Abstract 抽象
Environmental chemicals comprise a major portion of the human exposome,
with some shown to impact the health of susceptible populations, including
pregnant women and developing fetuses. The placenta and cord blood serve as
important biological windows into the maternal and fetal environments. In this
article we review how environmental chemicals (defined here to include man-made
chemicals [e.g., flame retardants, pesticides/herbicides, per- and
polyfluoroalkyl substances], toxins, metals, and other xenobiotic compounds)
contribute to the prenatal exposome and highlight future directions to advance
this research field. Our findings from a survey of recent literature indicate
the need to better understand the breadth of environmental chemicals that reach
the placenta and cord blood, as well as the linkages between prenatal exposures,
mechanisms of toxicity, and subsequent health outcomes. Research efforts
tailored towards addressing these needs will provide a more comprehensive
understanding of how environmental chemicals impact maternal and fetal
health.
环境化学物质构成了人类暴露组学的主要部分,其中一些被证明会影响易感人群的健康,包括孕妇和发育中的胎儿。胎盘和脐带血是了解母体和胎儿环境的重要生物窗口。在本文中,我们回顾了环境化学品(此处定义为包括人造化学品 [例如,阻燃剂、杀虫剂/除草剂、全氟和多氟烷基物质]、毒素、金属和其他外源性化合物)如何促进产前暴露组学,并强调了推进该研究领域的未来方向。我们对近期文献的调查结果表明,需要更好地了解到达胎盘和脐带血的环境化学物质的广度,以及产前暴露、毒性机制和随后的健康结果之间的联系。为满足这些需求而量身定制的研究工作将提供更全面的了解环境化学物质如何影响母婴健康。
Keywords: Exposome, Environment, Fetus, Pregnancy, Developmental toxicity
关键字:暴露组学, 环境, 胎儿, 妊娠, 发育毒性
1. Introduction 1. 引言
1.1. Introduction to the exposome
1.1. 暴露组简介
The concept of the “exposome” was introduced in 2005 as
“encompass[ing] life-course environmental exposures (including lifestyle
factors), from the prenatal period onwards” [1]. This initial definition has since been expanded
upon to include three categories: (i) the general external exposome (e.g.,
lifestyle and socioeconomic factors), (ii) the specific external exposome (e.g.,
environmental exposures, infectious agents, and therapeutics), and (iii) the
internal exposome (e.g., metabolism, circulating hormones, gut microflora,
inflammation, and oxidative stress) [2,3]. In short, the exposome
is currently a broad term reflecting any non-genetic factor that may play a role
in the development of disease.
“暴露组学”的概念于 2005 年提出,“包括从产前开始的生命历程环境暴露(包括生活方式因素)”[1]。此后,这一初始定义被扩展为包括三类:(i) 一般外部暴露组(例如,生活方式和社会经济因素),(ii) 特定的外部暴露组(例如,环境暴露、感染因子和治疗),以及 (iii) 内部暴露组(例如,代谢、循环激素、肠道菌群、炎症和氧化应激)[2,3].简而言之,暴露组学目前是一个广义的术语,反映了可能在疾病发展中发挥作用的任何非遗传因素。
Evaluating environmental chemicals as part of the human exposome is of
high interest, as environmental factors are known to significantly contribute to
the global burden of disease [2,4]. At the same time, the majority of
chemicals in the environment remain understudied and lack data in relation to
both exposures and associated toxicological/health outcomes [5,6]. Methods
are currently being expanded upon to evaluate broader chemical domains at
increasingly higher rates of throughput [7]. Some recent exposome-relevant examples include studies that have
evaluated chemicals in the environment, such as household dust [8–11],
drinking water [12–14], and household consumer products [15]. Other notable studies have evaluated exposome
measures in biological samples, including circulating blood, teeth, and saliva,
and have started linking these measures to health outcomes [16–18].
评估环境化学物质作为人类暴露组学的一部分具有高度意义,因为已知环境因素对全球疾病负担有重大影响[2,4]。与此同时,环境中的大多数化学物质仍未得到充分研究,并且缺乏与暴露和相关毒理学/健康结局相关的数据 [5,6]。目前正在扩展方法,以越来越高的通量率评估更广泛的化学领域 [7]。最近一些与暴露组学相关的例子包括评估环境中化学物质的研究,例如家庭灰尘[8–11]、饮用水[12–14]和家用消费品[15]。其他值得注意的研究评估了生物样本(包括循环血液、牙齿和唾液)中的暴露组指标,并开始将这些指标与健康结果联系起来[16–18]。
A critical research topic that remains understudied is the impact of the
prenatal environmental exposome on maternal and fetal health outcomes.
Addressing this research gap is of high interest as pregnant women and
developing fetuses represent populations that are particularly susceptible to
potential effects resulting from environmental factors. For example, pregnant
women experience shifts in immune cell responses, a hormone-regulated process
known to reduce risk of fetal rejection and promote transfer of maternal
antibodies to the fetus [19]. With this
overall reduction in immune cell responses, pregnant women can exhibit increased
risk of certain diseases, including infectious disease [19]. As certain environmental exposures are known to
disrupt immune function [20,21], these effects may become exacerbated
in pregnant women [22,23]. Within the embryonic environment, rapid cell
turnover and tissue growth can cause harmful effects to occur in the developing
fetus resulting from certain chemical exposures at lower concentrations than
those required to elicit effects in adults [24,25]. These disruptions can
also influence health outcomes later in life, a concept also known as the
Developmental Origins of Health and Disease (DOHaD) [26]. It is therefore of utmost importance to evaluate
the composition and resulting impact of prenatal environmental exposures.
一个仍未得到充分研究的关键研究课题是产前环境暴露组对母体和胎儿健康结果的影响。解决这一研究差距非常有趣,因为孕妇和发育中的胎儿代表了特别容易受到环境因素潜在影响的人群。例如,孕妇会经历免疫细胞反应的变化,这是一种激素调节过程,已知可以降低胎儿排斥反应的风险并促进母体抗体向胎儿的转移 [19]。随着免疫细胞反应的整体减少,孕妇患某些疾病的风险会增加,包括传染病[19]。由于已知某些环境暴露会破坏免疫功能[20,21],因此这些影响在孕妇中可能会加剧[22,23]。在胚胎环境中,细胞的快速更新和组织生长会导致发育中的胎儿发生有害影响,因为某些化学物质暴露的浓度低于成人所需的浓度[24,25]。这些干扰也会影响以后的健康结果,这个概念也被称为健康和疾病的发育起源 (DOHaD) [26]。因此,评估产前环境暴露的成分和由此产生的影响至关重要。
1.2. The prenatal exposure space and chemical transport
1.2. 产前暴露空间和化学运输
On a daily basis, pregnant women may be exposed to a vast number of
environmental chemicals via ingestion, inhalation, or dermal absorption. Tissue
partitioning and excretion rates, which determine how and where these chemicals
travel through the maternal body, are influenced by physiochemical properties of
chemicals as well as maternal and fetal-specific influences. Important chemical
properties that play roles in tissue partitioning and/or excretion include
charge [27], lipophilicity [28], protein binding affinity [29], solubility [30], and size/length [31]. Maternal and fetal-specific influences include metabolic
capabilities [32], as well as parameters
that commonly change throughout pregnancy, including tissue volumes and blood
flow rates, among others [33]. Exposures
during pregnancy include complex mixtures of environmental chemicals, many of
which partition into maternal blood and are able to reach the placental barrier
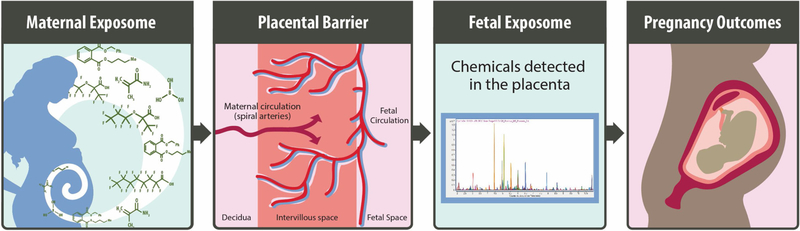
through the maternal circulation system [34] (Fig. 1). These types of
exposures contribute to the ‘prenatal exposome’, which includes
the ‘maternal exposome’ (or exposures to pregnant women) and the
‘fetal exposome’ (or exposures that reach the developing fetus).
Therefore, chemicals that contribute to the prenatal exposome include those that
may impact overall pregnancy health or other pregnancy-relevant outcomes,
including impacts on the developing fetus.
每天,孕妇可能通过摄入、吸入或皮肤吸收接触大量环境化学物质。组织分区和排泄率决定了这些化学物质在母体中的传播方式和位置,受化学物质的理化特性以及母体和胎儿特异性影响的影响。在组织分配和/或排泄中发挥作用的重要化学性质包括电荷 [27]、亲脂性 [28]、蛋白质结合亲和力 [29]、溶解度 [30] 和大小/长度 [31]。母体和胎儿特异性影响因素包括代谢能力 [32],以及整个妊娠期间通常会变化的参数,包括组织体积和血流速率等 [33]。怀孕期间的暴露包括环境化学物质的复杂混合物,其中许多混合物会分流到母体血液中,并能够通过母体循环系统到达胎盘屏障[34](图1)。这些类型的暴露导致了“产前暴露组”,其中包括“母体暴露组”(或对孕妇的暴露)和“胎儿暴露组”(或到达发育中的胎儿的暴露)。因此,导致产前暴露组的化学物质包括那些可能影响整体妊娠健康或其他妊娠相关结果的化学物质,包括对发育中的胎儿的影响。
Fig. 1. 图 1.
Overview of environmental contaminants reaching the maternal and fetal
exposomes (e.g. prenatal exposome), potentially influencing pregnancy outcomes
(i.e., maternal and fetal health).
到达母体和胎儿暴露组的环境污染物概述(例如 产前暴露组),可能影响妊娠结局(即母体和胎儿健康)。
Of particular relevance to the developing fetus, environmental chemicals
in circulating maternal serum can potentially cross the placenta. Similar to
maternal tissue partitioning, this translocation is dependent upon properties of
the chemical (e.g., charge, lipophilicity, and size) as well as maternal and
placental factors such as metabolism and transporter expression [35,36]. As is
the case for other biological barriers, small, lipophilic compounds, such as
methylmercury, readily cross the placenta and may even accumulate in the fetal
space [37]. The placenta is the interface
between mother and fetus, and it serves a multitude of functions, including the
regulation of nutrient transfer to the fetus and disposal of fetal waste.
Therefore, chemicals passing through the placenta must cross several barriers to
reach the fetal circulation. These barriers specifically include: (i) the
syncytiotrophoblasts, a fused, multicellular layer that lines the chorionic
villi, (ii) the interstitial tissue consisting of cytotrophoblasts, fibroblasts,
and connective tissue, collectively comprising the inner mass of the villi, and
(iii) the fetal endothelial cells that are present within the villi. Because of
their capacity for active transport, the specific barrier function of the
placenta is largely regulated by syncytiotrophoblasts [35,36].
与发育中的胎儿特别相关,循环母体血清中的环境化学物质有可能穿过胎盘。与母体组织分配类似,这种易位取决于化学物质的性质(如电荷、亲脂性和大小)以及母体和胎盘因素,如代谢和转运蛋白表达[35,36]。与其他生物屏障一样,甲基汞等亲脂性小化合物很容易穿过胎盘,甚至可能在胎儿间隙中积累[37]。胎盘是母亲和胎儿之间的接口,它具有多种功能,包括调节营养物质向胎儿的转移和胎儿废物的处理。因此,通过胎盘的化学物质必须穿过几个障碍才能到达胎儿循环。这些屏障具体包括:(i) 合体滋养层,排列在绒毛膜绒毛上的融合多细胞层,(ii) 由细胞滋养层、成纤维细胞和结缔组织组成的间质组织,共同构成绒毛的内部质量,以及 (iii) 存在于绒毛内的胎儿内皮细胞。由于胎盘具有主动运输能力,因此胎盘的特异性屏障功能主要受合体滋养层细胞的调节[35,36]。
Chemical translocation across the placenta can be passive, relying solely
on chemical gradients, and/or diffusive, actively driven by transport proteins
using ATP or ion gradients as chemical energy sources. Syncytiotrophoblasts
express a wide range of xenobiotic transporters that control translocation
across the placenta [38]. These include
both uptake transporters, such as solute carrier family 6 member 2 (SLC6A2) and
solute carrier family 22 member 5 (SLC22A5), and efflux transporters such as ATP
binding cassette subfamily B member 1 (ABCB1) and G member 2 (ABCG2) [38]. Of importance to regulating chemical
influx/efflux, the apical (maternal facing) and basolateral (fetal facing)
membranes of syncytiotrophoblasts each contain a unique profile of transporters.
