.org/10.1016/j.chemphyslip.2014.04.001IF: 3.4 Q1
Crottes D, Guizouarn H, Martin P, Borgese F, Soriani O (2013) The sigma-1 receptor: a regulator of cancer cell electrical plasticity? Front Physiol 4:175. https://doi.org/10.3389/fphys.2013.00175IF: 3.2 Q2
Csordas G, Renken C, Varnai P, Walter L, Weaver D, Buttle KF, Balla T, Mannella CA, Hajnoczky G (2006) Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol 174(7):915–921. https://doi.org/10.1083/jcb.200604016IF: 7.4 Q1
Csordas G, Golenar T, Seifert EL, Kamer KJ, Sancak Y, Perocchi F, Moffat C, Weaver D, de la Fuente PS, Bogorad R, Koteliansky V, Adijanto J, Mootha VK, Hajnoczky G (2013) MICU1 controls both the threshold and cooperative activation of the mitochondrial Ca(2)(+) uniporter. Cell Metab 17(6):976–987. https://doi.org/10.1016/j.cmet.2013.04.020IF: 27.7 Q1
Cuadrado A, Rojo AI, Wells G, Hayes JD, Cousin SP, Rumsey WL, Attucks OC, Franklin S, Levonen AL, Kensler TW, Dinkova-Kostova AT (2019) Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat Rev Drug Discov 18(4):295–317. https://doi.org/10.1038/s41573-018-0008-xIF: 122.7 Q1
Cui C, Merritt R, Fu L, Pan Z (2017) Targeting calcium signaling in cancer therapy. Acta Pharm Sin B 7(1):3–17. https://doi.org/10.1016/j.apsb.2016.11.001IF: 14.7 Q1
Danese A, Patergnani S, Bonora M, Wieckowski MR, Previati M, Giorgi C, Pinton P (2017) Calcium regulates cell death in cancer: roles of the mitochondria and mitochondria-associated membranes (MAMs). Biochim Biophys Acta Bioenerg 1858(8):615–627. https://doi.org/10.1016/j.bbabio.2017.01.003IF: 3.4 Q1
De Pinto V, Guarino F, Guarnera A, Messina A, Reina S, Tomasello FM, Palermo V, Mazzoni C (2010) Characterization of human VDAC isoforms: a peculiar function for VDAC3? Biochim Biophys Acta 1797(6–7):1268–1275. https://doi.org/10.1016/j.bbabio.2010.01.031IF: 3.4 Q1
De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R (2011) A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476(7360):336–340. https://doi.org/10.1038/nature10230IF: 50.5 Q1
De Stefani D, Bononi A, Romagnoli A, Messina A, De Pinto V, Pinton P, Rizzuto R (2012) VDAC1 selectively transfers apoptotic Ca2+ signals to mitochondria. Cell Death Differ 19(2):267–273. https://doi.org/10.1038/cdd.2011.92IF: 13.7 Q1
De Stefani D, Patron M, Rizzuto R (2015) Structure and function of the mitochondrial calcium uniporter complex. Biochim Biophys Acta 1853(9):2006–2011. https://doi.org/10.1016/j.bbamcr.2015.04.008IF: 4.6 Q1
Denton RM (2009) Regulation of mitochondrial dehydrogenases by calcium ions. Biochim Biophys Acta 1787(11):1309–1316. https://doi.org/10.1016/j.bbabio.2009.01.005IF: 3.4 Q1
Ding Z, Yuan J, Liang Y, Wu J, Gong H, Ye Y, Jiang G, Yin P, Li Y, Zhang G, Yang C, Guo J, Chen Z, Wang X, Weng L, Zou Y (2017) Ryanodine receptor type 2 plays a role in the development of cardiac fibrosis under mechanical stretch through TGFbeta-1. Int Heart J 58(6):957–961. https://doi.org/10.1536/ihj.16-572IF: 1.2 Q3
Doghman-Bouguerra M, Granatiero V, Sbiera S, Sbiera I, Lacas-Gervais S, Brau F, Fassnacht M, Rizzuto R, Lalli E (2016) FATE1 antagonizes calcium- and drug-induced apoptosis by uncoupling ER and mitochondria. EMBO Rep 17(9):1264–1280. https://doi.org/10.15252/embr.201541504IF: 6.5 Q1
Dong XY, Su YR, Qian XP, Yang XA, Pang XW, Wu HY, Chen WF (2003) Identification of two novel CT antigens and their capacity to elicit antibody response in hepatocellular carcinoma patients. Br J Cancer 89(2):291–297. https://doi.org/10.1038/sj.bjc.6601062IF: 6.4 Q1
Eckenrode EF, Yang J, Velmurugan GV, Foskett JK, White C (2010) Apoptosis protection by Mcl-1 and Bcl-2 modulation of inositol 1,4,5-trisphosphate receptor-dependent Ca2+ signaling. J Biol Chem 285(18):13678–13684. https://doi.org/10.1074/jbc.M109.096040IF: 4.0 Q2
Eisenberg-Bord M, Shai N, Schuldiner M, Bohnert M (2016) A tether is a tether is a tether: tethering at membrane contact sites. Dev Cell 39(4):395–409. https://doi.org/10.1016/j.devcel.2016.10.022IF: 10.7 Q1
Enogieru AB, Omoruyi SI, Hiss DC, Ekpo OE (2019) GRP78/BIP/HSPA5 as a therapeutic target in models of Parkinson’s disease: a mini review. Adv Pharm Sci 2019:2706783. https://doi.org/10.1155/2019/2706783
Everett RD, Chelbi-Alix MK (2007) PML and PML nuclear bodies: implications in antiviral defence. Biochimie 89(6–7):819–830. https://doi.org/10.1016/j.biochi.2007.01.004IF: 3.3 Q2
Fan G, Baker ML, Wang Z, Baker MR, Sinyagovskiy PA, Chiu W, Ludtke SJ, Serysheva II (2015) Gating machinery of InsP3R channels revealed by electron cryomicroscopy. Nature 527(7578):336–341. https://doi.org/10.1038/nature15249IF: 50.5 Q1
Feng S, Li H, Tai Y, Huang J, Su Y, Abramowitz J, Zhu MX, Birnbaumer L, Wang Y (2013) Canonical transient receptor potential 3 channels regulate mitochondrial calcium uptake. Proc Natl Acad Sci U S A 110(27):11011–11016. https://doi.org/10.1073/pnas.1309531110IF: 9.4 Q1
Feng YX, Jin DX, Sokol ES, Reinhardt F, Miller DH, Gupta PB (2017) Cancer-specific PERK signaling drives invasion and metastasis through CREB3L1. Nat Commun 8(1):1079. https://doi.org/10.1038/s41467-017-01052-yIF: 14.7 Q1
Flachbartova Z, Kovacech B (2013) Mortalin – a multipotent chaperone regulating cellular processes ranging from viral infection to neurodegeneration. Acta Virol 57(1):3–15. https://doi.org/10.4149/av_2013_01_3IF: 1.1 Q4
Foskett JK, White C, Cheung KH, Mak DO (2007) Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev 87(2):593–658. https://doi.org/10.1152/physrev.00035.2006IF: 29.9 Q1
Frenzel A, Grespi F, Chmelewskij W, Villunger A (2009) Bcl2 family proteins in carcinogenesis and the treatment of cancer. Apoptosis 14(4):584–596. https://doi.org/10.1007/s10495-008-0300-zIF: 6.1 Q1
Gandre-Babbe S, van der Bliek AM (2008) The novel tail-anchored membrane protein Mff controls mitochondrial and peroxisomal fission in mammalian cells. Mol Biol Cell 19(6):2402–2412. https://doi.org/10.1091/mbc.E07-12-1287IF: 3.1 Q3
Giacomello M, Pellegrini L (2016) The coming of age of the mitochondria-ER contact: a matter of thickness. Cell Death Differ 23(9):1417–1427. https://doi.org/10.1038/cdd.2016.52IF: 13.7 Q1
Gincel D, Silberberg SD, Shoshan-Barmatz V (2000) Modulation of the voltage-dependent anion channel (VDAC) by glutamate. J Bioenerg Biomembr 32(6):571–583. https://doi.org/10.1023/a:1005670527340IF: 2.9 Q2
Giorgi C, Ito K, Lin HK, Santangelo C, Wieckowski MR, Lebiedzinska M, Bononi A, Bonora M, Duszynski J, Bernardi R, Rizzuto R, Tacchetti C, Pinton P, Pandolfi PP (2010) PML regulates apoptosis at endoplasmic reticulum by modulating calcium release. Science 330(6008):1247–1251. https://doi.org/10.1126/science.1189157IF: 44.7 Q1
