- 1Division of Nephrology, Department of Internal Medicine, People’s Hospital of Xinjiang Uygur Autonomous Region, Urumqi, China
1 新疆维吾尔自治区人民医院内科肾内科,中国乌鲁木齐 - 2Division of Nephrology, Department of Internal Medicine, The First Affiliated Hospital of Xinjiang Medical University, Urumqi, China
2 新疆医科大学第一附属医院内科肾内科,中国乌鲁木齐 - 3Section of Nephrology, Department of Internal Medicine, Yale University School of Medicine, New Haven, CT, United States
3 美国康涅狄格州纽黑文市耶鲁大学医学院内科肾脏科
The glomerular filtration barrier, comprising the inner layer of capillary fenestrated endothelial cells, outermost podocytes, and the glomerular basement membrane between them, plays a pivotal role in kidney function. Podocytes, terminally differentiated epithelial cells, are challenging to regenerate once injured. They are essential for maintaining the integrity of the glomerular filtration barrier. Damage to podocytes, resulting from intrinsic or extrinsic factors, leads to proteinuria in the early stages and eventually progresses to chronic kidney disease (CKD). Immune-mediated podocyte injury is a primary pathogenic mechanism in proteinuric glomerular diseases, including minimal change disease, focal segmental glomerulosclerosis, membranous nephropathy, and lupus nephritis with podocyte involvement. An extensive body of evidence indicates that podocytes not only contribute significantly to the maintenance of the glomerular filtration barrier and serve as targets of immune responses but also exhibit immune cell-like characteristics, participating in both innate and adaptive immunity. They play a pivotal role in mediating glomerular injury and represent potential therapeutic targets for CKD. This review aims to systematically elucidate the mechanisms of podocyte immune injury in various podocyte lesions and provide an overview of recent advances in podocyte immunotherapy. It offers valuable insights for a deeper understanding of the role of podocytes in proteinuric glomerular diseases, and the identification of new therapeutic targets, and has significant implications for the future clinical diagnosis and treatment of podocyte-related disorders.
肾小球滤过屏障包括毛细血管有孔内皮细胞内层、最外层足细胞和它们之间的肾小球基底膜,在肾功能中起关键作用。足细胞是终末分化的上皮细胞,一旦损伤就难以再生。它们对于维持肾小球滤过屏障的完整性至关重要。由内在或外在因素引起的足细胞损伤在早期阶段导致蛋白尿,并最终进展为慢性肾病(CKD)。免疫介导的足细胞损伤是蛋白尿性肾小球疾病的主要致病机制,包括微小病变疾病、局灶节段性肾小球硬化、膜性肾病和伴有足细胞受累的狼疮性肾炎。 大量证据表明,足细胞不仅对肾小球滤过屏障的维持有重要作用,并作为免疫应答的靶点,而且还表现出免疫细胞样特征,参与先天性和适应性免疫。它们在介导肾小球损伤中起关键作用,并代表CKD的潜在治疗靶点。本文旨在系统阐述足细胞免疫损伤在各种足细胞病变中的机制,并对足细胞免疫治疗的最新进展进行综述。它为更深入地了解足细胞在蛋白尿性肾小球疾病中的作用以及识别新的治疗靶点提供了有价值的见解,并对未来足细胞相关疾病的临床诊断和治疗具有重要意义。
1 Introduction 1引言
The glomerulus, a specialized unit within the kidney, serves as a crucial filtration system that eliminates waste products from the blood. Maintaining internal stability by clearing external and internally produced waste is its primary function. Its filtration structure relies on the intricate network of glomerular capillaries, comprising glomerular endothelial cells, the glomerular basement membrane (GBM), and glomerular podocytes, collectively forming the unique filtration barrier of the glomerulus (1). Any impairment in this barrier may result in protein loss. Notably, proteinuric kidney disease originating from glomerular dysfunction accounts for 80% of individuals who eventually progress to end-stage kidney disease (ESKD), necessitating kidney replacement therapy (2).
肾小球是肾脏内的一个专门单位,是一个重要的过滤系统,可以清除血液中的废物。通过清除外部和内部产生的废物来维持内部稳定是其主要功能。其过滤结构依赖于肾小球毛细血管的复杂网络,包括肾小球内皮细胞、肾小球基底膜(GBM)和肾小球足细胞,共同形成肾小球的独特过滤屏障(1)。这种屏障的任何损伤都可能导致蛋白质损失。值得注意的是,源于肾小球功能障碍的蛋白尿性肾病占最终进展为终末期肾病(ESKD)的个体的80%,需要肾脏替代治疗(2)。
Podocytes, the largest cells in the glomerulus, attach to the outer layer of the GBM. They consist of a cell body housing a nucleus, several prominent primary processes branching out from the cell, and secondary processes that further branch to form interdigitating foot processes (tertiary processes) on the GBM (3). The podocyte cytoskeleton plays a vital role in stabilizing its morphology and maintaining its biological function. It contributes to the integrity of the glomerular filtration barrier through interactions with cell-cell junctions and cell-matrix proteins (4). Podocytes can be categorized into three distinct membrane domains: the basal domain, the apical domain, and the intercellular/slit diaphragm domain. The slit diaphragm, a complex structure comprising multiple protein molecules, plays a pivotal role in regulating glomerular permeability, in coordination with the glomerular basement membrane and the glomerular endothelial cells. Key protein molecules within the slit diaphragm of podocytes include nephrin, neph1, neph2, CD2-associated protein(CD2AP), and podocin, among others (5). Furthermore, emerging evidence suggests that podocytes possess diverse functions beyond their mechanical supportive role, contributing to the innate and adaptive immune system to maintain glomerular health homeostasis (6). The immune malfunction of podocytes is considered to be associated with the pathogenesis of several common proteinuric glomerular diseases. Immune complexes, complement factors, various immune cells, and even the immune attributes of podocytes might contribute to the development of these proteinuric glomerular diseases. However, the intricate mechanisms of immune responses leading to podocyte injury remain incompletely understood. This review aims to consolidate current insights into the pathogenic mechanisms of immune responses triggering podocyte injury and the latest therapeutic strategies targeting the immune system.