For example, ABCB1 and ABCG2 are both localized on the apical surface of
syncytiotrophoblasts, and this, combined with their affinity for a wide range of
substrates, allows these proteins to serve in a fetoprotective manner. This
fetoprotective activity occurs through the prevention of trans-placental
transfer of toxicants or active removal of toxicants from fetal circulation
[38]. For instance, ABCG2 prevents
the fetal accumulation of medications and environmental contaminants, including
glyburide, a medication that may be prescribed for the treatment of
pre-gestational or gestational diabetes mellitus, and zearalenone, an estrogenic
mycotoxin common in cereal crops [39–41]. Despite the
high degree of chemical transport regulation across the placenta, many types of
chemicals, including pharmaceuticals and various environmental toxicants,
readily pass through the placenta from mother to fetus, potentially impacting
fetal health.
胎盘上的化学易位可以是被动的,仅依赖于化学梯度,和/或扩散的,由使用 ATP 或离子梯度作为化学能源的转运蛋白主动驱动。合体滋养层细胞表达多种外源性转运蛋白,这些转运蛋白控制胎盘的易位[38]。这些包括摄取转运蛋白,如溶质载体家族 6 成员 2 (SLC6A2) 和溶质载体家族 22 成员 5 (SLC22A5),以及外排转运蛋白,如 ATP 结合盒亚家族 B 成员 1 (ABCB1) 和 G 成员 2 (ABCG2) [38]。合体滋养层细胞的顶端(面向母体)和基底外侧(面向胎儿)膜对于调节化学内流/外流很重要,每个膜都包含独特的转运蛋白谱。例如,ABCB1 和 ABCG2 都位于合体滋养层细胞的顶端表面,这与它们对多种底物的亲和力相结合,使这些蛋白质能够以胎儿保护方式发挥作用。这种胎儿保护活性是通过防止毒物经胎盘转移或从胎儿循环中主动清除毒物来实现的[38]。例如,ABCG2 可防止药物和环境污染物在胎儿体内积累,包括格列本脲(一种可用于治疗孕前或妊娠期糖尿病的药物)和玉米赤霉烯酮(一种在谷类作物中常见的雌激素霉菌毒素)[39–41]。尽管胎盘中化学运输监管程度很高,但许多类型的化学物质,包括药物和各种环境毒物,很容易通过胎盘从母亲传给胎儿,从而可能影响胎儿健康。
1.3. Focus of review: the prenatal exposome
1.3. 综述重点:产前暴露组学
The current review focuses on the evaluation of environmental chemicals
(defined here to include man-made chemicals [e.g., flame retardants,
pesticides/herbicides, per- and polyfluoroalkyl substances], toxins, metals, and
other xenobiotic compounds to which humans come into contact) that reach the
placental barrier and travel into human cord blood. The placenta and cord blood
were selected for focused review as they represent tissues that are feasible to
obtain for research purposes with minimally invasive procedures. Furthermore,
these tissues are directly applicable as target tissues involved in pregnancy
health outcomes and prenatal developmental toxicity, with focus on the prenatal
period, as detailed above. It is important to note that certain previous studies
have evaluated the pregnancy exposome through analyses of maternal circulating
blood [42,43]. These research approaches contribute valuable information
towards understanding relationships between exposure to environmental
contaminants and maternal and fetal health outcomes, as noted within a recent
review [44]. Here, we focus on studies
and future research efforts aimed at the evaluation of environmental chemicals
that reach and travel through the placental barrier, as these types of
contaminants have the potential to directly impact prenatal development in
addition to maternal health.
本综述的重点是评估到达胎盘屏障并进入人类脐带血的环境化学品(此处定义为包括人造化学品 [例如,阻燃剂、杀虫剂/除草剂、全氟烷基和多氟烷基物质]、毒素、金属和人类接触的其他外源性化合物)。选择胎盘和脐带血进行重点审查,因为它们代表了可通过微创手术获得用于研究目的的组织。此外,这些组织直接适用于参与妊娠健康结果和产前发育毒性的靶组织,重点是产前,如上所述。值得注意的是,以前的一些研究通过分析母体循环血来评估妊娠暴露组学[42,43]。正如最近的一篇综述所指出的,这些研究方法为理解环境污染物暴露与母体和胎儿健康结局之间的关系提供了有价值的信息[44]。在这里,我们专注于研究和未来的研究工作,旨在评估到达和穿过胎盘屏障的环境化学物质,因为除了孕产妇健康之外,这些类型的污染物还有可能直接影响产前发育。
This review is designed to provide a high-level understanding of the
types of research that have been completed aimed at evaluating effects from
prenatal exposures to environmental chemicals on maternal and infant health.
Included studies represent those that serve as examples from the published
literature, selected from PubMed and Google Scholar search queries. Example
studies were included if researchers examined environmental chemical exposures
during the prenatal period, and either measured chemicals and/or
exposure-related effects in the placenta or cord blood. A subset of
environmental chemicals was selected as the focus of this review to serve as
important case studies based on their known relationships to adverse effects
resulting from exposures during the prenatal period. Furthermore, this review
comments on critical data gaps and future research directions in the fields of
environmental exposure science and pregnancy health outcomes.
本综述旨在提供对已完成的研究类型的高层次理解,这些研究旨在评估产前暴露于环境化学物质对母婴健康的影响。纳入的研究代表那些从已发表的文献中作为示例的研究,从 PubMed 和 Google Scholar 搜索查询中选择。如果研究人员检查产前期间的环境化学物质暴露,并测量胎盘或脐带血中的化学物质和/或暴露相关影响,则包括示例研究。根据环境化学物质与产前暴露引起的不良反应的已知关系,选择了一个环境化学物质子集作为本综述的重点,作为重要的案例研究。此外,本综述评论了环境暴露科学和妊娠健康结果领域的关键数据差距和未来研究方向。
2. Environmental chemicals measured in placenta and cord blood
2. 胎盘和脐带血中测量的环境化学物质
Certain classes of environmental chemicals have been shown to travel via
maternal circulation and through the placental barrier, impacting the internal
environment of pregnant mothers and developing fetuses. Important examples of these
chemical classes are provided below and include flame retardants, metals, pesticide
and herbicides, per- and polyfluoroalkyl substances (PFAS), as well as naturally
occurring toxins. Though other types of environmental chemicals may be present
within the prenatal exposome, these specific chemical classes were selected for
inclusion in this review as example chemicals with published evidence supporting
their presence within human placenta and/or cord blood.
某些类别的环境化学物质已被证明会通过母体循环和胎盘屏障传播,影响怀孕母亲和发育中的胎儿的内部环境。下面提供了这些化学类别的重要示例,包括阻燃剂、金属、杀虫剂和除草剂、全氟烷基和多氟烷基物质 (PFAS) 以及天然存在的毒素。尽管产前暴露组中可能存在其他类型的环境化学物质,但这些特定的化学类别被选为本综述的实例化学物质,并已发表证据支持它们存在于人体胎盘和/或脐带血中。
2.1. Flame retardants 2.1. 阻燃剂
Flame retardants represent a wide group of chemicals that are commonly
used within the indoor environment for the purpose of reducing the potential for
materials to ignite [45]. These chemicals
are commonly used in building materials, bedding, clothing, and furniture.
Exposure to flame retardants can occur dermally as well as via inhalation, for
instance through resuspension of chemicals in household dust [46]. Flame retardant exposure has been linked to a
variety of health consequences, including endocrine disruption, immune system
repression, and cancer [45,47,48]. Of particular interest, prenatal exposures to flame retardants
have been linked to adverse effects in pregnant mothers and in developing
fetuses, including impaired neurologic function, endocrine disruption, and low
birth weight for gestational age [45,48,49].
阻燃剂是室内环境中常用的一大类化学物质,目的是减少材料点燃的可能性[45]。这些化学品通常用于建筑材料、床上用品、服装和家具。阻燃剂的暴露可以通过皮肤和吸入发生,例如通过将化学物质重新悬浮在室内灰尘中[46]。阻燃剂暴露与多种健康后果有关,包括内分泌紊乱、免疫系统抑制和癌症[45,47,48]。特别值得一提的是,产前暴露于阻燃剂与孕妇和发育中的胎儿的不良反应有关,包括神经功能受损、内分泌紊乱和胎龄前低出生体重[45,48,49]。
Flame retardants are generally classified by the elements they contain such as bromine, chlorine, phosphorus, nitrogen, boron, or metals. Polybrominated diphenyl ethers (PBDEs) are a class of flame retardants containing bromine that are persistent organic pollutants and have been widely studied in relation to prenatal exposure [46–48,50–52]. Though PBDEs have been banned in US commerce, PBDEs can persist in the environment causing exposures to remain of high relevance. After exposure, PBDEs act as lipophilic compounds and bind to lipoproteins, including those present in circulating plasma. Because of this binding, measurements of PBDEs in the plasma are often normalized to plasma lipoprotein levels [53]. PBDEs can additionally sequester into lipid heavy compartments such as adipose tissue [54] and mother’s milk [55].
PBDEs have been evaluated in placental tissue and cord blood [46,47,50], including one study that evaluated cord blood and maternal serum samples across 50 samples and detected PBDEs in every sample, with brominated diphenyl ether 209 accounting for approximately half of all total PBDEs among samples [46]. Several other studies have measured PBDEs in newborn cord blood and placenta and have identified strong correlations between these and maternal exposures measured from circulating serum [47,50,51]. Additionally, studies have shown relationships between PBDE exposure and adverse fetal health outcomes, including low birth weight and impaired neurodevelopment [48,52].
There is a general lack of data surrounding other classes of flame retardants specifically in relation to the prenatal exposome. For example, other classes of brominated flame retardants, such as tetrabromobisphenol A and hexabromocyclododecane, as well as organophosphate flame retardants, are thought to be possible carcinogens and may induce similar adverse health outcomes as PBDEs [45]. There are significantly fewer studies, however, measuring concentrations and toxicity responses of these chemicals in humans [45]. Because of the potential for associated prenatal toxicity, future research efforts aimed at evaluating this understudied chemical space will contribute to the current understanding of pregnancy outcomes associated with flame retardants.
2.2. Metals
Various toxic metals, including arsenic, cadmium, chromium, lead, and mercury, have been shown to cross the placenta and accumulate in fetal tissues following prenatal exposure conditions [56–59]. This occurs because metals are relatively stable compounds that can travel to distal target tissues following exposure. For example, inorganic arsenic (iAs) and its metabolites, monomethylated and dimethylated arsenicals, can be present at levels associated with toxicity in target tissues throughout the body [60,61], including the placenta and cord blood [56,61]. Cadmium has been detected throughout several target tissues, including maternal blood, placenta, and cord blood, with absorption and distribution shown to be highly dependent upon cadmium binding to metallothionein (MT) metal transporters [62]. Of particular relevance to the prenatal exposome, cadmium concentrations have been identified as increased in placental and fetal tissues from MT knockout mice exposed during late gestation [63]. A number of studies have detected and quantified the concentrations of toxic metals that reach the placenta and/or cord blood as a result of exposure during pregnancy, including those that have identified potential exposure biomarkers and related health outcomes.
As an example study that has investigated iAs throughout several tissues from pregnant women, researchers evaluating a birth cohort in New Hampshire measured iAs concentrations within human placenta samples and related these measures to iAs concentrations in maternal urine, and in maternal and newborn toenail samples [56]. Placental concentrations ranged from below detection limits to 18.35 ng/g and were positively correlated with iAs concentrations in newborn and maternal urine and toenails [56]. Cadmium has also been measured in placenta and newborn cord blood as reported by a study conducted in Croatia among smoking and non-smoking pregnant mothers [57]. The concentrations of cadmium were elevated in placenta and cord blood samples among mothers who smoked during pregnancy and ranged from 8.42 to 15.5 ng/g in the placenta and 0.025–0.054 ng/mL in the cord blood [57]. Mercury is another well-researched metal that has been evaluated in relation to prenatal environmental exposures and specifically measured in placenta and cord blood samples [58,59]. For instance, a cohort study conducted in Saudi Arabia found that 13 % of ~1500 cord blood samples had mercury concentrations above the EPA reference dose of 5.8 μg/L [58]. Increasing concentrations of mercury were found to be correlated with seafood consumption and mothers’ work status, among other potential factors [58]. These relevant studies measured the ability of various metals to cross the placenta and accumulate in fetal tissues and serve as a basis for future evaluation of associated health outcomes in the context of environmental exposures.