Giorgi C, Wieckowski MR, Pandolfi PP, Pinton P (2011) Mitochondria associated membranes (MAMs) as critical hubs for apoptosis. Commun Integr Biol 4(3):334–335. https://doi.org/10.4161/cib.4.3.15021
Giorgi C, Bonora M, Missiroli S, Poletti F, Ramirez FG, Morciano G, Morganti C, Pandolfi PP, Mammano F, Pinton P (2015) Intravital imaging reveals p53-dependent cancer cell death induced by phototherapy via calcium signaling. Oncotarget 6(3):1435–1445. https://doi.org/10.18632/oncotarget.2935
Giorgi C, Danese A, Missiroli S, Patergnani S, Pinton P (2018a) Calcium dynamics as a machine for decoding signals. Trends Cell Biol 28(4):258–273. https://doi.org/10.1016/j.tcb.2018.01.002IF: 13.0 Q1
Giorgi C, Marchi S, Pinton P (2018b) The machineries, regulation and cellular functions of mitochondrial calcium. Nat Rev Mol Cell Biol 19(11):713–730. https://doi.org/10.1038/s41580-018-0052-8IF: 81.3 Q1
Gonzalez-Angulo AM, Ferrer-Lozano J, Stemke-Hale K, Sahin A, Liu S, Barrera JA, Burgues O, Lluch AM, Chen H, Hortobagyi GN, Mills GB, Meric-Bernstam F (2011) PI3K pathway mutations and PTEN levels in primary and metastatic breast cancer. Mol Cancer Ther 10(6):1093–1101. https://doi.org/10.1158/1535-7163.MCT-10-1089IF: 5.3 Q1
Gorrini C, Harris IS, Mak TW (2013) Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov 12(12):931–947. https://doi.org/10.1038/nrd4002IF: 122.7 Q1
Gueguinou M, Crottes D, Chantome A, Rapetti-Mauss R, Potier-Cartereau M, Clarysse L, Girault A, Fourbon Y, Jezequel P, Guerin-Charbonnel C, Fromont G, Martin P, Pellissier B, Schiappa R, Chamorey E, Mignen O, Uguen A, Borgese F, Vandier C, Soriani O (2017) The SigmaR1 chaperone drives breast and colorectal cancer cell migration by tuning SK3-dependent Ca(2+) homeostasis. Oncogene 36(25):3640–3647. https://doi.org/10.1038/onc.2016.501IF: 6.9 Q1
Guerra MT, Florentino RM, Franca A, Lima Filho AC, Dos Santos ML, Fonseca RC, Lemos FO, Fonseca MC, Kruglov E, Mennone A, Njei B, Gibson J, Guan F, Cheng YC, Ananthanarayanan M, Gu J, Jiang J, Zhao H, Lima CX, Vidigal PT, Oliveira AG, Nathanson MH, Leite MF (2019) Expression of the type 3 InsP3 receptor is a final common event in the development of hepatocellular carcinoma. Gut 68(9):1676–1687. https://doi.org/10.1136/gutjnl-2018-317811IF: 23.0 Q1
Gutierrez T, Simmen T (2018) Endoplasmic reticulum chaperones tweak the mitochondrial calcium rheostat to control metabolism and cell death. Cell Calcium 70:64–75. https://doi.org/10.1016/j.ceca.2017.05.015IF: 4.3 Q2
Hajnoczky G, Csordas G, Yi M (2002) Old players in a new role: mitochondria-associated membranes, VDAC, and ryanodine receptors as contributors to calcium signal propagation from endoplasmic reticulum to the mitochondria. Cell Calcium 32(5–6):363–377. https://doi.org/10.1016/s0143416002001872IF: 4.3 Q2
Hakamata Y, Nishimura S, Nakai J, Nakashima Y, Kita T, Imoto K (1994) Involvement of the brain type of ryanodine receptor in T-cell proliferation. FEBS Lett 352(2):206–210. https://doi.org/10.1016/0014-5793(94)00955-4IF: 3.0 Q2
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100(1):57–70. https://doi.org/10.1016/s0092-8674(00)81683-9IF: 45.5 Q1
Hendershot LM (2004) The ER function BiP is a master regulator of ER function. Mt Sinai J Med 71(5):289–297
Higo T, Hattori M, Nakamura T, Natsume T, Michikawa T, Mikoshiba K (2005) Subtype-specific and ER luminal environment-dependent regulation of inositol 1,4,5-trisphosphate receptor type 1 by ERp44. Cell 120(1):85–98. https://doi.org/10.1016/j.cell.2004.11.048IF: 45.5 Q1
Hsu KS, Kao HY (2018) Correction to: PML: regulation and multifaceted function beyond tumor suppression. Cell Biosci 8:18. https://doi.org/10.1186/s13578-018-0213-7IF: 6.1 Q1
Hu Y, Lu W, Chen G, Wang P, Chen Z, Zhou Y, Ogasawara M, Trachootham D, Feng L, Pelicano H, Chiao PJ, Keating MJ, Garcia-Manero G, Huang P (2012) K-ras(G12V) transformation leads to mitochondrial dysfunction and a metabolic switch from oxidative phosphorylation to glycolysis. Cell Res 22(2):399–412. https://doi.org/10.1038/cr.2011.145IF: 28.1 Q1
Huang H, Shah K, Bradbury NA, Li C, White C (2014) Mcl-1 promotes lung cancer cell migration by directly interacting with VDAC to increase mitochondrial Ca2+ uptake and reactive oxygen species generation. Cell Death Dis 5:e1482. https://doi.org/10.1038/cddis.2014.419IF: 8.1 Q1
Huebner K, Croce CM (2003) Cancer and the FRA3B/FHIT fragile locus: it’s a HIT. Br J Cancer 88(10):1501–1506. https://doi.org/10.1038/sj.bjc.6600937
Ismail IH, Davidson R, Gagne JP, Xu ZZ, Poirier GG, Hendzel MJ (2014) Germline mutations in BAP1 impair its function in DNA double-strand break repair. Cancer Res 74(16):4282–4294. https://doi.org/10.1158/0008-5472.CAN-13-3109IF: 12.5 Q1
Ivanova H, Vervliet T, Missiaen L, Parys JB, De Smedt H, Bultynck G (2014) Inositol 1,4,5-trisphosphate receptor-isoform diversity in cell death and survival. Biochim Biophys Acta 1843(10):2164–2183. https://doi.org/10.1016/j.bbamcr.2014.03.007IF: 4.6 Q1
Ivanova H, Kerkhofs M, La Rovere RM, Bultynck G (2017) Endoplasmic reticulum-mitochondrial Ca(2+) fluxes underlying cancer cell survival. Front Oncol 7:70. https://doi.org/10.3389/fonc.2017.00070IF: 3.5 Q2
Jin M, Wang J, Ji X, Cao H, Zhu J, Chen Y, Yang J, Zhao Z, Ren T, Xing J (2019) MCUR1 facilitates epithelial-mesenchymal transition and metastasis via the mitochondrial calcium dependent ROS/Nrf2/Notch pathway in hepatocellular carcinoma. J Exp Clin Cancer Res 38(1):136. https://doi.org/10.1186/s13046-019-1135-xIF: 11.4 Q1
Kakihana T, Nagata K, Sitia R (2012) Peroxides and peroxidases in the endoplasmic reticulum: integrating redox homeostasis and oxidative folding. Antioxid Redox Signal 16(8):763–771. https://doi.org/10.1089/ars.2011.4238IF: 5.9 Q1
Kamer KJ, Mootha VK (2015) The molecular era of the mitochondrial calcium uniporter. Nat Rev Mol Cell Biol 16(9):545–553. https://doi.org/10.1038/nrm4039IF: 81.3 Q1
Kang HY, Kim NS, Lee CO, Lee JY, Kang WH (2000) Expression and function of ryanodine receptors in human melanocytes. J Cell Physiol 185(2):200–206. https://doi.org/10.1002/1097-4652(200011)185:2<200::AID-JCP4>3.0.CO;2-6
Kang SS, Han KS, Ku BM, Lee YK, Hong J, Shin HY, Almonte AG, Woo DH, Brat DJ, Hwang EM, Yoo SH, Chung CK, Park SH, Paek SH, Roh EJ, Lee SJ, Park JY, Traynelis SF, Lee CJ (2010) Caffeine-mediated inhibition of calcium release channel inositol 1,4,5-trisphosphate receptor subtype 3 blocks glioblastoma invasion and extends survival. Cancer Res 70(3):1173–1183. https://doi.org/10.1158/0008-5472.CAN-09-2886IF: 12.5 Q1
Kerkhofs M, Giorgi C, Marchi S, Seitaj B, Parys JB, Pinton P, Bultynck G, Bittremieux M (2017) Alterations in Ca(2+) signalling via ER-mitochondria contact site remodelling in cancer. Adv Exp Med Biol 997:225–254. https://doi.org/10.1007/978-981-10-4567-7_17