足细胞是肾小球中最大的细胞,附着在GBM的外层。它们由一个细胞体、几个突出的初级突起和次级突起组成,初级突起从细胞中分支出来,次级突起进一步分支,在GBM上形成相互交错的足突(三级突起)(3)。足细胞的细胞骨架在稳定其形态和维持其生物学功能方面起着至关重要的作用。它通过与细胞-细胞连接和细胞-基质蛋白的相互作用促进肾小球滤过屏障的完整性(4)。足细胞可分为三个不同的膜结构域:基底结构域、顶端结构域和细胞间/缝隙隔膜结构域。狭缝隔膜是一种由多个蛋白质分子组成的复杂结构,与肾小球基底膜和肾小球内皮细胞协调,在调节肾小球渗透性方面发挥着关键作用。 足细胞裂孔隔膜内的关键蛋白质分子包括nephrin、neph1、neph2、CD2相关蛋白(CD2AP)和podocin等(5)。此外,新出现的证据表明,足细胞具有超越其机械支持作用的多种功能,有助于先天性和适应性免疫系统维持肾小球健康稳态(6)。足细胞的免疫功能障碍被认为与几种常见的蛋白尿性肾小球疾病的发病机制有关。免疫复合物,补体因子,各种免疫细胞,甚至足细胞的免疫属性可能有助于这些蛋白尿性肾小球疾病的发展。然而,导致足细胞损伤的免疫反应的复杂机制仍不完全清楚。 本文综述了足细胞损伤的免疫反应机制和针对免疫系统的最新治疗策略。
2 Immunological mechanisms in podocyte
2足细胞的免疫机制
2.1 Innate immune responses in podocytes
2.1足细胞中的天然免疫应答
An expanding body of evidence underscores the significant role of podocytes in the glomerular filtration barrier and their involvement in innate immune responses. Podocytes have been found to express various pattern recognition receptors (PRRs), including Toll-like Receptors (TLRs), enabling them to actively participate in innate immune responses by recognizing and eliminating foreign pathogens or endogenous danger signals (7). Notably, Toll-like Receptor 4 (TLR4) is a specific subtype capable of recognizing bacterial lipopolysaccharide (LPS) (8). Furthermore, exposure to a high-glucose milieu has been demonstrated to directly enhance the activation of TLR4. This activation is posited to contribute to the pathogenesis of podocyte damage and the subsequent progression of interstitial fibrosis (9). TLRs are present on the cell surface or intracellularly and are expressed by a range of cell types, including dendritic cells, macrophages, fibroblasts, B cells, T cells, as well as endothelial and epithelial cells (10). They play a pivotal role in recognizing pathogen-associated biomarkers (11). For instance, cell surface TLRs mainly recognize microbial membrane components like LPS, lipids, and proteins, while intracellular TLRs primarily detect bacterial and viral nucleic acids (12). However, it’s worth noting that some studies have reported that stimuli like puromycin aminonucleoside (PAN) can induce glomerular podocyte injury, increase Toll-like Receptor 9 (TLR9) expression, activate Nuclear factor kappa-B (NF-κB) and p38,mitogen-activated protein kinase (MAPK) signaling pathways, and potentially use endogenous mitochondrial DNA as a ligand. This results in damage to glomerular podocytes and leads to apoptosis, suggesting that TLRs can have a dual effect on podocytes—both protecting the integrity of the glomerular filtration barrier and potentially promoting glomerulosclerosis (13). Moreover, Masum and colleagues utilized BXSB/MpJ-Yaa (Yaa) mice to demonstrate that overexpression of TLR9 correlated with podocyte injury and the development of typical membranoproliferative glomerulonephritis (MPGN) lesions. In contrast, BXSB control mice, with weak TLR9 expression, showed no MPGN lesions. These findings suggest a potential role of TLR9 in podocyte injury and MPGN development (14).