Specific linkages have been made between measures of prenatal exposures to metals and fetal health outcomes, including exposures to arsenic, cadmium, chromium, lead, and mercury [64–67]. For example, the effects of prenatal iAs exposure were evaluated in a pregnancy cohort located in Gómez Palacio, Mexico, and maternal urinary concentrations of iAs and select iAs metabolites showed negative associations with newborn birth weight, newborn length, and gestational age [68]. Further evaluation of newborn cord blood identified molecular signaling patterns that may drive certain mechanisms linking prenatal iAs exposure to fetal health outcomes [20,69]. The potential effects of prenatal exposure to cadmium have also been evaluated, with previous studies finding associations between concentrations in newborn cord blood and decreased newborn birth weight [57,70]. These represent select example studies from a pool of research on prenatal exposure to toxic metals that has been reviewed elsewhere [65,65,66,67,71,72]. Clearly there is evidence to support relationships between toxic metals exposures and adverse impacts on maternal and child health outcomes; though there are important aspects of metals research that remain understudied. Critical gaps in research include the impacts of metals as potentially beneficial nutrients versus toxic agents; individual metals that humans are routinely exposed to that still lack data; and metals as mixtures and/or components of complex mixtures within the environment.
2.3. Pesticides and herbicides
The increasing usage of pesticides and herbicides has resulted in the accumulation of these chemicals in ecological systems and other environments, causing increases in human exposures and potential related health outcomes. Maternal exposure to herbicides and pesticides classified as persistent organic pollutants (POPs) such as hexachlorocyclohexane (HCH), dichlorodiphenyltrichloroethane (DDT), and dichlorodiphenyldichloroethylene (DDE), among others, may pose a health risk to the developing fetus. Exposures to POPs such as DDT, DDE, or HCH are most likely to occur through consumption of fruits and vegetables, fatty meats, dairy, and fish, especially those imported from countries that allow the use of these chemicals [73]. These chemicals have been shown to reach placenta tissues and cross into cord blood, with potential associations with adverse fetal development and health outcomes among newborns, such as birth weight under 10th percentile for gestational age and decreased head and chest circumference [51,74–76].
Some studies have evaluated levels of pesticides and herbicides in placenta and cord blood. For instance, a birth cohort study conducted in Delhi, India measured and compared the concentrations of the organochlorine pesticides, HCH, DDT, and DDE, in placental tissue and cord blood between small for gestational age newborns and of appropriate size for gestational age newborns [74]. Total HCH concentration in placenta tissues among low birthweight babies averaged ~20 ng/mL; the concentration of DDT averaged ~2 ng/mL; and the concentration of DDE averaged ~4 ng/mL; all representing concentrations higher than those observed in the control newborns [74]. Similarly, a study within a cohort in Canada was able to measure concentrations of certain pesticide and herbicide POPs in cord blood including DDT, DDE, as well as oxychlordane [76]. The concentration of oxychlordane in cord blood was reported to reach up to 50 μg/kg [76]. These studies among others have provided critical evidence surrounding maternal exposures to certain POPs in pesticides and herbicides that are capable of reaching human placenta and cord blood tissues [51,74–76].
Other types of pesticides that are not currently considered POPs have also been evaluated, to an extent, in the context of prenatal exposures, including chlorpyrifos and glyphosate. Chlorpyrifos is an organophosphate pesticide and is presently one of the most widely used herbicides worldwide. Previous studies have associated prenatal exposure to chlorpyrifos to adverse birth outcomes such as decreased birth weight and length, as well as reduced sensory function and neurodevelopment [77–80]. An example study evaluating pesticide exposure among urban minorities in New York City reported concentrations of chlorpyrifos in newborn cord blood averaging 3.7 pg/g. These cord blood concentrations were significantly associated with concentrations in maternal blood, providing further evidence that chlorpyrifos is capable of crossing the placenta [77]. Glyphosate is currently the most used herbicide worldwide, however, fetal risks of exposure remain largely understudied in humans. Few studies have evaluated the ability of glyphosate to reach cross the placental barrier, however, one study in Thailand detected glyphosate in maternal serum and cord blood among farmworkers [81]. The wide use of pesticides and herbicides such as chlorpyrifos and glyphosate and their demonstrated potential to reach the fetus and impact health outcomes calls for further studies investigating these relationships.
2.4. PFAS
PFAS are a diverse chemical family of fluorine substituted organic structures that have been used for a wide variety of industrial applications in the United States and worldwide [82]. Only a small portion of PFAS have been evaluated for presence in target tissues relevant to prenatal exposure-induced toxicity, including the perfluoroalkyl acids, perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) [83]. However, over 3000 PFAS are estimated to be in global production [82]. Of timely relevance, a chemical category-based prioritization approach was recently developed by the US EPA to prioritize 75 PFAS for tiered toxicity and toxicokinetic testing based on relevance to human exposure, toxicity, and structural diversity [84]; though this prioritization effort has yet to be carried out with specific focus on the prenatal exposure space. While there is much attention now being focused on PFAS exposure and toxicity, there remains a limited understanding of how PFAS enter and affect the target tissues of relevance to maternal and fetal health. Some initial findings from recent studies are summarized below.
PFAS are known to be proteinophilic, associating with proteins such as albumin, fatty acid binding proteins, and organic anion transporters [85,86]. Because circulating blood contains many of these aforementioned proteins, PFAS can be detected at high concentrations in maternal blood, allowing for maternal circulation to carry PFAS to the placental interface where transfer into the fetal exposome occurs throughout pregnancy. The strong affinity of PFAS for maternal blood allows these substances to travel to the placenta and into cord blood circulation as is indicated by high correlations between maternal serum and cord blood serum [87,88]. Literature suggests transporters such as the breast cancer resistance protein (BCRP) can play a role in transfer of PFAS across the placental barrier, but overall very little is known concerning the mechanism of PFAS transfer from maternal circulation to fetal circulation [89]. Current data highlight that PFAS with linear structures are usually observed in biological fluids at higher concentrations in comparison to their matching branched isomers [90] and have been identified at higher concentrations in fetal serum relative to maternal serum [91]. This trend may reflect differences in efficiencies between compounds crossing the placenta [92], making PFAS uniquely structured to potentially accumulate in target tissues relevant to the fetal exposome. In addition, mothers with gestational diabetes have shown higher transfer efficiencies to cord serum for several PFAS compared to mothers without gestational diabetes [93]. PFAS concentrations have even been shown to increase in the fetal compartment throughout gestation [94], supporting the need for research to better understand potential implications of exposure on fetal health.
A variety of health-related endpoints have been evaluated in relation to PFAS accumulation in human cord blood and placenta with mixed results. For example, studies evaluating prenatal PFAS exposure in pregnancy cohorts in Canada have identified both significant [95] and non-significant [83] associations between PFAS concentrations in cord blood and infant birth weight. Potential alterations in hormonerelated signaling have also been evaluated in a few studies. For example, researchers evaluating a Japanese pregnancy cohort, containing 189 infant-mother pairs, identified associations between cord blood concentrations of PFAS and altered levels of cord blood hormones including estradiol, progesterone, and prolactin, and testosterone [96]. Studies carried out in Taiwan also identified significant relationships between PFAS and a number of endpoints in cord blood including thyroxine, thyroid stimulating hormone, and possible disruption in infant IgE levels [97,98].
Later-in-life outcomes have been evaluated in relation to PFAS to a limited extent and with mixed results. For instance, the study in Taiwan additionally investigated potential associations between cord blood PFAS and neurodevelopment at two years of age and identified decreased gross motor function associated with PFOS [99]. A recent systematic review also found associations between prenatal or early-life PFAS exposure and a range of outcomes including dyslipidemia, immunity (e.g., asthma and vaccine response), renal function, and age at menarche [100]. Other PFAS studies, though, found no associations between cord blood PFAS concentrations and a number of outcomes including attention deficit hyperactivity disorder [101], congenital cryptorchidism [102], and altered endocrine function assessed through circulating thyroid hormone levels [103]. Clearly, further research is needed to better understand prenatal PFAS exposure and its potential impacts on fetal growth and childhood development.
2.5. Toxins
Naturally occurring toxins in the environment can be a significant concern, as exposure to these chemicals can induce a variety of adverse health outcomes, inducing developmental toxicity resulting from exposures in utero [104–108]. Compared to the synthetic chemicals and metals previously discussed, studies on the presence of naturally occurring toxins in the prenatal exposome are relatively limited. This may be due to the severity of reactions to these exposures during pregnancy or a lack of understanding of the toxicity of toxins at low, chronic exposure levels. For example, ochratoxin A, a carcinogenic and neurotoxic food contaminant produced by Aspergillus and Penicillium, has been detected in human cord blood samples [109,110]. An example study carried out in Piacenza, Italy detected ochratoxin A in almost all cord blood samples (129 out of 130) evaluated [109]. The concentration of ochratoxin A was also significantly correlated with the participants’ intake of pork meat, soft drinks, and red wine.
Aflatoxins, a family of potent liver carcinogens also produced by Aspergillus, have also been assessed in pregnancy cohorts from multiple studies carried out in African countries. Fetal exposure to naturally occurring toxins is of particular interest for developing countries because of the challenges in food storage, both for human and livestock consumption, and the lack of testing and controls for these compounds. In terms of prevalence, aflatoxins M1, M2, B1, and B2 were detected in 12 % of cord blood samples analyzed in a cohort study conducted in Nigeria [71]. Aflatoxin-albumin adducts were also detected in cord blood samples collected from study participants located in Gambia [72–74]. Potential seasonal effects have been identified, with aflatoxins measured at significantly higher levels in cord blood samples collected in wet vs. dry months in Kenya [76]. Beyond African countries, aflatoxin B1–DNA adducts have been detected in placenta and cord blood samples from a cohort in Taiwan [75]. Such studies indicate the worldwide prevalence of toxins as environmental contaminants that can potentially impact the maternal and fetal exposome.
3. Current status of the environmental prenatal exposome
3.1. Chemical coverage in the prenatal exposome
There is evidence supporting relationships between prenatal exposure to select environmental chemicals and adverse pregnancy outcomes, as described above. Studies of environmental chemicals in cord blood and placenta samples have generally used targeted analytical approaches, wherein hypotheses related to few specific chemical contaminants are set a priori. These studies have employed mass spectrometry (MS) platforms for compound detection, coupled with gas chromatography (GC) or liquid chromatography (LC) systems for analyte separation. Low-resolution quadrupole (Q) mass analyzers (single or triple Q) generally provide adequate sensitivity and specificity for unambiguous analyte detection. Yet, in all instances of targeted analysis, chemical standards are needed to confirm the presence of each analyte (e.g., via matching of chromatographic retention time and mass spectrum), and to produce quantitative estimates of chemical concentration in the target tissue. This dependence on chemical standards has limited the number of environmental chemicals that have been characterized in cord blood and placenta samples.