Kerkhofs M, Bittremieux M, Morciano G, Giorgi C, Pinton P, Parys JB, Bultynck G (2018) Emerging molecular mechanisms in chemotherapy: Ca(2+) signaling at the mitochondria-associated endoplasmic reticulum membranes. Cell Death Dis 9(3):334. https://doi.org/10.1038/s41419-017-0179-0IF: 8.1 Q1
Khan MT, Wagner L 2nd, Yule DI, Bhanumathy C, Joseph SK (2006) Akt kinase phosphorylation of inositol 1,4,5-trisphosphate receptors. J Biol Chem 281(6):3731–3737. https://doi.org/10.1074/jbc.M509262200IF: 4.0 Q2
Kim KH, Roberts CW (2016) Targeting EZH2 in cancer. Nat Med 22(2):128–134. https://doi.org/10.1038/nm.4036IF: 58.7 Q1
Kirichok Y, Krapivinsky G, Clapham DE (2004) The mitochondrial calcium uniporter is a highly selective ion channel. Nature 427(6972):360–364. https://doi.org/10.1038/nature02246IF: 50.5 Q1
Kiss DL, Baez W, Huebner K, Bundschuh R, Schoenberg DR (2017) Impact of FHIT loss on the translation of cancer-associated mRNAs. Mol Cancer 16(1):179. https://doi.org/10.1186/s12943-017-0749-xIF: 27.7 Q1
Koval OM, Nguyen EK, Santhana V, Fidler TP, Sebag SC, Rasmussen TP, Mittauer DJ, Strack S, Goswami PC, Abel ED, Grumbach IM (2019) Loss of MCU prevents mitochondrial fusion in G1-S phase and blocks cell cycle progression and proliferation. Sci Signal 12(579). https://doi.org/10.1126/scisignal.aav1439IF: 6.7 Q1
Krols M, Bultynck G, Janssens S (2016) ER-mitochondria contact sites: a new regulator of cellular calcium flux comes into play. J Cell Biol 214(4):367–370. https://doi.org/10.1083/jcb.201607124IF: 7.4 Q1
Kronidou NG, Oppliger W, Bolliger L, Hannavy K, Glick BS, Schatz G, Horst M (1994) Dynamic interaction between Isp45 and mitochondrial hsp70 in the protein import system of the yeast mitochondrial inner membrane. Proc Natl Acad Sci U S A 91(26):12818–12822. https://doi.org/10.1073/pnas.91.26.12818IF: 9.4 Q1
Kuchay S, Giorgi C, Simoneschi D, Pagan J, Missiroli S, Saraf A, Florens L, Washburn MP, Collazo-Lorduy A, Castillo-Martin M, Cordon-Cardo C, Sebti SM, Pinton P, Pagano M (2017) PTEN counteracts FBXL2 to promote IP3R3- and Ca(2+)-mediated apoptosis limiting tumour growth. Nature 546(7659):554–558. https://doi.org/10.1038/nature22965IF: 50.5 Q1
Kutomi G, Tamura Y, Tanaka T, Kajiwara T, Kukita K, Ohmura T, Shima H, Takamaru T, Satomi F, Suzuki Y, Torigoe T, Sato N, Hirata K (2013) Human endoplasmic reticulum oxidoreductin 1-alpha is a novel predictor for poor prognosis of breast cancer. Cancer Sci 104(8):1091–1096. https://doi.org/10.1111/cas.12177IF: 4.5 Q1
Kveiborg M, Thomas G (2018) PACS-2 functions in colorectal cancer. Aging (Albany NY) 10(6):1190–1191. https://doi.org/10.18632/aging.101489IF: 3.9 Q2
Lamriben L, Graham JB, Adams BM, Hebert DN (2016) N-glycan-based ER molecular chaperone and protein quality control system: the Calnexin binding cycle. Traffic 17(4):308–326. https://doi.org/10.1111/tra.12358IF: 3.6 Q3
Lee HS, Lee SA, Hur SK, Seo JW, Kwon J (2014) Stabilization and targeting of INO80 to replication forks by BAP1 during normal DNA synthesis. Nat Commun 5:5128. https://doi.org/10.1038/ncomms6128IF: 14.7 Q1
Li H, Chen Y, Jones AF, Sanger RH, Collis LP, Flannery R, McNay EC, Yu T, Schwarzenbacher R, Bossy B, Bossy-Wetzel E, Bennett MV, Pypaert M, Hickman JA, Smith PJ, Hardwick JM, Jonas EA (2008) Bcl-xL induces Drp1-dependent synapse formation in cultured hippocampal neurons. Proc Natl Acad Sci U S A 105(6):2169–2174. https://doi.org/10.1073/pnas.0711647105IF: 9.4 Q1
Li G, Mongillo M, Chin KT, Harding H, Ron D, Marks AR, Tabas I (2009) Role of ERO1-alpha-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J Cell Biol 186(6):783–792. https://doi.org/10.1083/jcb.200904060IF: 7.4 Q1
Liao Y, Hao Y, Chen H, He Q, Yuan Z, Cheng J (2015) Mitochondrial calcium uniporter protein MCU is involved in oxidative stress-induced cell death. Protein Cell 6(6):434–442. https://doi.org/10.1007/s13238-015-0144-6IF: 13.6 Q1
Lindquist S, Craig EA (1988) The heat-shock proteins. Annu Rev Genet 22:631–677. https://doi.org/10.1146/annurev.ge.22.120188.003215IF: 8.7 Q1
Lipskaia L, Keuylian Z, Blirando K, Mougenot N, Jacquet A, Rouxel C, Sghairi H, Elaib Z, Blaise R, Adnot S, Hajjar RJ, Chemaly ER, Limon I, Bobe R (2014) Expression of sarco (endo) plasmic reticulum calcium ATPase (SERCA) system in normal mouse cardiovascular tissues, heart failure and atherosclerosis. Biochim Biophys Acta 1843(11):2705–2718. https://doi.org/10.1016/j.bbamcr.2014.08.002IF: 4.6 Q1
Little E, Ramakrishnan M, Roy B, Gazit G, Lee AS (1994) The glucose-regulated proteins (GRP78 and GRP94): functions, gene regulation, and applications. Crit Rev Eukaryot Gene Expr 4(1):1–18. https://doi.org/10.1615/critreveukargeneexpr.v4.i1.10IF: 1.5 Q4
Liu JC, Liu J, Holmstrom KM, Menazza S, Parks RJ, Fergusson MM, Yu ZX, Springer DA, Halsey C, Liu C, Murphy E, Finkel T (2016) MICU1 serves as a molecular gatekeeper to prevent in vivo mitochondrial calcium overload. Cell Rep 16(6):1561–1573. https://doi.org/10.1016/j.celrep.2016.07.011IF: 7.5 Q1
Lu H, Chen I, Shimoda LA, Park Y, Zhang C, Tran L, Zhang H, Semenza GL (2017) Chemotherapy-induced Ca(2+) release stimulates breast cancer stem cell enrichment. Cell Rep 18(8):1946–1957. https://doi.org/10.1016/j.celrep.2017.02.001IF: 7.5 Q1
Luo S, Mao C, Lee B, Lee AS (2006) GRP78/BiP is required for cell proliferation and protecting the inner cell mass from apoptosis during early mouse embryonic development. Mol Cell Biol 26(15):5688–5697. https://doi.org/10.1128/MCB.00779-06IF: 3.2 Q2
Lynes EM, Bui M, Yap MC, Benson MD, Schneider B, Ellgaard L, Berthiaume LG, Simmen T (2012) Palmitoylated TMX and calnexin target to the mitochondria-associated membrane. EMBO J 31(2):457–470. https://doi.org/10.1038/emboj.2011.384IF: 9.4 Q1
Lynes EM, Raturi A, Shenkman M, Ortiz Sandoval C, Yap MC, Wu J, Janowicz A, Myhill N, Benson MD, Campbell RE, Berthiaume LG, Lederkremer GZ, Simmen T (2013) Palmitoylation is the switch that assigns calnexin to quality control or ER Ca2+ signaling. J Cell Sci 126(Pt 17):3893–3903. https://doi.org/10.1242/jcs.125856IF: 3.3 Q3
Mak DO, Foskett JK (2015) Inositol 1,4,5-trisphosphate receptors in the endoplasmic reticulum: a single-channel point of view. Cell Calcium 58(1):67–78. https://doi.org/10.1016/j.ceca.2014.12.008IF: 4.3 Q2
Maldonado EN, Sheldon KL, DeHart DN, Patnaik J, Manevich Y, Townsend DM, Bezrukov SM, Rostovtseva TK, Lemasters JJ (2013) Voltage-dependent anion channels modulate mitochondrial metabolism in cancer cells: regulation by free tubulin and erastin. J Biol Chem 288(17):11920–11929. https://doi.org/10.1074/jbc.M112.433847IF: 4.0 Q2