越来越多的证据强调了足细胞在肾小球滤过屏障中的重要作用及其参与先天性免疫反应。已发现足细胞表达各种模式识别受体(PRR),包括Toll样受体(TLR),使其能够通过识别和消除外来病原体或内源性危险信号积极参与先天免疫反应(7)。值得注意的是,Toll样受体4(TLR4)是能够识别细菌脂多糖(LPS)的特定亚型(8)。此外,暴露于高葡萄糖环境已被证明直接增强TLR4的活化。这种激活被认为有助于足细胞损伤的发病机制和随后的间质纤维化进展(9)。 TLR存在于细胞表面或细胞内,并由一系列细胞类型表达,包括树突细胞、巨噬细胞、成纤维细胞、B细胞、T细胞以及内皮细胞和上皮细胞(10)。它们在识别病原体相关生物标志物方面发挥着关键作用(11)。例如,细胞表面TLR主要识别微生物膜组分如LPS、脂质和蛋白质,而细胞内TLR主要检测细菌和病毒核酸(12)。然而,值得注意的是,一些研究报道嘌呤霉素氨基糖苷(PAN)等刺激物可诱导肾小球足细胞损伤,增加Toll样受体9(TLR 9)表达,激活核因子κ-B(NF-κB)和p38、丝裂原活化蛋白激酶(MAPK)信号通路,并可能利用内源性线粒体DNA作为配体。 这导致肾小球足细胞损伤并导致细胞凋亡,表明TLR对足细胞具有双重作用-既保护肾小球滤过屏障的完整性,又可能促进肾小球硬化(13)。此外,Masum及其同事利用BXSB/MpJ-Yaa(Yaa)小鼠证明TLR 9的过表达与足细胞损伤和典型膜增生性肾小球肾炎(MPGN)病变的发展相关。相比之下,BXSB对照小鼠,TLR 9表达弱,没有显示MPGN病变。这些发现表明TLR 9在足细胞损伤和MPGN发展中的潜在作用(14)。
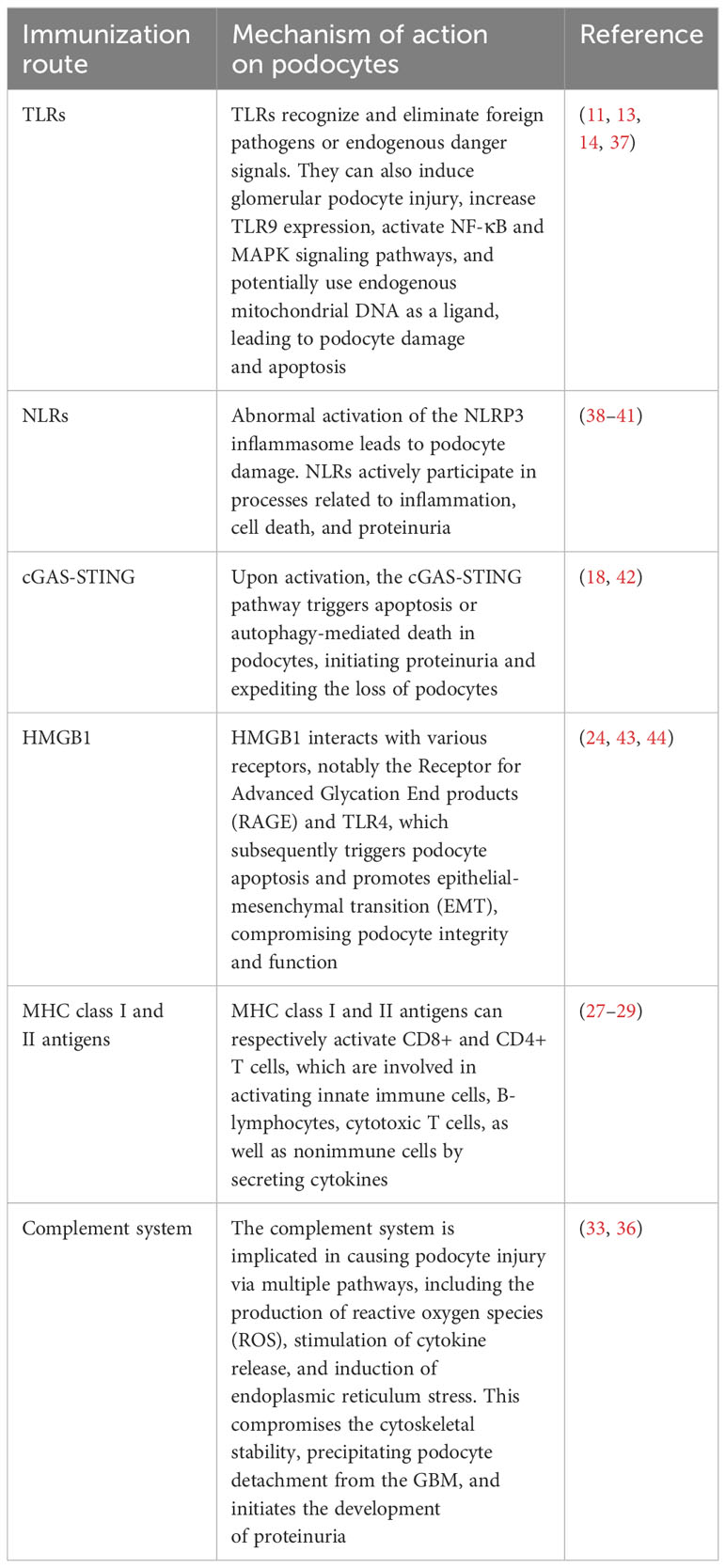
In addition to TLRs, podocytes also express other critical PRRs that actively participate in processes related to inflammation, cell death, and proteinuria (15). For example, Nod-like receptors (NLRs) have been implicated in podocyte injury, with abnormal activation of the NOD-like receptor thermal protein domain associated protein 3 (NLRP3) inflammasome leading to podocyte damage (16). The activation of the NLRP3 inflammasome contributes to aldosterone-induced podocyte injury, yet studies indicate that its targeted silencing can substantially reduce the severity of such damage. This finding underscores the potential therapeutic role of NLRP3 inhibition in conditions characterized by aldosterone-mediated damage to podocytes (17). More recently, researchers have uncovered the significance of the Cyclic GMP-AMP synthase (cGAS) - Stimulator of interferon genes (STING) axis in podocyte innate immunity (18). cGAS serves as a PRR and is a key sensor of cytosolic Deoxyribonucleic acid (DNA) (19). It can catalyze the production of the second messenger 2’3’-cyclic GMP-AMP (cGAMP), which binds to STING, activates TANK binding kinase 1 (TBK1) and interferon regulatory factor 3 (IRF3), ultimately leading to the production of type I interferons (20). Upon activation, the cGAS-STING pathway triggers apoptosis or autophagy-mediated death in