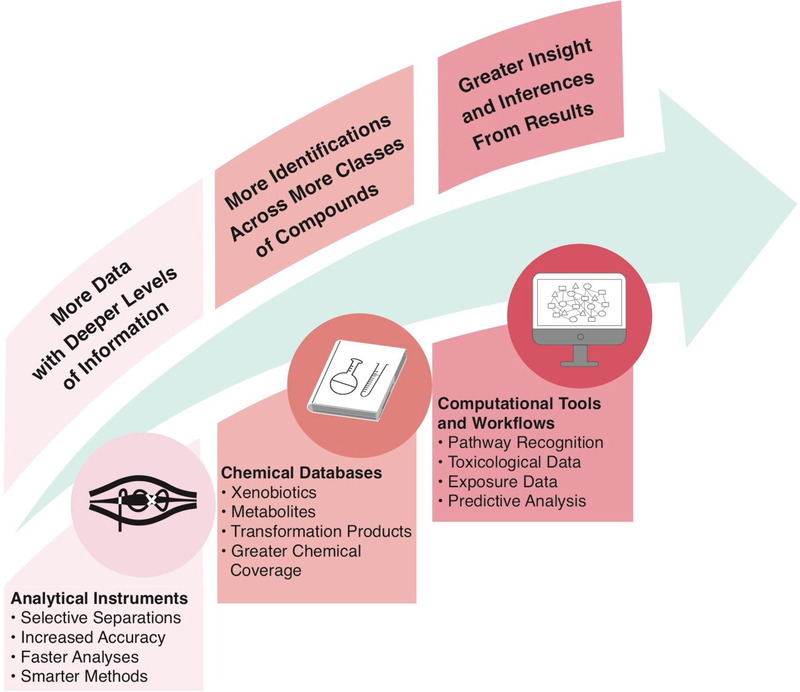
To improve the characterization of chemicals in the maternal and fetal exposomes, recent studies have implemented more global approaches based on “suspect screening” or “non-targeted analysis” methods [42,43]. By utilizing sophisticated analytical instrumentation, comprehensive chemical databases, and integrated computational workflows (Fig. 2), these complementary methods offer a means to rapidly examine poorly or never-before-studied compounds in high-interest samples [7,111]. In terms of evaluating the maternal/fetal exposome, studies have, to date, focused on the investigation of environmental chemicals in circulating maternal serum. As an important example, a recent study used a suspect screening approach based on liquid chromatography-quadrupole time-of-flight mass spectrometry (LC-QTOF/MS) to screen for environmental organic acids in maternal serum [43]. Researchers identified hundreds of potential environmental organic compounds among 20 samples, spanning phenols, phthalates, perfluorinated compounds, acidic pesticides, and related metabolites, the majority of which are not currently measured in large-scale biomonitoring studies. Targeted analyses confirmed the presence of benzophenone-1, bisphenol S, and monopentyl phthalate within maternal serum [43]. Similar methods were also employed by this research team to evaluate additional maternal serum samples, and an average of 56 suspect environmental organic compounds were reported per sample [42]. Six chemicals were then confirmed using targeted approaches: 2,4-Di-tert-butylphenol, 3,5-Di-tert-butylsalicylic acid, 2,4-Dinitrophenol, 4-Hydroxycoumarin, Pyrocatechol and 2′-Hydroxyacetophenone [42].
Fig. 2.
Integration of recent advances in analytical instrumentation, chemical databases, and computational tools and workflows to enable high-throughput chemical screening. The utilization of such integrative approaches will result in increased knowledge surrounding the fetal exposome.
These studies represent important examples of how researchers can use global analytical approaches that are less biased than targeted methods to characterize environmental chemicals relevant to prenatal exposures, with a focus on circulating maternal blood. Still, there remains a critical data gap surrounding which chemicals specifically reach the placental barrier and cross into the fetal circulation. As previously discussed, select chemicals have been evaluated in placenta and cord blood tissues using targeted approaches. Notable studies have evaluated hundreds of environmentally relevant chemicals in cord blood and infant meconium samples (for instance through the Maternal-Infant Research on Environmental Chemicals [MIREC] Research Platform) [76,112], but these studies have still utilized targeted analytical methods. Future studies could therefore leverage recent advances in analytical and computational methods that support suspect screening and non-targeted analysis to more fully characterize the fetal exposome.
3.2. Expanding methods to evaluate the environmental prenatal exposome
Mass spectrometry (MS) platforms are the primary tools for both suspect screening and non-targeted analysis applications [113]. Suspect screening utilizes reference spectra for individual analytes that have been previously acquired on an MS platform and stored in an accessible library. In suspect screening studies, empirical spectra are acquired for unknown “features” (i.e., an aggregation of associated m/z peaks) within a sample of interest, and then compared to library spectra to identify potential matches. All possible matches are scored based on the similarity between experimental and library spectra, with the best match (corroborated by manual review) often deemed a “tentative candidate” identification [114]. Additional (orthogonal) experimental data (e.g., retention behavior) may be used to elevate the tentative candidate to a “probable structure”, but examination using a reference standard is ultimately needed to list a feature of interest as a “confirmed structure” [114].
Suspect screening is a popular technique for exposomic investigations, as it can be implemented using both GC and LC separation, and both low- and high-resolution MS detection. Low-resolution MS is well-suited for suspect screening when using GC with an electron ionization (EI) source. Unlike “soft” ionization techniques, EI-MS generates rich fragmentation spectra that can be readily used to identify tentative candidates in samples of interest. Massive libraries of reference EI spectra exist for hundreds-of-thousands of known substances [115]. Thus, GC EI-MS applications have broad reach when screening for the presence of small molecules that are relatively volatile and non-polar (e.g., PBDEs and select pesticides/herbicides). Conversely, LC–MS techniques are best suited when seeking a chemical space that is less volatile, more polar, and inclusive of larger compounds (e.g., conjugated xenobiotic metabolites, hormones, lipids, peptides, and select environmental compounds, including PFAS). Unlike most GC-based methods, LC-based methods utilize softer ionization techniques (e.g., electrospray ionization [ESI], atmospheric pressure chemical ionization [APCI]) that produce the molecular ion with limited in-source fragmentation. Tandem mass spectrometry techniques are therefore used to generate fragmentation spectra (i.e., MS/MS spectra) that can be compared to library spectra for tentative compound identification. Similar numbers of reference spectra exist when comparing EI-MS and soft-ionization MS/MS libraries [115]. Yet, far fewer substances are represented in MS/MS libraries, owing to the variable nature of parameters used for spectral acquisition.
In principle, suspect screening works best when reference spectra exist for compounds of interest in an accessible library. Yet, exposome studies routinely show the existence of large numbers of empirical features that do not match with library entries; these unmatched features are described as the “dark matter” of the exposome [115,116]. Non-targeted analysis is the appropriate technique for proposing formulae and eventually structures for these dark matter compounds. Whether using GC or LC separation, non-targeted analysis relies on high-resolution mass spectrometry (HRMS) with soft ionization techniques. Here, the accurate mass, isotope profile, and available fragmentation spectra corresponding to an unknown feature is first used to proffer one or more candidate molecular formulae for that feature. Next, databases of known chemical structures are queried using the tentatively assigned formula and/or the observed accurate mass. Individual databases like PubChem [117] and ChemSpider [118] contain structures for millions of known compounds, whereas the CompTox Chemicals Dashboard (hereafter called the “Dashboard”) is “a one-stopshop for chemistry, toxicity and exposure information for over 875,000 chemicals” of interest to the US EPA [119]. Specific aspects of the Dashboard have been specifically developed to facilitate non-targeted analysis and help improve data organization, integration, and interpretation, making it a valuable resource for the exposomics community [119]. Articles describing this functionality and demonstrating enhancements to specific non-targeted analysis studies can be found in the published literature [7,120–122].
While non-targeted analysis can theoretically allow identification of millions of structures, many MS features remain unidentified even after exhaustive examination. Some of these unidentified features are by-products of the analytical tools (e.g., adducts, multimers and in-source fragments) and may be partially addressed using stringent data filtering procedures [123]. Others are truly unknown substances that may be produced from environmental transformation or degradation processes, or metabolically-aided processes that occur within biological systems. Various computational tools exist that allow prediction of transformation products and/or metabolites given a list of expected parent compounds [124]. Other tools exist that allow prediction of theoretical spectra for these anticipated products/metabolites [125,126]. When used together, these tools enable the identification of truly novel compounds in priority media and can help shed light on the dark matter of the human exposome.
3.3. Linking chemical exposures to toxicological responses and disease outcomes
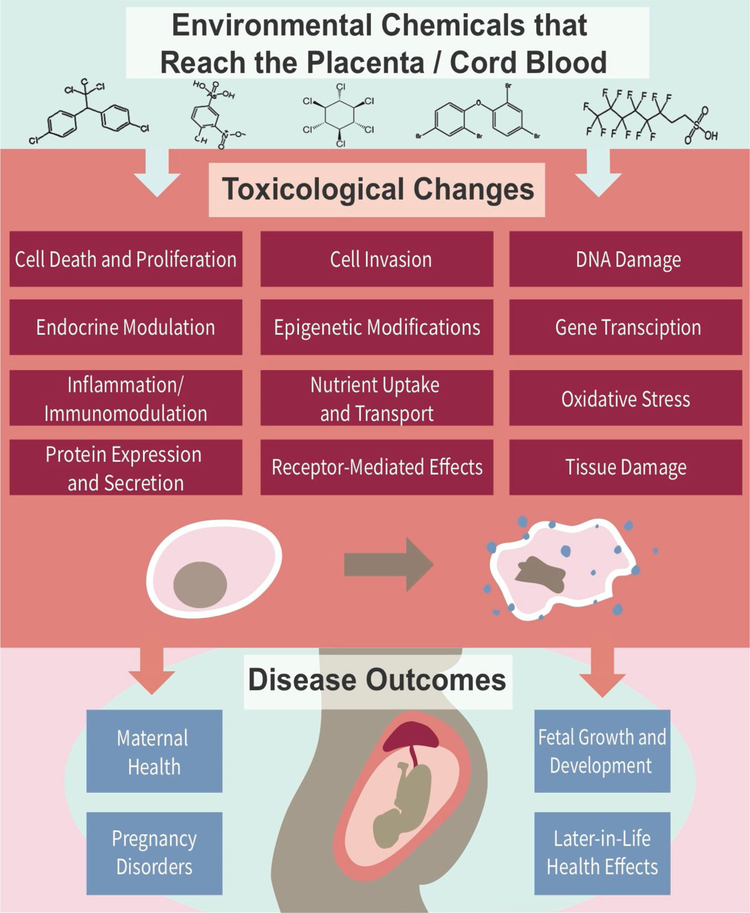
Many studies that have evaluated environmental chemicals reaching the prenatal exposome have focused on the targeted measurement of these chemicals, with some studies linking chemical measurements to disease outcomes. However, fewer studies have made full linkages between: (i) environmental measures, (ii) maternal/fetal health outcomes, and (iii) biological mechanisms of toxicity mechanisms underlying potential health outcomes. A more comprehensive understanding of how environmental exposures impact maternal and child health can be achieved by linking measured environmental chemicals that cross human placenta and cord blood with toxicological changes, elucidating mechanisms linking exposure to disease, and identifying biomarkers of exposure associated with adverse maternal and fetal health outcomes (Fig. 3). Select studies reviewed here implemented these types of approaches and aimed to anchor exposures to toxicological responses and disease outcomes.
Fig. 3.
Example toxicological endpoints and disease outcomes that can be related to measures of the prenatal exposome to better understand relationships and mechanisms underlying environmental exposure-induced disease.
Example studies that have evaluated prenatal exposure to iAs include research conducted in the Ron Pibul and Bangkok districts of Thailand, and in Gómez Palacio, Mexico that have associated internal exposure metrics to toxicological mechanisms of iAs-induced disease [20,127]. These studies related maternal iAs exposure through drinking water to altered gene expression profiles in newborn cord blood and related these to associated signaling pathways relevant to iAs-induced disease. More specifically, in Thailand, 11 transcripts in newborn cord blood were determined to be predictive biomarkers for iAs exposure and correlated with biological pathways including cell death, cell signaling, inflammation, and other stress responses [20,127]. Similarly, among the cohort in Mexico, iAs concentrations in maternal urine samples were found to be associated with differential expression of 334 transcripts, a portion of which were predicted to be regulated by epigenetic mediators. Pathway analysis resulted in the identification of similar signaling alterations including those associated with stress, inflammation, and cytokine activity. Interestingly, this study also found a decrease in the expression levels of genes involved in immune response, with general immunosuppression known to play a role known in diseases relevant to iAs exposure, such as increased risk of infectious disease and cancer [20].
In general, there are few studies that have employed mechanistic toxicology approaches to connect prenatal exposures to molecular mediators of disease. A recent example study evaluated the effects of prenatal exposure to PFAS and identified alterations in cord blood CpG methylation patterns within several genes, including those involved in endocrine system signaling, signal transduction, and immune signaling; though this research did not directly link these changes to observable health outcomes [128]. Future research that incorporates more toxicology-driven approaches, using in vitro, animal, and human study designs, can play an important role in elucidating how environmental exposures impact maternal and child health outcomes. The biological plausibility linking exposures to disease outcomes can be strengthened through in vitro methods aimed at identifying mechanisms of action, for instance through the systems biology-based approaches [129] and/or incorporation of recent genome editing technologies [130]. Indeed, many of the example chemicals discussed in this review have been examined across several models, including in vitro systems, and therefore have more robust information linking them to plausible adverse maternal and fetal health outcomes. Chemicals that are less studied, however, could significantly benefit from the inclusion of mechanistic toxicity studies, derived from several models, as well as human tissues. Such mechanistic-based studies will greatly contribute to the available evidence surrounding prenatal chemical exposures, toxicity responses, and adverse health outcomes.