Mallilankaraman K, Doonan P, Cardenas C, Chandramoorthy HC, Muller M, Miller R, Hoffman NE, Gandhirajan RK, Molgo J, Birnbaum MJ, Rothberg BS, Mak DO, Foskett JK, Madesh M (2012a) MICU1 is an essential gatekeeper for MCU-mediated mitochondrial Ca(2+) uptake that regulates cell survival. Cell 151(3):630–644. https://doi.org/10.1016/j.cell.2012.10.011IF: 45.5 Q1
Mallilankaraman K, Cardenas C, Doonan PJ, Chandramoorthy HC, Irrinki KM, Golenar T, Csordas G, Madireddi P, Yang J, Muller M, Miller R, Kolesar JE, Molgo J, Kaufman B, Hajnoczky G, Foskett JK, Madesh M (2012b) MCUR1 is an essential component of mitochondrial Ca2+ uptake that regulates cellular metabolism. Nat Cell Biol 14(12):1336–1343. https://doi.org/10.1038/ncb2622IF: 17.3 Q1
Marchi S, Marinello M, Bononi A, Bonora M, Giorgi C, Rimessi A, Pinton P (2012) Selective modulation of subtype III IP(3)R by Akt regulates ER Ca(2)(+) release and apoptosis. Cell Death Dis 3:e304. https://doi.org/10.1038/cddis.2012.45IF: 8.1 Q1
Marchi S, Lupini L, Patergnani S, Rimessi A, Missiroli S, Bonora M, Bononi A, Corra F, Giorgi C, De Marchi E, Poletti F, Gafa R, Lanza G, Negrini M, Rizzuto R, Pinton P (2013) Downregulation of the mitochondrial calcium uniporter by cancer-related miR-25. Curr Biol 23(1):58–63. https://doi.org/10.1016/j.cub.2012.11.026IF: 8.1 Q1
Marchi S, Patergnani S, Pinton P (2014) The endoplasmic reticulum-mitochondria connection: one touch, multiple functions. Biochim Biophys Acta 1837(4):461–469. https://doi.org/10.1016/j.bbabio.2013.10.015IF: 3.4 Q1
Marchi S, Bittremieux M, Missiroli S, Morganti C, Patergnani S, Sbano L, Rimessi A, Kerkhofs M, Parys JB, Bultynck G, Giorgi C, Pinton P (2017) Endoplasmic reticulum-mitochondria communication through Ca(2+) signaling: the importance of mitochondria-associated membranes (MAMs). Adv Exp Med Biol 997:49–67. https://doi.org/10.1007/978-981-10-4567-7_4
Marchi S, Patergnani S, Missiroli S, Morciano G, Rimessi A, Wieckowski MR, Giorgi C, Pinton P (2018) Mitochondrial and endoplasmic reticulum calcium homeostasis and cell death. Cell Calcium 69:62–72. https://doi.org/10.1016/j.ceca.2017.05.003IF: 4.3 Q2
Marchi S, Corricelli M, Branchini A, Vitto VAM, Missiroli S, Morciano G, Perrone M, Ferrarese M, Giorgi C, Pinotti M, Galluzzi L, Kroemer G, Pinton P (2019a) Akt-mediated phosphorylation of MICU1 regulates mitochondrial Ca(2+) levels and tumor growth. EMBO J 38(2). https://doi.org/10.15252/embj.201899435IF: 9.4 Q1
Marchi S, Vitto VAM, Patergnani S, Pinton P (2019b) High mitochondrial Ca(2+) content increases cancer cell proliferation upon inhibition of mitochondrial permeability transition pore (mPTP). Cell Cycle 18(8):914–916. https://doi.org/10.1080/15384101.2019.1598729IF: 3.4 Q3
Marchi S, Giorgi C, Galluzzi L, Pinton P (2020) Ca(2+) fluxes and cancer. Mol Cell 78(6):1055–1069. https://doi.org/10.1016/j.molcel.2020.04.017IF: 14.5 Q1
Marino M, Stoilova T, Giorgi C, Bachi A, Cattaneo A, Auricchio A, Pinton P, Zito E (2015) SEPN1, an endoplasmic reticulum-localized selenoprotein linked to skeletal muscle pathology, counteracts hyperoxidation by means of redox-regulating SERCA2 pump activity. Hum Mol Genet 24(7):1843–1855. https://doi.org/10.1093/hmg/ddu602IF: 3.1 Q2
Mariot P, Prevarskaya N, Roudbaraki MM, Le Bourhis X, Van Coppenolle F, Vanoverberghe K, Skryma R (2000) Evidence of functional ryanodine receptor involved in apoptosis of prostate cancer (LNCaP) cells. Prostate 43(3):205–214. https://doi.org/10.1002/(sici)1097-0045(20000515)43:3<205::aid-pros6>3.0.co;2-m
Matyash M, Matyash V, Nolte C, Sorrentino V, Kettenmann H (2002) Requirement of functional ryanodine receptor type 3 for astrocyte migration. FASEB J 16(1):84–86. https://doi.org/10.1096/fj.01-0380fjeIF: 4.4 Q1
Mazure NM (2017) VDAC in cancer. Biochim Biophys Acta Bioenerg 1858(8):665–673. https://doi.org/10.1016/j.bbabio.2017.03.002IF: 3.4 Q1
McCarthy TV, Datar S, Mackrill JJ (2003) Activation of ryanodine receptor/Ca2+ release channels downregulates CD38 in the Namalwa B lymphoma. FEBS Lett 554(1–2):133–137. https://doi.org/10.1016/s0014-5793(03)01122-0IF: 3.0 Q2
Mekahli D, Bultynck G, Parys JB, De Smedt H, Missiaen L (2011) Endoplasmic-reticulum calcium depletion and disease. Cold Spring Harb Perspect Biol 3(6). https://doi.org/10.1101/cshperspect.a004317IF: 6.9 Q1
Messina A, Reina S, Guarino F, De Pinto V (2012) VDAC isoforms in mammals. Biochim Biophys Acta 1818(6):1466–1476. https://doi.org/10.1016/j.bbamem.2011.10.005IF: 2.8 Q2
Mikoshiba K (2007) The IP3 receptor/Ca2+ channel and its cellular function. Biochem Soc Symp 74:9–22. https://doi.org/10.1042/BSS0740009
Missiroli S, Bonora M, Patergnani S, Poletti F, Perrone M, Gafa R, Magri E, Raimondi A, Lanza G, Tacchetti C, Kroemer G, Pandolfi PP, Pinton P, Giorgi C (2016) PML at mitochondria-associated membranes is critical for the repression of autophagy and cancer development. Cell Rep 16(9):2415–2427. https://doi.org/10.1016/j.celrep.2016.07.082IF: 7.5 Q1
Missiroli S, Danese A, Iannitti T, Patergnani S, Perrone M, Previati M, Giorgi C, Pinton P (2017) Endoplasmic reticulum-mitochondria Ca(2+) crosstalk in the control of the tumor cell fate. Biochim Biophys Acta Mol Cell Res 1864(6):858–864. https://doi.org/10.1016/j.bbamcr.2016.12.024IF: 4.6 Q1
Miyakawa T, Maeda A, Yamazawa T, Hirose K, Kurosaki T, Iino M (1999) Encoding of Ca2+ signals by differential expression of IP3 receptor subtypes. EMBO J 18(5):1303–1308. https://doi.org/10.1093/emboj/18.5.1303IF: 9.4 Q1
Moller JV, Olesen C, Winther AM, Nissen P (2010) The sarcoplasmic Ca2+-ATPase: design of a perfect chemi-osmotic pump. Q Rev Biophys 43(4):501–566. https://doi.org/10.1017/S003358351000017XIF: 7.2 Q1
Monaco G, Decrock E, Arbel N, van Vliet AR, La Rovere RM, De Smedt H, Parys JB, Agostinis P, Leybaert L, Shoshan-Barmatz V, Bultynck G (2015) The BH4 domain of anti-apoptotic Bcl-XL, but not that of the related Bcl-2, limits the voltage-dependent anion channel 1 (VDAC1)-mediated transfer of pro-apoptotic Ca2+ signals to mitochondria. J Biol Chem 290(14):9150–9161. https://doi.org/10.1074/jbc.M114.622514IF: 4.0 Q2
Monet M, Lehen'kyi V, Gackiere F, Firlej V, Vandenberghe M, Roudbaraki M, Gkika D, Pourtier A, Bidaux G, Slomianny C, Delcourt P, Rassendren F, Bergerat JP, Ceraline J, Cabon F, Humez S, Prevarskaya N (2010) Role of cationic channel TRPV2 in promoting prostate cancer migration and progression to androgen resistance. Cancer Res 70(3):1225–1235. https://doi.org/10.1158/0008-5472.CAN-09-2205IF: 12.5 Q1
Monteith GR, McAndrew D, Faddy HM, Roberts-Thomson SJ (2007) Calcium and cancer: targeting Ca2+ transport. Nat Rev Cancer 7(7):519–530. https://doi.org/10.1038/nrc2171IF: 72.5 Q1