podocytes. This event initiates proteinuria and expedites the loss of podocytes (21). Consequently, these chain reactions contribute significantly to the acceleration of the development and the progression of glomerular diseases. The cGAS-STING pathway is a critical innate immune pathway that senses cytosolic DNA, contributing to antiviral immunity and inflammatory responses. High-mobility group box 1 (HMGB1), a nuclear protein that is overexpressed in podocytes, becomes actively secreted or passively released into the extracellular milieu following cellular damage (22). Upon its release, HMGB1 interacts with various receptors, notably the Receptor for Advanced Glycation End products (RAGE) and TLR4, which subsequently triggers podocyte apoptosis and promotes epithelial-mesenchymal transition (EMT)—processes that compromise podocyte integrity and function (23, 24). Therapeutic strategies that interrupt the HMGB1-receptor signaling axis have demonstrated potential in ameliorating podocyte injury, consequently enhancing the integrity of the glomerular filtration barrier (25). This avenue represents a promising therapeutic target for ameliorating the progression of CKD (Figure 1).
除TLR外,足细胞还表达其他关键PRR,这些PRR积极参与炎症、细胞死亡和蛋白尿相关过程(15)。例如,NOD样受体(NLR)与足细胞损伤有关,NOD样受体热蛋白结构域相关蛋白3(NLRP 3)炎性体的异常激活导致足细胞损伤(16)。NLRP 3炎性体的激活有助于醛固酮诱导的足细胞损伤,但研究表明,其靶向沉默可以大大降低这种损伤的严重程度。这一发现强调了NLRP 3抑制在以醛固酮介导的足细胞损伤为特征的病症中的潜在治疗作用(17)。最近,研究人员发现了环GMP-AMP合酶(cGAS)-干扰素基因刺激因子(STING)轴在足细胞先天免疫中的重要性(18)。 cGAS作为PRR,是细胞溶质脱氧核糖核酸(DNA)的关键传感器(19)。它可以催化第二信使2 '3'-环GMP-AMP(cGAMP)的产生,其结合STING,激活TANK结合激酶1(TBK 1)和干扰素调节因子3(IRF 3),最终导致I型干扰素的产生(20)。激活后,cGAS-STING通路触发足细胞的凋亡或自噬介导的死亡。该事件引发蛋白尿并加速足细胞的损失(21)。因此,这些连锁反应显著加速肾小球疾病的发展和进展。cGAS-STING途径是一种重要的先天免疫途径,可感知胞质DNA,有助于抗病毒免疫和炎症反应。 高迁移率族蛋白1(HMGB 1)是一种在足细胞中过表达的核蛋白,在细胞损伤后主动分泌或被动释放到细胞外环境中(22)。在其释放后,HMGB 1与各种受体相互作用,特别是晚期糖基化终产物受体(RECEPTOR for Advanced Glycation End products,EMT)和TLR 4,其随后触发足细胞凋亡并促进上皮-间质转化(EMT)-损害足细胞完整性和功能的过程(23,24)。中断HMGB 1受体信号传导轴的治疗策略已被证明有可能改善足细胞损伤,从而增强肾小球滤过屏障的完整性(25)。该途径代表了改善CKD进展的有前景的治疗靶点(图1)。
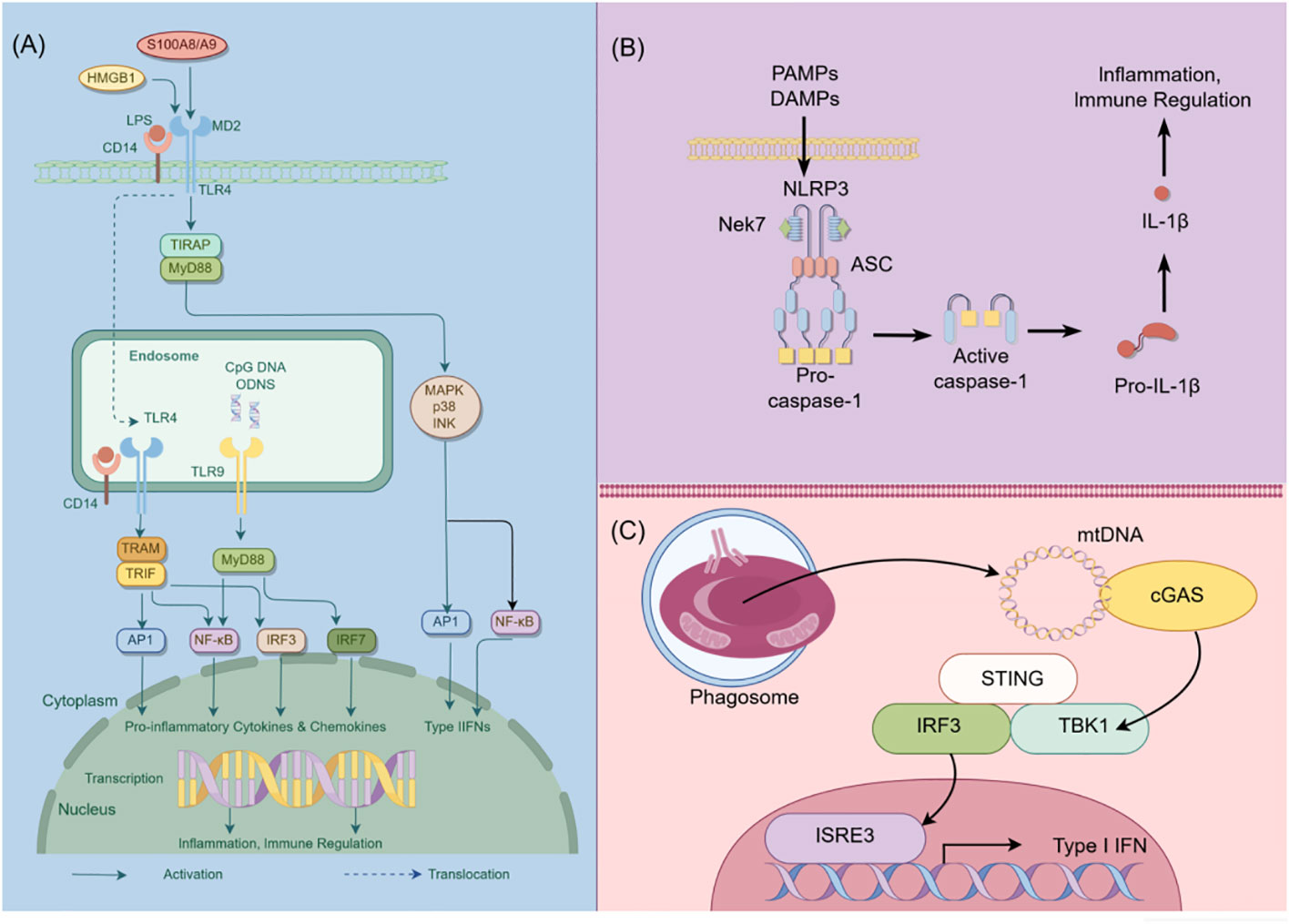