3.4. Other limitations and future directions
The prenatal exposome represents an exciting area of investigation that is rapidly expanding to better address maternal and fetal health impacted by environmental chemicals. As reviewed above, there are many gaps that persist within this research field, including current limitations surrounding: (i) chemical coverage, (ii) anchorage of environmental monitoring data to toxicological endpoints, and (iii) related disease outcomes. Additionally, there remain limited data surrounding the temporality of exposome measures. Limited studies have evaluated how environmental chemicals and/or biomarkers of exposure change over time, particularly in biological matrices relevance to prenatal exposures and perinatal health. For example, the placenta represents a temporary organ that undergoes profound and dynamic changes in molecular expression, cell differentiation, structure, and function during pregnancy; causing the accumulation and impact of environmental chemicals on this organ to also change over time [62]. The majority of studies, to date, have focused on the evaluation of biological samples at a single point in time (i.e., biased towards time of infant birth). Studies could leverage the use of animal models or select alternative in vitro models to obtain information surrounding placenta and cord blood responses throughout pregnancy. In humans, other biological tissues could be used to inform issues surrounding temporality, including maternal blood and urine.
Data are also limited surrounding later-in-life health effects that could result from prenatal exposure conditions, with most studies evaluating effects during pregnancy or at birth. These obstetric outcomes are still critical endpoints to evaluate, as many of these are also associated with later-in-life health effects (e.g., associations between preeclampsia / pre-term birth and suboptimal childhood neurodevelopment [131]). The influence of other factors that play a role in chemicals reaching the placenta/fetus, including genetics and sex-specific traits, also represents an important data gap. Other factors that potentially interact with and influence the prenatal exposome remain understudied, including nutrition and socioeconomic status. These research topics represent important areas that could be addressed in future studies through the implementation of aforementioned advances in analytical chemistry approaches, expanded epidemiological measures, toxicological endpoints, and temporal influences on exposures and disease outcomes.
4. Conclusion
In conclusion, this review provides an overview on environmental chemicals representing the prenatal exposome. Emphasis is placed on chemicals reaching the human placenta and cord blood, as these represent important target tissues that heavily regulate maternal and fetal health outcomes. Current studies have shown that certain classes of chemicals, including flame retardants, metals, pesticides and herbicides, PFAS, and toxins, have the potential to impact the maternal and fetal exposome. However, there is a lack of knowledge surrounding the global space of chemicals that may reach the human placenta and cord blood that could be addressed through non-targeted / suspect screening analytical approaches. Further studies are also needed to clearly link chemical exposures to potential adverse pregnancy and developmental toxicity outcomes. Advances in this field of research will significantly contribute towards the understanding of environmental chemicals in the fetal exposome and their impact on maternal and fetal health.
Acknowledgement
This research was supported by grants from the National Institute of Environmental Health Sciences (P30ES010126, P42ES005948, R01-MD011609, K24-ES031131).
Abbreviations
- DOHaD
Developmental Origins of Health and Disease
- SLC6A2
Solute Carrier Family 6 Member 2
- SLC22A5
Solute Carrier Family 22 Member 5
- ABCG2
ATP Binding Cassette Subfamily G Member 2
- ABCB1
ATP Binding Cassette Subfamily B Member 1
- PFAS
Per- and Polyfluoroalkyl Substances
- PBDEs
Polybrominated diphenyl ethers
- iAs
Inorganic Arsenic
- POPs
Persistent Organic Pollutants
- HCH
Hexachlorocyclohexane
- DDT
Dichlorodiphenyltrichloroethane
- DDE
Dichlorodiphenyldichloroethylene
- PFOS
Perfluorooctane Sulfonate
- PFOA
Perfluorooctanoic Acid
- MS
Mass Spectrometry
- GC
Gas Chromatography
- LC
Liquid Chromatography
- Q
Quadrupole
- LC-QTOF/MS
Liquid Chromatography-Quadrupole Time-Of-Flight Mass Spectrometry
- MIREC
Maternal-Infant Research on Environmental Chemicals
- EI
Electron Ionization
- ESI
Electrospray Ionization
- APCI
tmospheric Pressure Chemical Ionization
- HRMS
High-Resolution Mass Spectrometry
Footnotes
Declaration of Competing Interest
The authors have no conflict of interest.
References
- [1].Wild CP, Complementing the Genome With an “exposome”: the Outstanding Challenge of Environmental Exposure Measurement in Molecular Epidemiology, AACR, 2005. [DOI] [PubMed] [Google Scholar]
- [2].Rappaport SM, Smith MT, Epidemiology. Environment and disease risks, Science 330 (6003) (2010) 460–461 Epub 2010/10/23. doi: 10.1126/science.1192603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wild CP, The exposome: from concept to utility, Int. J. Epidemiol. 41 (1) (2012) 24–32. [DOI] [PubMed] [Google Scholar]
- [4].Briggs D, Environmental pollution and the global burden of disease, Br. Med. Bull. 68 (2003) 1–24, 10.1093/bmb/ldg019Epub2004/02/06, [DOI] [PubMed] [Google Scholar]
- [5].Egeghy PP, Judson R, Gangwal S, Mosher S, Smith D, Vail J, Cohen Hubal EA, The exposure data landscape for manufactured chemicals, Sci. Total Environ. 414 (2012) 159–166 Epub 2011/11/23. doi: 10.1016/j.scitotenv.2011.10.046. [DOI] [PubMed] [Google Scholar]
- [6].Judson R, Richard A, Dix DJ, Houck K, Martin M, Kavlock R, Dellarco V, Henry T, Holderman T, Sayre P, Tan S, Carpenter T, Smith E, The toxicity data landscape for environmental chemicals, Environ. Health Perspect. 117 (5) (2009) 685–695 Epub 2009/05/30. doi: 10.1289/ehp.0800168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sobus JR, Wambaugh JF, Isaacs KK, Williams AJ, McEachran AD, Richard AM, Grulke CM, Ulrich EM, Rager JE, Strynar MJ, Newton SR, Integrating tools for non-targeted analysis research and chemical safety evaluations at the US EPA, J. Expo. Sci. Environ. Epidemiol 28 (5) (2018) 411–426 Epub 2017/12/31. doi: 10.1038/s41370-017-0012-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rager JE, Strynar MJ, Liang S, McMahen RL, Richard AM, Grulke CM, Wambaugh JF, Isaacs KK, Judson R, Williams AJ, Sobus JR, Linking high resolution mass spectrometry data with exposure and toxicity forecasts to advance high-throughput environmental monitoring, Environ. Int 88 (2016) 269–280 Epub 2016/01/27. doi: 10.1016/j.envint.2015.12.008. [DOI] [PubMed] [Google Scholar]
- [9].Moschet C, Anumol T, Lew BM, Bennett DH, Young TM, Household dust as a repository of chemical accumulation: new insights from a comprehensive high-resolution mass spectrometric study, Environ. Sci. Technol. 52 (5) (2018) 2878–2887 Epub 2018/02/14. doi: 10.1021/acs.est.7b05767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ouyang X, Weiss JM, de Boer J, Lamoree MH, Leonards PEG, Non-target analysis of household dust and laundry dryer lint using comprehensive two-dimensional liquid chromatography coupled with time-of-flight mass spectrometry, Chemosphere. 166 (2017) 431–437 Epub 2016/10/06. doi: 10.1016/j.chemosphere.2016.09.107. [DOI] [PubMed] [Google Scholar]
- [11].Rostkowski P, Haglund P, Aalizadeh R, Alygizakis N, Thomaidis N, Arandes JB, Nizzetto PB, Booij P, Budzinski H, Brunswick P, Covaci A, Gallampois C, Grosse S, Hindle R, Ipolyi I, Jobst K, Kaserzon SL, Leonards P, Lestremau F, Letzel T, Magner J, Matsukami H, Moschet C, Oswald P, Plassmann M, Slobodnik J, Yang C, The strength in numbers: comprehensive characterization of house dust using complementary mass spectrometric techniques, Anal. Bioanal. Chem. 411 (10) (2019) 1957–1977 Epub 2019/03/05. doi: 10.1007/s00216-019-01615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Newton SR, McMahen RL, Sobus JR, Mansouri K, Williams AJ, McEachran AD, Strynar MJ, Suspect screening and non-targeted analysis of drinking water using point-of-use filters, Environ Pollut. 234 (2018) 297–306 Epub 2017/11/29. doi: 10.1016/j.envpol.2017.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Brunner AM, Baken KA Dingemans MML, van Wezel AP, Prioritizing anthropogenic chemicals in drinking water and sources through combined use of mass spectrometry and ToxCast toxicity data, J. Hazard. Mater. 364 (2019) 332–338 Epub 2018/11/02. doi: 10.1016/j.jhazmat.2018.10.044. [DOI] [PubMed] [Google Scholar]
- [14].Kimura SY, Cuthbertson AA, Byer JD, Richardson SD, The DBP exposome: development of a new method to simultaneously quantify priority disinfection by-products and comprehensively identify unknowns, Water Res. 148 (2019) 324–333 Epub 2018/11/06. doi: 10.1016/j.watres.2018.10.057. [DOI] [PubMed] [Google Scholar]
- [15].Phillips KA, Yau A, Favela KA, Isaacs KK, McEachran A, Grulke C, Richard AM, Williams AJ, Sobus JR, Thomas RS, Wambaugh JF, Suspect screening analysis of chemicals in consumer products, Environ. Sci. Technol. 52 (5) (2018) 3125–3135 Epub 2018/02/07. doi: 10.1021/acs.est.7b04781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bessonneau V, Pawliszyn J, Rappaport SM, The saliva exposome for monitoring of individuals’ health trajectories, Environ. Health Perspect. 125 (7) (2017) 077014 Epub 2017/07/27. doi: 10.1289/EHP1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rappaport SM, Barupal DK, Wishart D, Vineis P, Scalbert A, The blood exposome and its role in discovering causes of disease, Environ. Health Perspect. 122 (8) (2014) 769–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Andra SS, Austin C, Arora M, The tooth exposome in children’s health research, Curr. Opin. Pediatr 28 (2) (2016) 221–227 Epub 2016/02/10. doi: 10.1097/MOP.0000000000000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Robinson DP, Klein SL, Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis, Horm. Behav 62 (3) (2012) 263–271 Epub 2012/03/13. doi: 10.1016/j.yhbeh.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rager JE, Bailey KA, Smeester L, Miller SK, Parker JS, Laine JE, Drobna Z, Currier J, Douillet C, Olshan AF, Rubio-Andrade M, Styblo M, Garcia-Vargas G, Fry RC, Prenatal arsenic exposure and the epigenome: altered microRNAs associated with innate and adaptive immune signaling in newborn cord blood, Environ. Mol. Mutagen. 55 (3) (2014) 196–208 Epub 2013/12/12. doi: 10.1002/em.21842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mattison DR, Environmental exposures and development, Curr. Opin. Pediatr 22 (2) (2010) 208–218 Epub 2010/03/11. doi: 10.1097/MOP.0b013e32833779bf. PubMed PMID: 20216314; PMCID: PMC2887611.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kourtis AP, Read JS, Jamieson DJ, Pregnancy and infection, N. Engl. J. Med 370 (23) (2014) 2211–2218 Epub 2014/06/05. doi: 10.1056/NEJMra1213566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sappenfield E, Jamieson DJ, Kourtis AP, Pregnancy and susceptibility to infectious diseases, Infect. Dis. Obstet. Gynecol. 2013 (2013) 752852 Epub 2013/08/13. doi: 10.1155/2013/752852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Brent RL, Tanski S, Weitzman M, A pediatric perspective on the unique vulnerability and resilience of the embryo and the child to environmental toxicants: the importance of rigorous research concerning age and agent, Pediatrics. 113 (4 Suppl) (2004) 935–944 [PubMed] [Google Scholar]