Monteith GR, Davis FM, Roberts-Thomson SJ (2012) Calcium channels and pumps in cancer: changes and consequences. J Biol Chem 287(38):31666–31673. https://doi.org/10.1074/jbc.R112.343061IF: 4.0 Q2
Morciano G, Marchi S, Morganti C, Sbano L, Bittremieux M, Kerkhofs M, Corricelli M, Danese A, Karkucinska-Wieckowska A, Wieckowski MR, Bultynck G, Giorgi C, Pinton P (2018) Role of mitochondria-associated ER membranes in calcium regulation in Cancer-specific settings. Neoplasia 20(5):510–523. https://doi.org/10.1016/j.neo.2018.03.005IF: 6.3 Q1
Munoz-Maldonado C, Zimmer Y, Medova M (2019) A comparative analysis of individual RAS mutations in cancer biology. Front Oncol 9:1088. https://doi.org/10.3389/fonc.2019.01088IF: 3.5 Q2
Newton CL, Mignery GA, Sudhof TC (1994) Co-expression in vertebrate tissues and cell lines of multiple inositol 1,4,5-trisphosphate (InsP3) receptors with distinct affinities for InsP3. J Biol Chem 269(46):28613–28619
Ning X, Shi Z, Liu X, Zhang A, Han L, Jiang K, Kang C, Zhang Q (2015) DNMT1 and EZH2 mediated methylation silences the microRNA-200b/a/429 gene and promotes tumor progression. Cancer Lett 359(2):198–205. https://doi.org/10.1016/j.canlet.2015.01.005IF: 9.1 Q1
Niu Z, Wang M, Zhou L, Yao L, Liao Q, Zhao Y (2015) Elevated GRP78 expression is associated with poor prognosis in patients with pancreatic cancer. Sci Rep 5:16067. https://doi.org/10.1038/srep16067IF: 3.8 Q1
Oropeza-Almazan Y, Vazquez-Garza E, Chapoy-Villanueva H, Torre-Amione G, Garcia-Rivas G (2017) Small interfering RNA targeting mitochondrial calcium uniporter improves cardiomyocyte cell viability in hypoxia/reoxygenation injury by reducing calcium overload. Oxid Med Cell Longev 2017:5750897. https://doi.org/10.1155/2017/5750897
Oxenoid K, Dong Y, Cao C, Cui T, Sancak Y, Markhard AL, Grabarek Z, Kong L, Liu Z, Ouyang B, Cong Y, Mootha VK, Chou JJ (2016) Architecture of the mitochondrial calcium uniporter. Nature 533(7602):269–273. https://doi.org/10.1038/nature17656IF: 50.5 Q1
Palmgren MG, Nissen P (2011) P-type ATPases. Annu Rev Biophys 40:243–266. https://doi.org/10.1146/annurev.biophys.093008.131331IF: 10.4 Q1
Park J, Lee Y, Park T, Kang JY, Mun SA, Jin M, Yang J, Eom SH (2020) Structure of the MICU1-MICU2 heterodimer provides insights into the gatekeeping threshold shift. IUCrJ 7(Pt 2):355–365. https://doi.org/10.1107/S2052252520001840IF: 2.9 Q1
Paupe V, Prudent J, Dassa EP, Rendon OZ, Shoubridge EA (2015) CCDC90A (MCUR1) is a cytochrome c oxidase assembly factor and not a regulator of the mitochondrial calcium uniporter. Cell Metab 21(1):109–116. https://doi.org/10.1016/j.cmet.2014.12.004IF: 27.7 Q1
Penston J, Wormsley KG (1986) H2-receptor antagonists and gastric cancer. Med Toxicol 1(3):163–168. https://doi.org/10.1007/bf03259835
Perocchi F, Gohil VM, Girgis HS, Bao XR, McCombs JE, Palmer AE, Mootha VK (2010) MICU1 encodes a mitochondrial EF hand protein required for Ca(2+) uptake. Nature 467(7313):291–296. https://doi.org/10.1038/nature09358IF: 50.5 Q1
Petrungaro C, Zimmermann KM, Kuttner V, Fischer M, Dengjel J, Bogeski I, Riemer J (2015) The Ca(2+)-dependent release of the Mia40-induced MICU1-MICU2 Dimer from MCU regulates mitochondrial Ca(2+) uptake. Cell Metab 22(4):721–733. https://doi.org/10.1016/j.cmet.2015.08.019IF: 27.7 Q1
Pfaffenbach KT, Lee AS (2011) The critical role of GRP78 in physiologic and pathologic stress. Curr Opin Cell Biol 23(2):150–156. https://doi.org/10.1016/j.ceb.2010.09.007IF: 6.0 Q1
Pierro C, Cook SJ, Foets TC, Bootman MD, Roderick HL (2014) Oncogenic K-Ras suppresses IP(3)-dependent Ca(2)(+) release through remodelling of the isoform composition of IP(3)Rs and ER luminal Ca(2)(+) levels in colorectal cancer cell lines. J Cell Sci 127(Pt 7):1607–1619. https://doi.org/10.1242/jcs.141408
Pinton P (2018) Mitochondria-associated membranes (MAMs) and pathologies. Cell Death Dis 9(4):413. https://doi.org/10.1038/s41419-018-0424-1
Pinton P, Leo S, Wieckowski MR, Di Benedetto G, Rizzuto R (2004) Long-term modulation of mitochondrial Ca2+ signals by protein kinase C isozymes. J Cell Biol 165(2):223–232. https://doi.org/10.1083/jcb.200311061
Pinton P, Giorgi C, Pandolfi PP (2011) The role of PML in the control of apoptotic cell fate: a new key player at ER-mitochondria sites. Cell Death Differ 18(9):1450–1456. https://doi.org/10.1038/cdd.2011.31
Ponte S, Carvalho L, Gagliardi M, Campos I, Oliveira PJ, Jacinto A (2020) Drp1-mediated mitochondrial fission regulates calcium and F-actin dynamics during wound healing. Biol Open 9(5). https://doi.org/10.1242/bio.048629IF: 1.8 Q3
Poston CN, Krishnan SC, Bazemore-Walker CR (2013) In-depth proteomic analysis of mammalian mitochondria-associated membranes (MAM). J Proteomics 79:219–230. https://doi.org/10.1016/j.jprot.2012.12.018IF: 2.8 Q2
Prevarskaya N, Ouadid-Ahidouch H, Skryma R, Shuba Y (2014) Remodelling of Ca2+ transport in cancer: how it contributes to cancer hallmarks? Philos Trans R Soc Lond B Biol Sci 369(1638):20130097. https://doi.org/10.1098/rstb.2013.0097IF: 5.4 Q1
Prole DL, Taylor CW (2016) Inositol 1,4,5-trisphosphate receptors and their protein partners as signalling hubs. J Physiol 594(11):2849–2866. https://doi.org/10.1113/JP271139IF: 4.7 Q1
Prudent J, Popgeorgiev N, Gadet R, Deygas M, Rimokh R, Gillet G (2016) Mitochondrial Ca(2+) uptake controls actin cytoskeleton dynamics during cell migration. Sci Rep 6:36570. https://doi.org/10.1038/srep36570IF: 3.8 Q1
Qiu J, Tan YW, Hagenston AM, Martel MA, Kneisel N, Skehel PA, Wyllie DJ, Bading H, Hardingham GE (2013) Mitochondrial calcium uniporter Mcu controls excitotoxicity and is transcriptionally repressed by neuroprotective nuclear calcium signals. Nat Commun 4:2034. https://doi.org/10.1038/ncomms3034IF: 14.7 Q1
Raffaello A, De Stefani D, Sabbadin D, Teardo E, Merli G, Picard A, Checchetto V, Moro S, Szabo I, Rizzuto R (2013) The mitochondrial calcium uniporter is a multimer that can include a dominant-negative pore-forming subunit. EMBO J 32(17):2362–2376. https://doi.org/10.1038/emboj.2013.157IF: 9.4 Q1
Rai K, Pilarski R, Cebulla CM, Abdel-Rahman MH (2016) Comprehensive review of BAP1 tumor predisposition syndrome with report of two new cases. Clin Genet 89(3):285–294. https://doi.org/10.1111/cge.12630IF: 2.9 Q2
Ramos-Franco J, Fill M, Mignery GA (1998) Isoform-specific function of single inositol 1,4,5-trisphosphate receptor channels. Biophys J 75(2):834–839. https://doi.org/10.1016/S0006-3495(98)77572-1IF: 3.2 Q2
Ran Q, Wadhwa R, Kawai R, Kaul SC, Sifers RN, Bick RJ, Smith JR, Pereira-Smith OM (2000) Extramitochondrial localization of mortalin/mthsp70/PBP74/GRP75. Biochem Biophys Res Commun 275(1):174–179. https://doi.org/10.1006/bbrc.2000.3237IF: 2.5 Q3