Figure 1 Schematic diagram of innate immunity in podocytes. This figure illustrates the role of TLR, NLRP3, and cGAS-STING signaling pathways in podocyte innate immunity. (A) Intracellular TLR signaling pathway: TLR, a pattern recognition receptor, can identify pathogen-related molecular patterns (PAMPs) and damage-related molecular patterns (DAMPs) within and outside cells, such as lipopolysaccharides (LPS). Upon TLR activation, downstream signaling molecules, including IRF3, IRF7, NF-κB, among others, are stimulated through various adapter proteins like MyD88, TRIF, etc., leading to the expression of antiviral and inflammatory genes. (B) NLRP3 Inflammasome activation: NLRP3, a member of the NOD-like receptor (NLR) family, is capable of recognizing intracellular PAMPs and DAMPS. Following NLRP3 activation, it assembles an inflammasome complex with ASC and Caspase-1, thereby triggering Caspase-1 activity and facilitating the maturation and secretion of IL-1β. (C) CGAS-STING signal pathway: CGAS, a cyclic GMP-AMP synthase, can detect DNA within cells. Upon eGAS binding to DNA, it synthesizes cyclic GMP-AMP (cGAMP) as a second messenger, which activates the STING protein. The STING protein subsequently triggers downstream signaling molecules like TBK1 and IRF3, leading to the expression of antiviral and inflammatory genes. HMGB1, High mobility group box-1 protein; LPS, Lipopolysaccharide; TIRAP, Toll-interleukin Receptor domain-containing adaptor protein; MyD88, Myeloid Differentiation Factor 88; MAPK, mitogen-activated protein kinases; AP1, activator protein-1; NF-κB, Nuclear factor kappa-B; IRF3, Interferon regulatory Factor 3; IRF7, Interferon regulatory Factor 7; PAMPs, pathogen-associated molecular patterns; DAMPs, damage-associated molecular patterns; NLRP3, NOD-like receptor thermal protein domain associated protein 3; Nek7, never in mitosis gene a-related kinase 7; ASC, apoptosis-associated speck-like protein containing a CARD domain; CGAS, cyclic guanosine monophosphate-adenosine monophosphate synthase; TBK1, TANK-binding kinase 1; STING, Stimulator of interferon genes; By Figdraw (www.figdraw.com).