- [25].Falck AJ, Mooney S, Kapoor SS, White KM, Bearer C, El Metwally D, Developmental exposure to environmental toxicants, Pediatr. Clin. North Am 62 (5) (2015) 1173–1197 Epub 2015/09/01. doi: 10.1016/j.pcl.2015.05.005. [DOI] [PubMed] [Google Scholar]
- [26].Hoffman DJ, Reynolds RM, Hardy DB, Developmental origins of health and disease: current knowledge and potential mechanisms, Nutr. Rev 75 (12) (2017) 951–970 Epub 2017/12/01. doi: 10.1093/nutrit/nux053. [DOI] [PubMed] [Google Scholar]
- [27].McKinney J, Rogers R, ES&T metal bioavailability, Environ. Sci. Technol. 26 (7) (1992) 1298–1299. [Google Scholar]
- [28].Aylward L, Hays S, LaKind J, Ryan J, Rapid communication: partitioning of persistent lipophilic compounds, including dioxins, between human milk lipid and blood lipid: an initial assessment, J. Toxicol. Environ. Health Part A 66 (1) (2003) 1–5. [DOI] [PubMed] [Google Scholar]
- [29].Han X, Snow TA, Kemper RA, Jepson GW, Binding of perfluorooctanoic acid to rat and human plasma proteins, Chem. Res. Toxicol. 16 (6) (2003) 775–781. [DOI] [PubMed] [Google Scholar]
- [30].Peters SA, Ungell AL, Dolgos H, Physiologically based pharmacokinetic (PBPK) modeling and simulation: applications in lead optimization, Curr. Opin. Drug Discov. Devel 12 (4) (2009) 509–518 [PubMed] [Google Scholar]
- [31].Conder JM, Hoke RA, Wolf Wd M.H. Russell, Buck RC, Are PFCAs bioaccumulative? A critical review and comparison with regulatory criteria and persistent lipophilic compounds, Environ. Sci. Technol. 42 (4) (2008) 995–1003. [DOI] [PubMed] [Google Scholar]
- [32].Frederiksen H, Skakkebaek NE, Andersson AM, Metabolism of phthalates in humans, Mol. Nutr. Food Res. 51 (7) (2007) 899–911. [DOI] [PubMed] [Google Scholar]
- [33].Kapraun DF, Wambaugh JF, Setzer RW, Judson RS, Empirical models for anatomical and physiological changes in a human mother and fetus during pregnancy and gestation, PLoS One 14 (5) (2019) e0215906 Epub 2019/05/03. doi: 10.1371/journal.pone.0215906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Needham LL, Grandjean P, Heinzow B, Jørgensen PJ, Nielsen F, Patterson DG Jr, Sjödin A, Turner WE, Weihe P, Partition of environmental chemicals between maternal and fetal blood and tissues, Environ. Sci. Technol. 45 (3) (2010) 1121–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Eshkoli T, Sheiner E, Ben-Zvi Z, Holcberg G, Drug transport across the placenta, Curr. Pharm. Biotechnol 12 (5) (2011) 707–714 [DOI] [PubMed] [Google Scholar]
- [36].Al-Enazy S, Ali S, Albekairi N, El-Tawil M, Rytting E, Placental control of drug delivery, Adv. Drug Deliv. Rev. 116 (2017) 63–72 Epub 2016/08/17. doi: 10.1016/j.addr.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Dock L, Rissanen RL, Vahter M, Demethylation and placental transfer of methyl mercury in the pregnant hamster, Toxicology 94 (1994) 131–142. [DOI] [PubMed] [Google Scholar]
- [38].Iqbal M, Audette MC, Petropoulos S, Gibb W, Matthews SG, Placental drug transporters and their role in fetal protection, Placenta. 33 (3) (2012) 137–142 Epub 2012/01/24. doi: 10.1016/j.placenta.2012.01.008. [DOI] [PubMed] [Google Scholar]
- [39].Bircsak KM, Gupta V, Yuen PY, Gorczyca L, Weinberger BI, Vetrano AM, Aleksunes LM, Genetic and dietary regulation of glyburide efflux by the human placental breast Cancer resistance protein transporter, J. Pharmacol. Exp. Ther 357 (1) (2016) 103–113 Epub 2016/02/07. doi: 10.1124/jpet.115.230185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Szilagyi JT, Gorczyca L, Brinker A, Buckley B, Laskin JD, Aleksunes LM, Placental BCRP/ABCG2 transporter prevents fetal exposure to the estrogenic mycotoxin zearalenone, Toxicol. Sci. (2018) Epub 2018/12/24. doi: 10.1093/toxsci/kfy303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Xiao J, Wang Q, Bircsak KM, Wen X, Aleksunes LM, In vitro screening of environmental chemicals identifies zearalenone as a novel substrate of the placental BCRP/ABCG2 transporter, Toxicol. Res. (Camb) 4 (3) (2015) 695–706 Epub 2015/06/09. doi: 10.1039/c4tx00147h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wang A, Gerona RR, Schwartz JM, Lin T, Sirota M, Morello-Frosch R, Woodruff TJ, A suspect screening method for characterizing multiple chemical exposures among a demographically diverse population of pregnant women in San Francisco, Environ. Health Perspect. 126 (7) (2018) 077009 Epub 2018/07/26. doi: 10.1289/EHP2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gerona RR, Schwartz JM, Pan J, Friesen MM, Lin T, Woodruff TJ, Suspect screening of maternal serum to identify new environmental chemical biomonitoring targets using liquid chromatography-quadrupole time-of-flight mass spectrometry, J. Expo. Sci. Environ. Epidemiol 28 (2) (2018) 101–108 Epub 2017/10/12. doi: 10.1038/jes.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wang A, Padula A, Sirota M, Woodruff TJ, Environmental influences on reproductive health: the importance of chemical exposures, Fertil. Steril. 106 (4) (2016) 905–929 Epub 2016/08/12. doi: 10.1016/j.fertnstert.2016.07.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sciences NIoEH. Flame Retardant Fact Sheet, (2016) [cited 2019 April 11]. Available from: https://www.niehs.nih.gov/health/materials/flame_retardants_508.pdf.
- [46].Frederiksen M, Thomsen M, Vorkamp K, Knudsen LE, Patterns and concentration levels of polybrominated diphenyl ethers (PBDEs) in placental tissue of women in Denmark, Chemosphere 76 (11) (2009) 1464–1469 Epub 2009/08/18. doi: 10.1016/j.chemosphere.2009.07.017. [DOI] [PubMed] [Google Scholar]
- [47].Frederiksen M, Thomsen C, Froshaug M, Vorkamp K, Thomsen M, Becher G, Knudsen LE, Polybrominated diphenyl ethers in paired samples of maternal and umbilical cord blood plasma and associations with house dust in a Danish cohort, Int. J. Hyg. Environ. Health 213 (4) (2010) 233–242 Epub 2010/05/18. doi: 10.1016/j.ijheh.2010.04.008. [DOI] [PubMed] [Google Scholar]
- [48].Eskenazi B, Chevrier J, Rauch SA, Kogut K, Harley KG, Johnson C, Trujillo C, Sjodin A, Bradman A, In utero and childhood polybrominated diphenyl ether (PBDE) exposures and neurodevelopment in the CHAMACOS study, Environ. Health Perspect. 121 (2) (2013) 257–262 Epub 2012/11/17. doi: 10.1289/ehp.1205597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Mitro SD, Johnson T, Zota AR, Cumulative chemical exposures during pregnancy and early development, Curr. Environ. Health Rep. 2 (4) (2015) 367–378 Epub 2015/09/06. doi: 10.1007/s40572-015-0064-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Antignac JP, Cariou R, Zalko D, Berrebi A, Cravedi JP, Maume D, Marchand P, Monteau F, Riu A, Andre F, Le Bizec B, Exposure assessment of French women and their newborn to brominated flame retardants: determination of tri- to deca- polybromodiphenylethers (PBDE) in maternal adipose tissue, serum, breast milk and cord serum, Environ Pollut. 157 (1) (2009) 164–173 Epub 2008/09/23. doi: 10.1016/j.envpol.2008.07.008. [DOI] [PubMed] [Google Scholar]
- [51].Polder A Muller MHB, Brynildsrud OB, Gronnestad R, Karimi M, Lie E, Manyilizu WB, Mdegela RH, Mokiti F, Murtadha M, Nonga HE, Skaare JU, Solhaug A, Lyche JL, Prenatal exposure to persistent organic pollutants in Northern Tanzania and their distribution between breast milk, maternal blood, placenta and cord blood, Environ. Res. 170 (2019) 433–442 Epub 2019/01/12. doi: 10.1016/j.envres.2018.12.026. [DOI] [PubMed] [Google Scholar]
- [52].Herbstman JB, Sjodin A, Kurzon M, Lederman SA, Jones RS, Rauh V, Needham LL, Tang D, Niedzwiecki M, Wang RY, Perera F, Prenatal exposure to PBDEs and neurodevelopment, Environ. Health Perspect. 118 (5) (2010) 712–719 Epub 2010/01/09. doi: 10.1289/ehp.0901340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Butt CM, Miranda ML, Stapleton HM, Development of an analytical method to quantify PBDEs, OH-BDEs, HBCDs, 2, 4, 6-TBP, EH-TBB, and BEH-TEBP in human serum, Anal. Bioanal. Chem. 408 (10) (2016) 2449–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Brown JF, Lawton RW, Polychlorinated biphenyl (PCB) partitioning between adipose tissue and serum, Bull. Environ. Contam. Toxicol. 33 (1) (1984) 277–280. [DOI] [PubMed] [Google Scholar]
- [55].Schecter A, Pavuk M, Päpke O, Ryan JJ, Birnbaum L, Rosen R, Polybrominated diphenyl ethers (PBDEs) in US mothers’ milk, Environ. Health Perspect. 111 (14) (2003) 1723–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Punshon T, Davis MA, Marsit CJ, Theiler SK, Baker ER, Jackson BP, Conway DC, Karagas MR, Placental arsenic concentrations in relation to both maternal and infant biomarkers of exposure in a US cohort, J. Expo. Sci. Environ. Epidemiol 25 (6) (2015) 599–603 Epub 2015/03/26. doi: 10.1038/jes.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Piasek M, Mikolic A, Sekovanic A, Sulimanec Grgec A, Jurasovic J, Cadmium in placenta- a valuable biomarker of exposure during pregnancy in biomedical research, J Toxicol Environ Health A. 77 (18) (2014) 1071–1074 Epub 2014/07/30. doi: 10.1080/15287394.2014.915779. [DOI] [PubMed] [Google Scholar]
- [58].Al-Saleh I, Shinwari N, Mashhour A, Mohamed Gel D, Rabah A, Heavy metals (lead, cadmium and mercury) in maternal, cord blood and placenta of healthy women, Int. J. Hyg. Environ. Health 214 (2) (2011) 79–101 Epub 2010/11/26. doi: 10.1016/j.ijheh.2010.10.001. [DOI] [PubMed] [Google Scholar]