Raphael M, Lehen'kyi V, Vandenberghe M, Beck B, Khalimonchyk S, Vanden Abeele F, Farsetti L, Germain E, Bokhobza A, Mihalache A, Gosset P, Romanin C, Clezardin P, Skryma R, Prevarskaya N (2014) TRPV6 calcium channel translocates to the plasma membrane via Orai1-mediated mechanism and controls cancer cell survival. Proc Natl Acad Sci U S A 111(37):E3870–E3879. https://doi.org/10.1073/pnas.1413409111
Rapizzi E, Pinton P, Szabadkai G, Wieckowski MR, Vandecasteele G, Baird G, Tuft RA, Fogarty KE, Rizzuto R (2002) Recombinant expression of the voltage-dependent anion channel enhances the transfer of Ca2+ microdomains to mitochondria. J Cell Biol 159(4):613–624. https://doi.org/10.1083/jcb.200205091
Raturi A, Gutierrez T, Ortiz-Sandoval C, Ruangkittisakul A, Herrera-Cruz MS, Rockley JP, Gesson K, Ourdev D, Lou PH, Lucchinetti E, Tahbaz N, Zaugg M, Baksh S, Ballanyi K, Simmen T (2016) TMX1 determines cancer cell metabolism as a thiol-based modulator of ER-mitochondria Ca2+ flux. J Cell Biol 214(4):433–444. https://doi.org/10.1083/jcb.201512077
Ren T, Zhang H, Wang J, Zhu J, Jin M, Wu Y, Guo X, Ji L, Huang Q, Zhang H, Yang H, Xing J (2017) MCU-dependent mitochondrial Ca(2+) inhibits NAD(+)/SIRT3/SOD2 pathway to promote ROS production and metastasis of HCC cells. Oncogene 36(42):5897–5909. https://doi.org/10.1038/onc.2017.167
Ren T, Wang J, Zhang H, Yuan P, Zhu J, Wu Y, Huang Q, Guo X, Zhang J, Ji L, Li J, Zhang H, Yang H, Xing J (2018) MCUR1-mediated mitochondrial calcium signaling facilitates cell survival of hepatocellular carcinoma via reactive oxygen species-dependent P53 degradation. Antioxid Redox Signal 28(12):1120–1136. https://doi.org/10.1089/ars.2017.6990IF: 5.9 Q1
Revathidevi S, Munirajan AK (2019) Akt in cancer: mediator and more. Semin Cancer Biol 59:80–91. https://doi.org/10.1016/j.semcancer.2019.06.002
Rezuchova I, Hudecova S, Soltysova A, Matuskova M, Durinikova E, Chovancova B, Zuzcak M, Cihova M, Burikova M, Penesova A, Lencesova L, Breza J, Krizanova O (2019) Type 3 inositol 1,4,5-trisphosphate receptor has antiapoptotic and proliferative role in cancer cells. Cell Death Dis 10(3):186. https://doi.org/10.1038/s41419-019-1433-4
Rimessi A, Marchi S, Fotino C, Romagnoli A, Huebner K, Croce CM, Pinton P, Rizzuto R (2009) Intramitochondrial calcium regulation by the FHIT gene product sensitizes to apoptosis. Proc Natl Acad Sci U S A 106(31):12753–12758. https://doi.org/10.1073/pnas.0906484106
Rimessi A, Marchi S, Patergnani S, Pinton P (2014) H-Ras-driven tumoral maintenance is sustained through caveolin-1-dependent alterations in calcium signaling. Oncogene 33(18):2329–2340. https://doi.org/10.1038/onc.2013.192
Rimessi A, Pedriali G, Vezzani B, Tarocco A, Marchi S, Wieckowski MR, Giorgi C, Pinton P (2020) Interorganellar calcium signaling in the regulation of cell metabolism: a cancer perspective. Semin Cell Dev Biol 98:167–180. https://doi.org/10.1016/j.semcdb.2019.05.015
Ringer S (1883) A further contribution regarding the influence of the different constituents of the blood on the contraction of the heart. J Physiol 4(1):29–42.3. https://doi.org/10.1113/jphysiol.1883.sp000120IF: 4.7 Q1
Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T (1998) Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 280(5370):1763–1766. https://doi.org/10.1126/science.280.5370.1763IF: 44.7 Q1
Salmena L, Carracedo A, Pandolfi PP (2008) Tenets of PTEN tumor suppression. Cell 133(3):403–414. https://doi.org/10.1016/j.cell.2008.04.013IF: 45.5 Q1
Sancak Y, Markhard AL, Kitami T, Kovacs-Bogdan E, Kamer KJ, Udeshi ND, Carr SA, Chaudhuri D, Clapham DE, Li AA, Calvo SE, Goldberger O, Mootha VK (2013) EMRE is an essential component of the mitochondrial calcium uniporter complex. Science 342(6164):1379–1382. https://doi.org/10.1126/science.1242993IF: 44.7 Q1
Schein SJ, Colombini M, Finkelstein A (1976) Reconstitution in planar lipid bilayers of a voltage-dependent anion-selective channel obtained from paramecium mitochondria. J Membr Biol 30(2):99–120. https://doi.org/10.1007/bf01869662IF: 2.3 Q3
Scherer PE, Manning-Krieg UC, Jeno P, Schatz G, Horst M (1992) Identification of a 45-kDa protein at the protein import site of the yeast mitochondrial inner membrane. Proc Natl Acad Sci U S A 89(24):11930–11934. https://doi.org/10.1073/pnas.89.24.11930
Scherr AL, Gdynia G, Salou M, Radhakrishnan P, Duglova K, Heller A, Keim S, Kautz N, Jassowicz A, Elssner C, He YW, Jaeger D, Heikenwalder M, Schneider M, Weber A, Roth W, Schulze-Bergkamen H, Koehler BC (2016) Bcl-xL is an oncogenic driver in colorectal cancer. Cell Death Dis 7(8):e2342. https://doi.org/10.1038/cddis.2016.233
Schneider HC, Westermann B, Neupert W, Brunner M (1996) The nucleotide exchange factor MGE exerts a key function in the ATP-dependent cycle of mt-Hsp70-Tim44 interaction driving mitochondrial protein import. EMBO J 15(21):5796–5803
Schuettengruber B, Chourrout D, Vervoort M, Leblanc B, Cavalli G (2007) Genome regulation by polycomb and trithorax proteins. Cell 128(4):735–745. https://doi.org/10.1016/j.cell.2007.02.009
Sebag SC, Koval OM, Paschke JD, Winters CJ, Comellas AP, Grumbach IM (2018) Inhibition of the mitochondrial calcium uniporter prevents IL-13 and allergen-mediated airway epithelial apoptosis and loss of barrier function. Exp Cell Res 362(2):400–411. https://doi.org/10.1016/j.yexcr.2017.12.003IF: 3.3 Q2
Shay G, Lynch CC, Fingleton B (2015) Moving targets: emerging roles for MMPs in cancer progression and metastasis. Matrix Biol 44-46:200–206. https://doi.org/10.1016/j.matbio.2015.01.019
Shibao K, Fiedler MJ, Nagata J, Minagawa N, Hirata K, Nakayama Y, Iwakiri Y, Nathanson MH, Yamaguchi K (2010) The type III inositol 1,4,5-trisphosphate receptor is associated with aggressiveness of colorectal carcinoma. Cell Calcium 48(6):315–323. https://doi.org/10.1016/j.ceca.2010.09.005
Shimizu S, Konishi A, Kodama T, Tsujimoto Y (2000) BH4 domain of antiapoptotic Bcl-2 family members closes voltage-dependent anion channel and inhibits apoptotic mitochondrial changes and cell death. Proc Natl Acad Sci U S A 97(7):3100–3105. https://doi.org/10.1073/pnas.97.7.3100
Shoshan-Barmatz V, Gincel D (2003) The voltage-dependent anion channel: characterization, modulation, and role in mitochondrial function in cell life and death. Cell Biochem Biophys 39(3):279–292. https://doi.org/10.1385/CBB:39:3:279
Shoshan-Barmatz V, De Pinto V, Zweckstetter M, Raviv Z, Keinan N, Arbel N (2010) VDAC, a multi-functional mitochondrial protein regulating cell life and death. Mol Aspects Med 31(3):227–285. https://doi.org/10.1016/j.mam.2010.03.002
Simmen T, Aslan JE, Blagoveshchenskaya AD, Thomas L, Wan L, Xiang Y, Feliciangeli SF, Hung CH, Crump CM, Thomas G (2005) PACS-2 controls endoplasmic reticulum-mitochondria communication and Bid-mediated apoptosis. EMBO J 24(4):717–729. https://doi.org/10.1038/sj.emboj.7600559
Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ (2005) Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer 5(8):615–625. https://doi.org/10.1038/nrc1669
Singh A, Chagtoo M, Tiwari S, George N, Chakravarti B, Khan S, Lakshmi S, Godbole MM (2017a) Inhibition of inositol 1, 4, 5-trisphosphate receptor induce breast cancer cell death through deregulated autophagy and cellular bioenergetics. J Cell Biochem 118(8):2333–2346. https://doi.org/10.1002/jcb.25891
Singh A, Sharma RK, Chagtoo M, Agarwal G, George N, Sinha N, Godbole MM (2017b) 1H NMR metabolomics reveals Association of High Expression of inositol 1, 4, 5 trisphosphate receptor and metabolites in breast cancer patients. PLoS One 12(1):e0169330. https://doi.org/10.1371/journal.pone.0169330
Siprashvili Z, Sozzi G, Barnes LD, McCue P, Robinson AK, Eryomin V, Sard L, Tagliabue E, Greco A, Fusetti L, Schwartz G, Pierotti MA, Croce CM, Huebner K (1997) Replacement of Fhit in cancer cells suppresses tumorigenicity. Proc Natl Acad Sci U S A 94(25):13771–13776. https://doi.org/10.1073/pnas.94.25.13771
Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, Neve RM, Kuo WL, Davies M, Carey M, Hu Z, Guan Y, Sahin A, Symmans WF, Pusztai L, Nolden LK, Horlings H, Berns K, Hung MC, van de Vijver MJ, Valero V, Gray JW, Bernards R, Mills GB, Hennessy BT (2008) An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res 68(15):6084–6091. https://doi.org/10.1158/0008-5472.CAN-07-6854
Su Y, Huang X, Huang Z, Huang T, Xu Y, Yi C (2020) STAT3 localizes in mitochondria-associated ER membranes instead of in mitochondria. Front Cell Dev Biol 8:274. https://doi.org/10.3389/fcell.2020.00274
Szabadkai G, Bianchi K, Varnai P, De Stefani D, Wieckowski MR, Cavagna D, Nagy AI, Balla T, Rizzuto R (2006) Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol 175(6):901–911. https://doi.org/10.1083/jcb.200608073
Szado T, Vanderheyden V, Parys JB, De Smedt H, Rietdorf K, Kotelevets L, Chastre E, Khan F, Landegren U, Soderberg O, Bootman MD, Roderick HL (2008) Phosphorylation of inositol 1,4,5-trisphosphate receptors by protein kinase B/Akt inhibits Ca2+ release and apoptosis. Proc Natl Acad Sci U S A 105(7):2427–2432. https://doi.org/10.1073/pnas.0711324105IF: 9.4 Q1
Takahashi N, Chen HY, Harris IS, Stover DG, Selfors LM, Bronson RT, Deraedt T, Cichowski K, Welm AL, Mori Y, Mills GB, Brugge JS (2018) Cancer cells co-opt the neuronal redox-sensing channel TRPA1 to promote oxidative-stress tolerance. Cancer Cell 33(6):985–1003. e1007. https://doi.org/10.1016/j.ccell.2018.05.001
Takei N, Yoneda A, Sakai-Sawada K, Kosaka M, Minomi K, Tamura Y (2017) Hypoxia-inducible ERO1alpha promotes cancer progression through modulation of integrin-beta1 modification and signalling in HCT116 colorectal cancer cells. Sci Rep 7(1):9389. https://doi.org/10.1038/s41598-017-09976-7
Tanaka T, Kutomi G, Kajiwara T, Kukita K, Kochin V, Kanaseki T, Tsukahara T, Hirohashi Y, Torigoe T, Okamoto Y, Hirata K, Sato N, Tamura Y (2017) Cancer-associated oxidoreductase ERO1-alpha promotes immune escape through up-regulation of PD-L1 in human breast cancer. Oncotarget 8(15):24706–24718. https://doi.org/10.18632/oncotarget.14960
Tang S, Wang X, Shen Q, Yang X, Yu C, Cai C, Cai G, Meng X, Zou F (2015) Mitochondrial Ca(2)(+) uniporter is critical for store-operated Ca(2)(+) entry-dependent breast cancer cell migration. Biochem Biophys Res Commun 458(1):186–193. https://doi.org/10.1016/j.bbrc.2015.01.092
Tosatto A, Sommaggio R, Kummerow C, Bentham RB, Blacker TS, Berecz T, Duchen MR, Rosato A, Bogeski I, Szabadkai G, Rizzuto R, Mammucari C (2016) The mitochondrial calcium uniporter regulates breast cancer progression via HIF-1alpha. EMBO Mol Med 8(5):569–585. https://doi.org/10.15252/emmm.201606255
Toyoshima C, Nakasako M, Nomura H, Ogawa H (2000) Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 a resolution. Nature 405(6787):647–655. https://doi.org/10.1038/35015017
Trisciuoglio D, Tupone MG, Desideri M, Di Martile M, Gabellini C, Buglioni S, Pallocca M, Alessandrini G, D'Aguanno S, Del Bufalo D (2017) BCL-XL overexpression promotes tumor progression-associated properties. Cell Death Dis 8(12):3216. https://doi.org/10.1038/s41419-017-0055-y
Tsujimoto Y, Shimizu S (2000) VDAC regulation by the Bcl-2 family of proteins. Cell Death Differ 7(12):1174–1181. https://doi.org/10.1038/sj.cdd.4400780
Tu H, Wang Z, Bezprozvanny I (2005) Modulation of mammalian inositol 1,4,5-trisphosphate receptor isoforms by calcium: a role of calcium sensor region. Biophys J 88(2):1056–1069. https://doi.org/10.1529/biophysj.104.049601
Ueasilamongkol P, Khamphaya T, Guerra MT, Rodrigues MA, Gomes DA, Kong Y, Wei W, Jain D, Trampert DC, Ananthanarayanan M, Banales JM, Roberts LR, Farshidfar F, Nathanson MH, Weerachayaphorn J (2020) Type 3 inositol 1,4,5-trisphosphate receptor is increased and enhances malignant properties in cholangiocarcinoma. Hepatology 71(2):583–599. https://doi.org/10.1002/hep.30839
Vandecaetsbeek I, Trekels M, De Maeyer M, Ceulemans H, Lescrinier E, Raeymaekers L, Wuytack F, Vangheluwe P (2009) Structural basis for the high Ca2+ affinity of the ubiquitous SERCA2b Ca2+ pump. Proc Natl Acad Sci U S A 106(44):18533–18538. https://doi.org/10.1073/pnas.0906797106
Verfaillie T, Rubio N, Garg AD, Bultynck G, Rizzuto R, Decuypere JP, Piette J, Linehan C, Gupta S, Samali A, Agostinis P (2012) PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress. Cell Death Differ 19(11):1880–1891. https://doi.org/10.1038/cdd.2012.74
Vervloessem T, Yule DI, Bultynck G, Parys JB (2015) The type 2 inositol 1,4,5-trisphosphate receptor, emerging functions for an intriguing Ca(2)(+)-release channel. Biochim Biophys Acta 1853(9):1992–2005. https://doi.org/10.1016/j.bbamcr.2014.12.006
Vogelstein B, Lane D, Levine AJ (2000) Surfing the p53 network. Nature 408(6810):307–310. https://doi.org/10.1038/35042675
Voos W, Rottgers K (2002) Molecular chaperones as essential mediators of mitochondrial biogenesis. Biochim Biophys Acta 1592(1):51–62. https://doi.org/10.1016/s0167-4889(02)00264-1
Vultur A, Gibhardt CS, Stanisz H, Bogeski I (2018) The role of the mitochondrial calcium uniporter (MCU) complex in cancer. Pflugers Arch 470(8):1149–1163. https://doi.org/10.1007/s00424-018-2162-8
Wadhwa R, Pereira-Smith OM, Reddel RR, Sugimoto Y, Mitsui Y, Kaul SC (1995) Correlation between complementation group for immortality and the cellular distribution of mortalin. Exp Cell Res 216(1):101–106. https://doi.org/10.1006/excr.1995.1013