图1足细胞先天免疫示意图。该图说明了TLR、NLRP 3和cGAS-STING信号通路在足细胞先天免疫中的作用。(A)细胞内TLR信号通路:TLR是一种模式识别受体,可以识别细胞内外的病原体相关分子模式(PAMP)和损伤相关分子模式(DAMP),如脂多糖(LPS)。TLR激活后,下游信号分子,包括IRF 3、IRF 7、NF-κB等,通过各种衔接蛋白如MyD 88、TRIF等被刺激,导致抗病毒和炎症基因的表达。(B)NLRP 3炎症体激活:NLRP 3是NOD样受体(NLR)家族的成员,能够识别细胞内PAMP和DAMPS。在NLRP 3活化后,它与ASC和Caspase-1组装炎性体复合物,从而触发Caspase-1活性并促进IL-1β的成熟和分泌。 (C)CGAS-STING信号通路:CGAS是一种环状GMP-AMP合酶,可以检测细胞内的DNA。eGAS与DNA结合后,它合成环状GMP-AMP(cGAMP)作为第二信使,激活STING蛋白。STING蛋白随后触发下游信号分子如TBK 1和IRF 3,导致抗病毒和炎症基因的表达。 HMGB 1,高迁移率族蛋白-1蛋白; LPS,脂多糖; TIRAP,含Toll-白细胞介素受体结构域的衔接蛋白; MyD 88,髓样分化因子88; MAPK,促分裂原活化蛋白激酶; AP 1,激活蛋白-1; NF-κB,核因子κ-B; IRF 3,干扰素调节因子3; IRF 7,干扰素调节因子7; PAMP,病原体相关分子模式; DAMPs,损伤相关分子模式; NLRP 3,NOD样受体热蛋白结构域相关蛋白3; Nek 7,从未参与有丝分裂基因a相关激酶7; ASC,含有CARD结构域的凋亡相关斑点样蛋白; CGAS,环鸟苷单磷酸-腺苷单磷酸合酶; TBK 1,TANK结合激酶1; STING,干扰素基因刺激因子; Figdraw(www.figdraw.com)。
2.2 Adaptive immune responses in podocytes
2.2足细胞的适应性免疫应答
In addition to their role in innate immunity, podocytes also participate in adaptive immune responses (26). Podocytes have the capacity to express major histocompatibility complex (MHC) class I and II antigens, which can respectively activate CD8+ and CD4+ T cells (27, 28). CD4+ T cells are involved in activating innate immune cells, B-lymphocytes, cytotoxic T cells, as well as nonimmune cells by secreting cytokines (29). On the other hand, CD8+ T cells exert specific cytotoxic effects, aiding in the body’s defense against pathogens and tumor cells (30). The activation of T cells relies on a two-signal process: the first signal involves the binding of MHC antigens to the T cell receptor (TCR), and the second signal entails the interaction between a co-stimulatory molecule and its ligand. Remarkably, podocytes can express B7-1 (CD80), which is a T cell co-stimulatory molecule that binds to CD28 on T cells, resulting in the generation of a positive synergistic stimulation signal. B7-1/CD80 is associated with B cells and antigen-presenting cells (APCs), and it can augment T cell responses to antigens presented by MHC class I/II (31). In contrast, B7-2 (CD86) is another co-stimulatory molecule found on B cells and APCs, which can also bind to CD28 (32) (Figure 2). These findings underscore the specific role of podocytes in adaptive immune regulation, which may have a significant impact on renal inflammation and injury. The complement system, an important integral component of innate and adaptive immune responses, has gained increasing attention in understanding the underlying pathogenesis of glomerular diseases. With a better understanding of the complement system and its role in glomerular diseases, treatments targeting complement activation are being explored for various glomerular conditions. Abnormal activation of the complement system is implicated in causing podocyte injury through multiple pathways. This involvement includes the production of reactive oxygen species (ROS), stimulation of cytokine release, and induction of endoplasmic reticulum stress. Collectively, these biochemical events compromise cytoskeletal stability, leading to podocyte detachment from the GBM and initiating the development of proteinuria (33). Studies investigating primary membranous nephropathy have demonstrated the critical involvement of the C3a/C3aR pathway in podocyte injury, noting that increased levels of plasma C3a and glomerular C3aR are associated with disease progression and serve as predictive indicators for patient prognosis (34). Podocytes serve a dual role in the narrative of complement-mediated damage: they are not only a target but also a source of complement system activation (35). Podocytes employ multiple defense mechanisms, including the expression of regulatory proteins like complement factor H and surface regulators such as CD46, CD55, and CD59, as well as processes like autophagy and actin-mediated endocytosis, to protect against and maintain homeostasis in the face of complement-induced damage (36). The interplay between the complement system and podocyte damage is intricate and comprises multiple dimensions. Elucidating the nuances of this relationship is pivotal for the innovation of therapeutic approaches aimed at treating conditions linked to podocyte damage (Table 1) (Figure 3).