- [59].Gilman CL, Soon R, Sauvage L, Ralston NV, Berry MJ, Umbilical cord blood and placental mercury, selenium and selenoprotein expression in relation to maternal fish consumption, J. Trace Elem. Med. Biol. 30 (2015) 17–24 Epub 2015/03/10. doi: 10.1016/j.jtemb.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Rager JE, Tilley SK, Tulenko SE, Smeester L, Ray PD, Yosim A, Currier JM, Ishida MC, Gonzalez-Horta Mdel C, Sanchez-Ramirez B, Ballinas-Casarrubias L, Gutierrez-Torres DS, Drobna Z, Del Razo LM, Garcia-Vargas GG, Kim WY, Zhou YH, Wright FA, Styblo M, Fry RC, Identification of novel gene targets and putative regulators of arsenic-associated DNA methylation in human urothelial cells and bladder cancer, Chem. Res. Toxicol. 28 (6) (2015) 1144–1155 Epub 2015/06/04. doi: 10.1021/tx500393y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Hall M, Gamble M, Slavkovich V, Liu X, Levy D, Cheng Z, van Geen A, Yunus M, Rahman M, Pilsner JR, Graziano J, Determinants of arsenic metabolism: blood arsenic metabolites, plasma folate, cobalamin, and homocysteine concentrations in maternal-newborn pairs, Environ. Health Perspect. 115 (10) (2007) 1503–1509 Epub 2007/10/17. doi: 10.1289/ehp.9906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Jacobo-Estrada T, Santoyo-Sanchez M, Thevenod F, Barbier O Cadmium Handling, Toxicity and molecular targets involved during pregnancy: lessons from experimental models, Int. J. Mol. Sci 18 (7) (2017) Epub 2017/07/25. doi: 10.3390/ijms18071590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Brako EE, Wilson AK, Jonah MM, Blum CA, Cerny EA, Williams KL, Bhattacharyya MH, Cadmium pathways during gestation and lactation in control versus metallothoinein 1,2-knockout mice, Toxicol. Sci. 71 (2) (2003) 154–163 Epub 2003/02/04. [DOI] [PubMed] [Google Scholar]
- [64].Patel NB, Xu Y, McCandless LC, Chen A, Yolton K, Braun J, Jones RL, Dietrich KN, Lanphear BP, Very low-level prenatal mercury exposure and behaviors in children: the HOME Study, Environ. Health 18 (1) (2019) 4 Epub 2019/01/11. doi: 10.1186/s12940-018-0443-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Wai KM, Mar O, Kosaka S, Umemura M, Watanabe C, Prenatal heavy metal exposure and adverse birth outcomes in Myanmar: a birth-cohort study, Int. J. Environ. Res. Public Health 14 (11) (2017) Epub 2017/11/04. doi: 10.3390/ijerph14111339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Bommarito PA, Martin E, Fry RC, Effects of prenatal exposure to endocrine disruptors and toxic metals on the fetal epigenome, Epigenomics 9 (3) (2017) 333–350 Epub 2017/02/25. doi: 10.2217/epi-2016-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Bank-Nielsen PI, Long M, Bonefeld-Jorgensen EC, Pregnant inuit women’s exposure to metals and association with fetal growth outcomes: ACCEPT 2010(−) 2015, Int. J. Environ. Res. Public Health 16 (7) (2019) Epub 2019/04/04. doi: 10.3390/ijerph16071171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Laine JE, Bailey KA, Rubio-Andrade M, Olshan AF, Smeester L, Drobna Z, Herring AH, Styblo M, Garcia-Vargas GG, Fry RC, Maternal arsenic exposure, arsenic methylation efficiency, and birth outcomes in the Biomarkers of Exposure to ARsenic (BEAR) pregnancy cohort in Mexico, Environ. Health Perspect. 123 (2) (2015) 186–192 Epub 2014/10/18. doi: 10.1289/ehp.1307476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Rojas D, Rager JE, Smeester L, Bailey KA, Drobna Z, Rubio-Andrade M, Styblo M, Garcia-Vargas G, Fry RC, Prenatal arsenic exposure and the epigenome: identifying sites of 5-methylcytosine alterations that predict functional changes in gene expression in newborn cord blood and subsequent birth outcomes, Toxicol. Sci. 143 (1) (2015) 97–106 Epub 2014/10/12. doi: 10.1093/toxsci/kfu210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Salpietro CD, Gangemi S, Minciullo PL, Briuglia S, Merlino MV, Stelitano A, Cristani M, Trombetta D, Saija A, Cadmium concentration in maternal and cord blood and infant birth weight: a study on healthy non-smoking women, J. Perinat. Med 30 (5) (2002) 395–399 Epub 2002/11/22. doi: 10.1515/JPM.2002.061. [DOI] [PubMed] [Google Scholar]
- [71].Gundacker C, Hengstschlager M, The role of the placenta in fetal exposure to heavy metals, Wien. Med. Wochenschr 162 (9–10) (2012) 201–206 Epub 2012/06/22. doi: 10.1007/s10354-012-0074-3. [DOI] [PubMed] [Google Scholar]
- [72].Rahman A, Kumarathasan P, Gomes J, Infant and mother related outcomes from exposure to metals with endocrine disrupting properties during pregnancy, Sci. Total Environ. 569–570 (2016) 1022–1031 Epub 2016/07/06. doi: 10.1016/j.scitotenv.2016.06.134. [DOI] [PubMed] [Google Scholar]
- [73].Registry AfTSaD. Public Health Statement for DDT, DDE, and DDD Center for Disease Control and Prevention, (2002) [cited 2019 April 15]. Available from: https://www.atsdr.cdc.gov/phs/phs.asp?id=79&tid=20.
- [74].Dewan P, Jain V, Gupta P, Banerjee BD, Organochlorine pesticide residues in maternal blood, cord blood, placenta, and breastmilk and their relation to birth size, Chemosphere 90 (5) (2013) 1704–1710 Epub 2012/11/13. doi: 10.1016/j.chemosphere.2012.09.083. [DOI] [PubMed] [Google Scholar]
- [75].Needham LL, Grandjean P, Heinzow B, Jorgensen PJ, Nielsen F, Patterson DG Jr., A. Sjodin, W.E. Turner, P. Weihe, Partition of environmental chemicals between maternal and fetal blood and tissues, Environ. Sci. Technol. 45 (3) (2011) 1121–1126 Epub 2010/12/21. doi: 10.1021/es1019614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Fisher M, Arbuckle TE, Liang CL, LeBlanc A, Gaudreau E, Foster WG, Haines D, Davis K, Fraser WD, Concentrations of persistent organic pollutants in maternal and cord blood from the maternal-infant research on environmental chemicals (MIREC) cohort study, Environ. Health 15 (1) (2016) 59 Epub 2016/05/05. doi: 10.1186/s12940-016-0143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Whyatt RM, Camann D, Perera FP, Rauh VA, Tang D, Kinney PL, Garfinkel R, Andrews H, Hoepner L, Barr DB, Biomarkers in assessing residential insecticide exposures during pregnancy and effects on fetal growth, Toxicol. Appl. Pharmacol. 206 (2) (2005) 246–254 Epub 2005/06/22. doi: 10.1016/j.taap.2004.11.027. [DOI] [PubMed] [Google Scholar]
- [78].Whyatt RM, Rauh V, Barr DB, Camann DE, Andrews HF, Garfinkel R, Hoepner LA, Diaz D, Dietrich J, Reyes A, Tang D, Kinney PL, Perera FP, Prenatal insecticide exposures and birth weight and length among an urban minority cohort, Environ. Health Perspect. 112 (10) (2004) 1125–1132 Epub 2004/07/09. doi: 10.1289/ehp.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Silver MK, Shao J, Ji C, Zhu B, Xu L, Li M, Chen M, Xia Y, Kaciroti N, Lozoff B, Meeker JD, Prenatal organophosphate insecticide exposure and infant sensory function, Int. J. Hyg. Environ. Health 221 (3) (2018) 469–478 Epub 2018/02/07. doi: 10.1016/j.ijheh.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Rauh VA, Garcia WE, Whyatt RM, Horton MK, Barr DB, Louis ED, Prenatal exposure to the organophosphate pesticide chlorpyrifos and childhood tremor, Neurotoxicology 51 (2015) 80–86 Epub 2015/09/20. doi: 10.1016/j.neuro.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Kongtip P, Nankongnab N, Phupancharoensuk R, Palarach C, Sujirarat D, Sangprasert S, Sermsuk M, Sawattrakool N, Woskie SR, Glyphosate and paraquat in maternal and fetal serums in Thai women, J. Agromed 22 (3) (2017) 282–289 Epub 2017/04/20. doi: 10.1080/1059924X.2017.1319315. [DOI] [PubMed] [Google Scholar]
- [82].KEMI S, Occurrence and Use of Highly Fluorinated Substances and Alternatives, Swedish Chemicals Agency Stockholm, Sweden, 2015. [Google Scholar]
- [83].Monroy R, Morrison K, Teo K, Atkinson S, Kubwabo C, Stewart B, Foster WG, Serum levels of perfluoroalkyl compounds in human maternal and umbilical cord blood samples, Environ. Res. 108 (1) (2008) 56–62. [DOI] [PubMed] [Google Scholar]
- [84].Patlewicz G, Richard AM, Williams AJ, Grulke CM, Sams R, Lambert J, Noyes PD, DeVito MJ, Hines RN, Strynar M, Guiseppi-Elie A, Thomas RS, A chemical category-based prioritization approach for selecting 75 per- and polyfluoroalkyl substances (PFAS) for tiered toxicity and toxicokinetic testing, Environ. Health Perspect. 127 (1) (2019) 14501 Epub 2019/01/12. doi: 10.1289/EHP4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].MacManus-Spencer LA, Tse ML, Hebert PC, Bischel HN, Luthy RG, Binding of Perfluorocarboxylates to serum albumin: a comparison of analytical methods, Anal. Chem. 82 (3) (2010) 974–981, 10.1021/ac902238u. [DOI] [PubMed] [Google Scholar]
- [86].Ng CA, Hungerbühler K, Bioconcentration of Perfluorinated Alkyl Acids: How Important Is Specific Binding? Environ. Sci. Technol. 47 (13) (2013) 7214–7223, 10.1021/es400981a. [DOI] [PubMed] [Google Scholar]
- [87].Glynn A, Berger U, Bignert A, Ullah S, Aune M, Lignell S, Darnerud PO, Perfluorinated alkyl acids in blood serum from primiparous women in Sweden: serial sampling during pregnancy and nursing, and temporal trends 1996–2010, Environ. Sci. Technol. 46 (16) (2012) 9071–9079. [DOI] [PubMed] [Google Scholar]
- [88].Porpora MG, Lucchini R, Abballe A, Ingelido AM, Valentini S, Fuggetta E, Cardi V, Ticino A, Marra V, Fulgenzi AR, Placental transfer of persistent organic pollutants: a preliminary study on mother-newborn pairs, Int. J. Environ. Res. Public Health 10 (2) (2013) 699–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Szilagyi JT, Composto-Wahler GM, Joseph LB, Wang B, Rosen T, Laskin JD, Aleksunes LM, Anandamide down-regulates placental transporter expression through CB2 receptor-mediated inhibition of cAMP synthesis, Pharmacol. Res. 141 (2019) 331–342, 10.1016/j.phrs.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Zhang Y, Beesoon S, Zhu L, Martin JW, Biomonitoring of perfluoroalkyl acids in human urine and estimates of biological half-life, Environ. Sci. Technol. 47 (18) (2013) 10619–10627. [DOI] [PubMed] [Google Scholar]
- [91].Gützkow KB, Haug LS, Thomsen C, Sabaredzovic A, Becher G, Brunborg G, Placental transfer of perfluorinated compounds is selective–a Norwegian Mother and Child sub-cohort study, Int. J. Hyg. Environ. Health 215 (2) (2012) 216–219. [DOI] [PubMed] [Google Scholar]
- [92].Kim S, Choi K, Ji K, Seo J, Kho Y, Park J, Kim S, Park S, Hwang I, Jeon J, Trans-placental transfer of thirteen perfluorinated compounds and relations with fetal thyroid hormones, Environ. Sci. Technol. 45 (17) (2011) 7465–7472. [DOI] [PubMed] [Google Scholar]
- [93].Eryasa B, Grandjean P, Nielsen F, Valvi D, Zmirou-Navier D, Sunderland E, Weihe P, Oulhote Y, Physico-chemical properties and gestational diabetes predict transplacental transfer and partitioning of perfluoroalkyl substances, Environ. Int 130 (2019) 104874,, 10.1016/j.envint.2019.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Mamsen LS, Björvang RD, Mucs D, Vinnars M-T, Papadogiannakis N, Lindh CH, Andersen CY, Damdimopoulou P, Concentrations of perfluoroalkyl substances (PFASs) in human embryonic and fetal organs from first, second, and third trimester pregnancies, Environ. Int 124 (2019) 482–492. [DOI] [PubMed] [Google Scholar]
- [95].Arbuckle TE, Kubwabo C, Walker M, Davis K, Lalonde K, Kosarac I, Wen SW, Arnold DL, Umbilical cord blood levels of perfluoroalkyl acids and polybrominated flame retardants, Int. J. Hyg. Environ. Health 216 (2) (2013) 184–194. [DOI] [PubMed] [Google Scholar]