Wadhwa R, Taira K, Kaul SC (2002) An Hsp70 family chaperone, mortalin/mthsp70/PBP74/Grp75: what, when, and where? Cell Stress Chaperones 7(3):309–316. https://doi.org/10.1379/1466-1268(2002)007<0309:ahfcmm>2.0.co;2
Wagner LE 2nd, Joseph SK, Yule DI (2008) Regulation of single inositol 1,4,5-trisphosphate receptor channel activity by protein kinase A phosphorylation. J Physiol 586(15):3577–3596. https://doi.org/10.1113/jphysiol.2008.152314
Wang X, Perez E, Liu R, Yan LJ, Mallet RT, Yang SH (2007) Pyruvate protects mitochondria from oxidative stress in human neuroblastoma SK-N-SH cells. Brain Res 1132(1):1–9. https://doi.org/10.1016/j.brainres.2006.11.032
Wang M, Wey S, Zhang Y, Ye R, Lee AS (2009) Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid Redox Signal 11(9):2307–2316. https://doi.org/10.1089/ARS.2009.2485
Wang PT, Garcin PO, Fu M, Masoudi M, St-Pierre P, Pante N, Nabi IR (2015) Distinct mechanisms controlling rough and smooth endoplasmic reticulum contacts with mitochondria. J Cell Sci 128(15):2759–2765. https://doi.org/10.1242/jcs.171132
Wang Y, Qi YX, Qi Z, Tsang SY (2019) TRPC3 regulates the proliferation and apoptosis resistance of triple negative breast cancer cells through the TRPC3/RASA4/MAPK pathway. Cancers (Basel) 11(4). https://doi.org/10.3390/cancers11040558
Weisthal S, Keinan N, Ben-Hail D, Arif T, Shoshan-Barmatz V (2014) Ca(2+)-mediated regulation of VDAC1 expression levels is associated with cell death induction. Biochim Biophys Acta 1843(10):2270–2281. https://doi.org/10.1016/j.bbamcr.2014.03.021
White C, Li C, Yang J, Petrenko NB, Madesh M, Thompson CB, Foskett JK (2005) The endoplasmic reticulum gateway to apoptosis by Bcl-X(L) modulation of the InsP3R. Nat Cell Biol 7(10):1021–1028. https://doi.org/10.1038/ncb1302
Whitehurst AW (2014) Cause and consequence of cancer/testis antigen activation in cancer. Annu Rev Pharmacol Toxicol 54:251–272. https://doi.org/10.1146/annurev-pharmtox-011112-140326
Xu N, Zhang D, Chen J, He G, Gao L (2019) Low expression of ryanodine receptor 2 is associated with poor prognosis in thyroid carcinoma. Oncol Lett 18(4):3605–3612. https://doi.org/10.3892/ol.2019.10732
Yoshimine S, Kikuchi E, Kosaka T, Mikami S, Miyajima A, Okada Y, Oya M (2013) Prognostic significance of Bcl-xL expression and efficacy of Bcl-xL targeting therapy in urothelial carcinoma. Br J Cancer 108(11):2312–2320. https://doi.org/10.1038/bjc.2013.216
Youker RT, Shinde U, Day R, Thomas G (2009) At the crossroads of homoeostasis and disease: roles of the PACS proteins in membrane traffic and apoptosis. Biochem J 421(1):1–15. https://doi.org/10.1042/BJ20081016
Yu H, Lee H, Herrmann A, Buettner R, Jove R (2014) Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer 14(11):736–746. https://doi.org/10.1038/nrc3818
Yu C, Wang Y, Peng J, Shen Q, Chen M, Tang W, Li X, Cai C, Wang B, Cai S, Meng X, Zou F (2017) Mitochondrial calcium uniporter as a target of microRNA-340 and promoter of metastasis via enhancing the Warburg effect. Oncotarget 8(48):83831–83844. https://doi.org/10.18632/oncotarget.19747
Yuan Z, Cao A, Liu H, Guo H, Zang Y, Wang Y, Wang Y, Wang H, Yin P, Peng W (2017) Calcium uptake via mitochondrial uniporter contributes to palmitic acid-induced apoptosis in mouse podocytes. J Cell Biochem 118(9):2809–2818. https://doi.org/10.1002/jcb.25930
Yule DI, Betzenhauser MJ, Joseph SK (2010) Linking structure to function: recent lessons from inositol 1,4,5-trisphosphate receptor mutagenesis. Cell Calcium 47(6):469–479. https://doi.org/10.1016/j.ceca.2010.04.005
Zanesi N, Pekarsky Y, Croce CM (2005) A mouse model of the fragile gene FHIT: from carcinogenesis to gene therapy and cancer prevention. Mutat Res 591(1–2):103–109. https://doi.org/10.1016/j.mrfmmm.2005.05.016
Zeng F, Chen X, Cui W, Wen W, Lu F, Sun X, Ma D, Yuan Y, Li Z, Hou N, Zhao H, Bi X, Zhao J, Zhou J, Zhang Y, Xiao RP, Cai J, Zhang X (2018) RIPK1 binds MCU to mediate induction of mitochondrial Ca(2+) uptake and promotes colorectal oncogenesis. Cancer Res 78(11):2876–2885. https://doi.org/10.1158/0008-5472.CAN-17-3082IF: 12.5 Q1
Zhang T, Zhao C, Luo L, Zhao H, Cheng J, Xu F (2012) The expression of Mcl-1 in human cervical cancer and its clinical significance. Med Oncol 29(3):1985–1991. https://doi.org/10.1007/s12032-011-0005-y
Zhang K, Jiao K, Xing Z, Zhang L, Yang J, Xie X, Yang L (2014) Bcl-xL overexpression and its association with the progress of tongue carcinoma. Int J Clin Exp Pathol 7(11):7360–7377
Zhao H, Li T, Wang K, Zhao F, Chen J, Xu G, Zhao J, Li T, Chen L, Li L, Xia Q, Zhou T, Li HY, Li AL, Finkel T, Zhang XM, Pan X (2019) AMPK-mediated activation of MCU stimulates mitochondrial Ca(2+) entry to promote mitotic progression. Nat Cell Biol 21(4):476–486. https://doi.org/10.1038/s41556-019-0296-3
Zhou X, Ren Y, Zhang J, Zhang C, Zhang K, Han L, Kong L, Wei J, Chen L, Yang J, Wang Q, Zhang J, Yang Y, Jiang T, Li M, Kang C (2015a) HOTAIR is a therapeutic target in glioblastoma. Oncotarget 6(10):8353–8365. https://doi.org/10.18632/oncotarget.3229
Zhou X, Ren Y, Kong L, Cai G, Sun S, Song W, Wang Y, Jin R, Qi L, Mei M, Wang X, Kang C, Li M, Zhang L (2015b) Targeting EZH2 regulates tumor growth and apoptosis through modulating mitochondria dependent cell-death pathway in HNSCC. Oncotarget 6(32):33720–33732. https://doi.org/10.18632/oncotarget.5606
Acknowledgments
PP is grateful to Camilla degli Scrovegni for continuous support. The Signal Transduction Laboratory is supported by the Italian Association for Cancer Research (AIRC: IG-23670 to P.P. and IG-19803 to C.G.), A-ROSE, Telethon (GGP11139B to P.P), Progetti di Rilevante Interesse Nazionale (PRIN2017E5L5P3 to P.P and PRIN20177E9EPY to C.G.), the Italian Ministry of Health (GR-2013-02356747 to C.G.), the European Research Council (ERC, 853057- InflaPML to C.G.), and local funds from the University of Ferrara to PP and C.G. SM is supported by the Italian Ministry of Health (GR-2016-02364602) and local funds from Marche Polytechnic University (Ancona, Italy). MRW was supported by the Internal Project of The Children’s Memorial Health Institute (No S141/2014).
The authors declare no conflict of interests. All authors read and approved the final version of the manuscript.
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Danese, A. et al. (2020). Cancer-Related Increases and Decreases in Calcium Signaling at the Endoplasmic Reticulum-Mitochondria Interface (MAMs). In: Pedersen, S.H.F., Barber, D.L. (eds) Organelles in Disease. Reviews of Physiology, Biochemistry and Pharmacology, vol 185. Springer, Cham. https://doi.org/10.1007/112_2020_43
Download citation
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-22594-9
Online ISBN: 978-3-031-22595-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)