除了它们在先天免疫中的作用外,足细胞还参与适应性免疫应答(26)。足细胞具有表达主要组织相容性复合体(MHC)I类和II类抗原的能力,其可分别激活CD8 + 和CD4 + T细胞(27,28)。CD4 + T细胞通过分泌细胞因子参与激活先天免疫细胞、B淋巴细胞、细胞毒性T细胞以及非免疫细胞(29)。另一方面,CD8 + T细胞发挥特定的细胞毒性作用,帮助身体防御病原体和肿瘤细胞(30)。T细胞的活化依赖于两个信号过程:第一个信号涉及MHC抗原与T细胞受体(TCR)的结合,第二个信号需要共刺激分子与其配体之间的相互作用。 值得注意的是,足细胞可以表达B7-1(CD 80),其是结合T细胞上的CD 28的T细胞共刺激分子,导致产生正协同刺激信号。B7-1/CD 80与B细胞和抗原呈递细胞(APC)相关,并且它可以增强T细胞对由MHC I/II类呈递的抗原的应答(31)。相比之下,B7-2(CD 86)是在B细胞和APC上发现的另一种共刺激分子,其也可以结合CD 28(32)(图2)。这些发现强调了足细胞在适应性免疫调节中的特殊作用,这可能对肾脏炎症和损伤产生重大影响。补体系统是先天性和适应性免疫反应的重要组成部分,在了解肾小球疾病的潜在发病机制方面越来越受到关注。 随着对补体系统及其在肾小球疾病中的作用的更好理解,正在探索针对各种肾小球病症的靶向补体激活的治疗。补体系统的异常激活涉及通过多种途径引起足细胞损伤。这种参与包括产生活性氧(ROS),刺激细胞因子释放和诱导内质网应激。总的来说,这些生化事件损害细胞骨架稳定性,导致足细胞从GBM脱离并引发蛋白尿的发展(33)。研究原发性膜性肾病的研究表明,C3 a/C3 aR途径在足细胞损伤中起关键作用,并指出血浆C3 a和肾小球C3 aR水平升高与疾病进展相关,可作为患者预后的预测指标(34)。 足细胞在补体介导的损伤中起双重作用:它们不仅是补体系统激活的靶点,也是补体系统激活的来源(35)。足细胞采用多种防御机制,包括表达调节蛋白(如补体因子H)和表面调节因子(如CD46、CD55和CD59),以及自噬和肌动蛋白介导的内吞作用等过程,以在面对补体诱导的损伤时保护和维持体内平衡(36)。补体系统和足细胞损伤之间的相互作用是错综复杂的,包括多个维度。阐明这种关系的细微差别对于旨在治疗与足细胞损伤相关的疾病的治疗方法的创新至关重要(表1)(图3)。
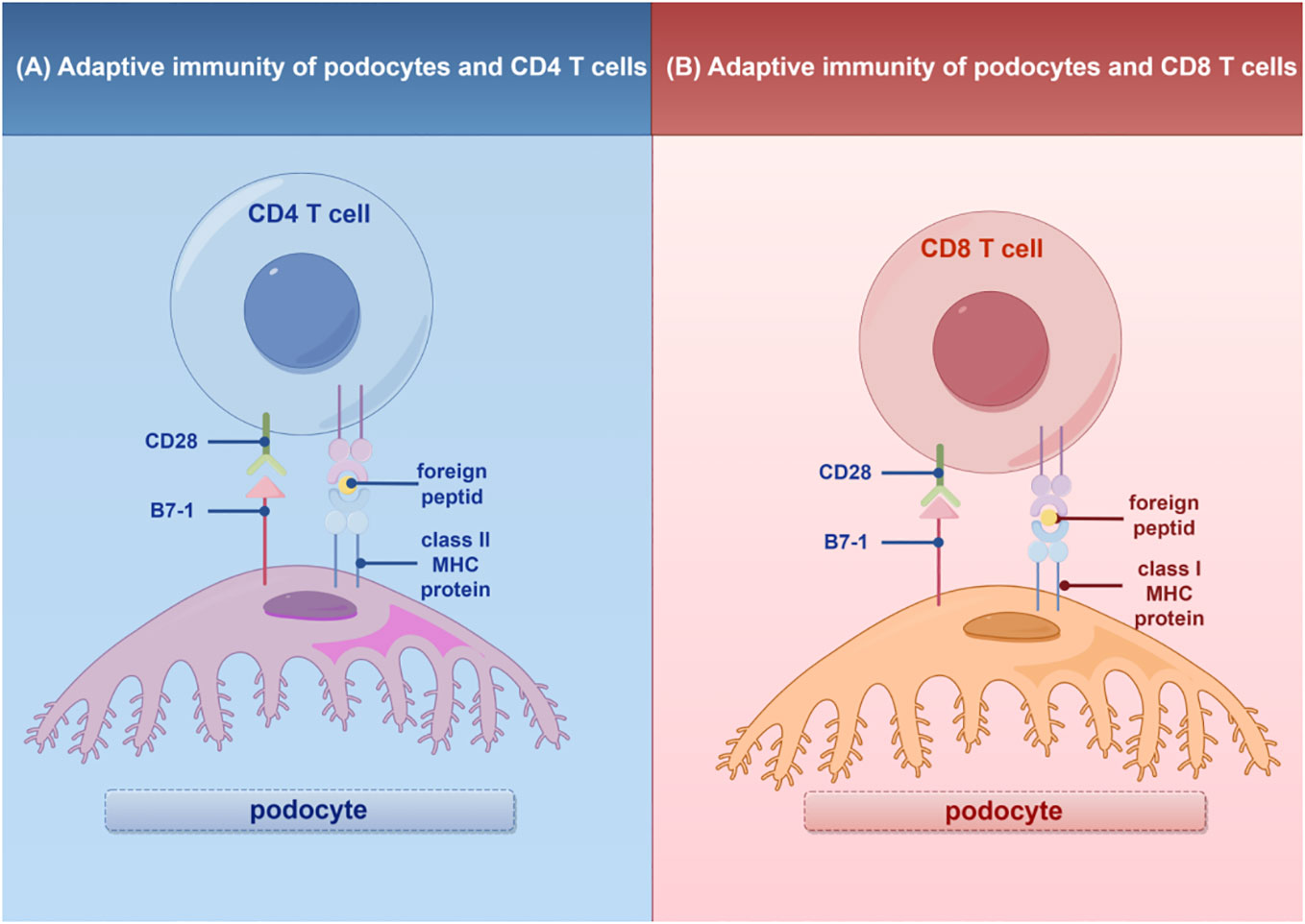
Figure 2 Adaptive immunity of T cells in podocytes. (A) Interaction of podocytes with CD4 T Cells: Podocytes express B7-1 and MHC II molecules, facilitating interactions with CD4 T cells and promoting glomerular inflammatory responses. (B) Interaction of podocytes with CD8 T Cells: Podocytes express B7-1 and MHC I molecules, enabling interactions with CD8 T cells and promoting glomerular inflammatory responses. MHC, major histocompatibility (www.figdraw.com). complex; By Figdraw.