- [96].Itoh S, Araki A, Mitsui T, Miyashita C, Goudarzi H, Sasaki S, Cho K, Nakazawa H, Iwasaki Y, Shinohara N, Association of perfluoroalkyl substances exposure in utero with reproductive hormone levels in cord blood in the Hokkaido Study on Environment and Children’s Health, Environ. Int 94 (2016) 51–59. [DOI] [PubMed] [Google Scholar]
- [97].Tsai M-S, Lin C-C, Chen M-H, Hsieh W-S, Chen P-C, Perfluoroalkyl substances and thyroid hormones in cord blood, Environ. Pollut. 222 (2017) 543–548. [DOI] [PubMed] [Google Scholar]
- [98].Wang I-J, Hsieh W-S, Chen C-Y, Fletcher T, Lien G-W, Chiang H-L, Chiang CF, Wu T-N, Chen P-C, The effect of prenatal perfluorinated chemicals exposures on pediatric atopy, Environ. Res. 111 (6) (2011) 785–791. [DOI] [PubMed] [Google Scholar]
- [99].Chen M-H, Ha E-H, Liao H-F, Jeng S-F, Su Y-N, Wen T-W, Lien G-W, Chen CY, Hsieh W-S, Chen P-C, Perfluorinated compound levels in cord blood and neurodevelopment at 2 years of age, Epidemiology 24 (6) (2013) 800–808. [DOI] [PubMed] [Google Scholar]
- [100].Rappazzo KM, Coffman E, Hines EP, Exposure to perfluorinated alkyl substances and health outcomes in children: a systematic review of the epidemiologic literature, Int. J. Environ. Res. Public Health 14 (7) (2017) 691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Ode A, Källén K, Gustafsson P, Rylander L, Jönsson BA, Olofsson P, Ivarsson SA, Lindh CH, Rignell-Hydbom A, Fetal exposure to perfluorinated compounds and attention deficit hyperactivity disorder in childhood, PLoS One 9 (4) (2014) e95891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Jensen DV, Christensen J, Virtanen HE, Skakkebæk NE, Main KM, Toppari J, Veje CW, Andersson A-M, Nielsen F, Grandjean P, No association between exposure to perfluorinated compounds and congenital cryptorchidism: a nested case–control study among 215 boys from Denmark and Finland, Reproduction. 147 (4) (2014) 411–417. [DOI] [PubMed] [Google Scholar]
- [103].Inoue K, Okada F, Ito R, Kato S, Sasaki S, Nakajima S, Uno A, Saijo Y, Sata F, Yoshimura Y, Perfluorooctane sulfonate (PFOS) and related perfluorinated compounds in human maternal and cord blood samples: assessment of PFOS exposure in a susceptible population during pregnancy, Environ. Health Perspect. 112 (11) (2004) 1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Belli P, Bellaton C, Durand J, Balleydier S, Milhau N, Mure M, Mornex JF, Benahmed M, Le Jan C, Fetal and neonatal exposure to the mycotoxin zearalenone induces phenotypic alterations in adult rat mammary gland, Food Chem. Toxicol. 48 (10) (2010) 2818–2826 Epub 2010/07/20. doi: 10.1016/j.fct.2010.07.012. [DOI] [PubMed] [Google Scholar]
- [105].Hilakivi-Clarke L, Cho E, Clarke R, Maternal genistein exposure mimics the effects of estrogen on mammary gland development in female mouse offspring, Oncol. Rep. 5 (3) (1998) 609–616 [DOI] [PubMed] [Google Scholar]
- [106].Szilagyi JT, Gorczyca L, Brinker A, Buckley B, Laskin JD, Aleksunes LM, Placental BCRP/ABCG2 transporter prevents fetal exposure to the estrogenic mycotoxin zearalenone, Toxicol. Sci. 168 (2) (2019) 394–404 Epub 2018/12/24. doi: 10.1093/toxsci/kfy303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Rogers EH, Zehr RD, Gage MI, Humpage AR, Falconer IR, Marr M, Chernoff N, The cyanobacterial toxin, cylindrospermopsin, induces fetal toxicity in the mouse after exposure late in gestation, Toxicon. 49 (6) (2007) 855–864 Epub 2007/02/13. doi: 10.1016/j.toxicon.2006.12.009. [DOI] [PubMed] [Google Scholar]
- [108].Wangikar PB, Dwivedi P, Sinha N, Effect in rats of simultaneous prenatal exposure to ochratoxin A and aflatoxin B1. I. Maternal toxicity and fetal malformations, Birth Defects Res. B Dev. Reprod. Toxicol 71 (6) (2004) 343–351 Epub 2004/12/24. doi: 10.1002/bdrb.20021. [DOI] [PubMed] [Google Scholar]
- [109].Biasucci G, Calabrese G, Di Giuseppe R, Carrara G, Colombo F, Mandelli B, Maj M, Bertuzzi T, Pietri A, Rossi F, The presence of ochratoxin A in cord serum and in human milk and its correspondence with maternal dietary habits, Eur. J. Nutr. 50 (3) (2011) 211–218 Epub 2010/09/03. doi: 10.1007/s00394-010-0130y. [DOI] [PubMed] [Google Scholar]
- [110].Jonsyn FE, Maxwell SM, Hendrickse RG, Human fetal exposure to ochratoxin A and aflatoxins, Ann. Trop. Paediatr 15 (1) (1995) 3–9 [DOI] [PubMed] [Google Scholar]
- [111].Hollender J, Schymanski EL, Singer HP, Ferguson PL, Nontarget Screening with High Resolution Mass Spectrometry in the Environment: Ready to Go? Environ. Sci. Technol. 51 (20) (2017) 11505–11512 Epub 2017/09/07. doi: 10.1021/acs.est.7b02184. [DOI] [PubMed] [Google Scholar]
- [112].Arbuckle TE, Fraser WD, Fisher M, Davis K, Liang CL, Lupien N, Bastien S, Velez MP, von Dadelszen P, Hemmings DG, Wang J, Helewa M, Taback S, Sermer M, Foster W, Ross G, Fredette P, Smith G, Walker M, Shear R, Dodds L, Ettinger AS, Weber JP, D’Amour M, Legrand M, Kumarathasan P, Vincent R, Luo ZC, Platt RW, Mitchell G, Hidiroglou N, Cockell K, Villeneuve M, Rawn DF, Dabeka R, Cao XL, Becalski A, Ratnayake N, Bondy G, Jin X, Wang Z, Tittlemier S, Julien P, Avard D, Weiler H, Leblanc A, Muckle G, Boivin M, Dionne G, Ayotte P, Lanphear B, Seguin JR, SaintAmour D, Dewailly E, Monnier P, Koren G, Ouellet E, Cohort profile: the maternal-infant research on environmental chemicals research platform, Paediatr. Perinat. Epidemiol 27 (4) (2013) 415–425 Epub 2013/06/19. doi: 10.1111/ppe.12061. [DOI] [PubMed] [Google Scholar]
- [113].Andra SS, Austin C, Patel D, Dolios G, Awawda M, Arora M, Trends in the application of high-resolution mass spectrometry for human biomonitoring: an analytical primer to studying the environmental chemical space of the human exposome, Environ. Int 100 (2017) 32–61 Epub 2017/01/08. doi: 10.1016/j.envint.2016.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Schymanski EL, Jeon J, Gulde R, Fenner K, Ruff M, Singer HP, Hollender J, Identifying small molecules via high resolution mass spectrometry: communicating confidence, Environ. Sci. Technol. 48 (4) (2014) 2097–2098 Epub 2014/01/31. doi: 10.1021/es5002105. [DOI] [PubMed] [Google Scholar]
- [115].Peisl BYL, Schymanski EL, Wilmes P, Dark matter in host-microbiome metabolomics: tackling the unknowns-A review, Anal. Chim. Acta 1037 (2018) 13–27 Epub 2018/10/08. doi: 10.1016/j.aca.2017.12.034. [DOI] [PubMed] [Google Scholar]
- [116].da Silva RR, Dorrestein PC, Quinn RA, Illuminating the dark matter in metabolomics, Proc. Natl. Acad. Sci. U. S. A. 112 (41) (2015) 12549–12550 Epub 2015/10/03. doi: 10.1073/pnas.1516878112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Kim S, Chen J, Cheng T, Gindulyte A, He J, He S, Li Q, Shoemaker BA, Thiessen PA, Yu B, Zaslavsky L, Zhang J, Bolton EE, PubChem 2019 update: improved access to chemical data, Nucleic Acids Res. 47 (D1) (2019) D1102–D9. Epub 2018/10/30. doi: 10.1093/nar/gky1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Williams AJ, ChemSpider, (2015).
- [119].Williams AJ, Grulke CM, Edwards J, McEachran AD, Mansouri K, Baker NC, Patlewicz G, Shah I, Wambaugh JF, Judson RS, Richard AM, The CompTox Chemistry Dashboard: a community data resource for environmental chemistry, J. Cheminform 9 (1) (2017) 61 Epub 2017/12/01. doi: 10.1186/s13321-017-02476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].McEachran AD, Sobus JR, Williams AJ, Identifying known unknowns using the US EPA’s CompTox chemistry dashboard, Anal. Bioanal. Chem. 409 (7) (2017) 1729–1735 Epub 2016/12/18. doi: 10.1007/s00216-016-0139-z. [DOI] [PubMed] [Google Scholar]
- [121].McEachran AD, Mansouri K, Grulke C, Schymanski EL, Ruttkies C, Williams AJ, MS-Ready” structures for non-targeted high-resolution mass spectrometry screening studies, J. Cheminform 10 (1) (2018) 45 Epub 2018/09/01. doi: 10.1186/s13321-018-0299-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Sobus JR, Grossman JN, Chao A, Singh R, Williams AJ, Grulke CM, Richard AM, Newton SR, McEachran AD, Ulrich EM, Using prepared mixtures of ToxCast chemicals to evaluate non-targeted analysis (NTA) method performance, Anal. Bioanal. Chem. 411 (4) (2019) 835–851 Epub 2019/01/07. doi: 10.1007/s00216-018-1526-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Mahieu NG, Patti GJ, Systems-level annotation of a metabolomics data set reduces 25000 features to fewer than 1000 unique metabolites, Anal. Chem. 89 (19) (2017) 10397–10406 Epub 2017/09/16. doi: 10.1021/acs.analchem.7b02380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Djoumbou-Feunang Y, Fiamoncini J, Gil-de-la-Fuente A, Greiner R, Manach C, Wishart DS, BioTransformer: a comprehensive computational tool for small molecule metabolism prediction and metabolite identification, J. Cheminform 11 (1) (2019) 2 Epub 2019/01/07. doi: 10.1186/s13321-018-0324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Blazenovic I, Kind T, Torbasinovic H, Obrenovic S, Mehta SS, Tsugawa H, Wermuth T, Schauer N, Jahn M, Biedendieck R, Jahn D, Fiehn O, Comprehensive comparison of in silico MS/MS fragmentation tools of the CASMI contest: database boosting is needed to achieve 93% accuracy, J. Cheminform 9 (1) (2017) 32 Epub 2017/11/01. doi: 10.1186/s13321-017-0219-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Schymanski EL, Ruttkies C, Krauss M, Brouard C, Kind T, Duhrkop K, Allen F, Vaniya A, Verdegem D, Bocker S, Rousu J, Shen H, Tsugawa H, Sajed T, Fiehn O, Ghesquiere B, Neumann S, Critical assessment of small molecule identification 2016: automated methods, J. Cheminform 9 (1) (2017) 22 Epub 2017/11/01. doi: 10.1186/s13321-017-0207-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Fry RC, Navasumrit P, Valiathan C, Svensson JP, Hogan BJ, Luo M, Bhattacharya S, Kandjanapa K, Soontararuks S, Nookabkaew S, Mahidol C, Ruchirawat M, Samson LD, Activation of inflammation/NF-kappaB signaling in infants born to arsenic-exposed mothers, PLoS Genet. 3 (11) (2007) e207 Epub 2007/11/28. doi: 10.1371/journal.pgen.0030207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Miura R, Araki A, Miyashita C, Kobayashi S, Kobayashi S, Wang S-L, Chen CH, Miyake K, Ishizuka M, Iwasaki Y, An epigenome-wide study of cord blood DNA methylations in relation to prenatal perfluoroalkyl substance exposure: the Hokkaido study, Environ. Int 115 (2018) 21–28. [DOI] [PubMed] [Google Scholar]
- [129].Rager JE, Fry RC, Systems biology and environmental exposures, in: Zhang W (Ed.), Network Biology, Nova Science Publishers, 2013, pp. 81–130. [Google Scholar]
- [130].Fry RC, Carberry C, Rager JE, Use of genome editing tools in environmental health research, Curr. Opin. Toxicol (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Tuovinen S, Eriksson JG, Kajantie E, Raikkonen K, Maternal hypertensive pregnancy disorders and cognitive functioning of the offspring: a systematic review, J. Am. Soc. Hypertens 8 (11) (2014) 832–847 e1. Epub 2014/12/03. doi: 10.1016/j.jash.2014.09.005. [DOI] [PubMed] [Google Scholar]