图2足细胞中T细胞的适应性免疫。(A)足细胞与CD 4 T细胞的相互作用:足细胞表达B7-1和MHC II分子,促进与CD 4 T细胞的相互作用并促进肾小球炎症反应。(B)足细胞与CD 8 T细胞的相互作用:足细胞表达B7-1和MHC I分子,能够与CD 8 T细胞相互作用并促进肾小球炎症反应。MHC,主要组织相容性(www.figdraw.com)。复杂;通过Figdraw.
Figure 3 The role of complement system in podocyte immunity. Diagrammatic representation of complement system pathways and their involvement in podocyte immunity, highlighting the classical, lectin, and alternative pathways leading to pathogen lysis and immune cell recruitment. By Figdraw (www.figdraw.com).
图3补体系统在足细胞免疫中的作用。补体系统途径及其参与足细胞免疫的图示,突出了导致病原体裂解和免疫细胞募集的经典、凝集素和替代途径。By Figdraw(www.figdraw.com).
3 Crosstalk between podocytes and other cells in the glomerulus
图3肾小球中足细胞和其他细胞之间的串扰
Cellular crosstalk is a fundamental process of intercellular signaling and interaction that exerts a significant influence on the behavior and function of various cells. This dynamic communication can occur between identical cell types or across different cell types (45). The interaction between other cells in the glomerulus and podocytes is critical in the development of glomerular diseases.Research has demonstrated a correlation between mitochondrial oxidative stress in glomerular endothelial cells and an increased expression of Endothelin-1 (Edn1) along with its receptor, Endothelin-1 receptor A (Ednra) (46). Targeted attenuation of mitochondrial oxidative stress or the disruption of the Edn1/Ednra signaling cascade has been shown to effectively ameliorate damage to glomerular endothelial cells, reduce the depletion of podocytes, decrease proteinuria levels, and attenuate the progression of glomerulosclerosis in D2 mice (47).
细胞串扰是细胞间信号传导和相互作用的基本过程,对各种细胞的行为和功能产生重大影响。这种动态通信可以发生在相同的细胞类型之间或不同的细胞类型之间(45)。肾小球中的其他细胞与足细胞之间的相互作用在肾小球疾病的发展中至关重要。研究表明,肾小球内皮细胞中的线粒体氧化应激与内皮素-1(Edn 1)沿着其受体内皮素-1受体A(Ednra)的表达增加之间存在相关性(46)。线粒体氧化应激的靶向减弱或Edn 1/Ednra信号级联的破坏已被证明可有效改善D2小鼠肾小球内皮细胞的损伤,减少足细胞的消耗,降低蛋白尿水平,并减缓肾小球硬化的进展(47)。
Vascular endothelial growth factor A (VEGF-A) and angiopoietin 1 (Ang-1) secreted by podocytes serve as protective factors that uphold the integrity and stability of endothelial cells by binding to receptors on the endothelial cells (48–50), Nevertheless, both the overexpression and deficiency of VEGF-A can lead to endothelial cell injury and proteinuria (51). Angiopoietin 2 (Ang2), primarily produced by glomerular endothelial cells, exhibits an antagonistic effect. It competitively inhibits the activation of the Tie2 receptor by Ang1, thereby increasing endothelial cell apoptosis and permeability (47). Additionally, vascular endothelial growth factor B (VEGF-B) secreted by podocytes acts as a pathogenic factor, fostering lipid accumulation and lipotoxicity, damaging the endothelial cell surface, and inducing proteinuria by binding to receptors on the endothelial cells (52).
足细胞分泌的血管内皮生长因子A(VEGF-A)和血管生成素1(Ang-1)作为保护因子,通过与内皮细胞上的受体结合来维持内皮细胞的完整性和稳定性(48- 50)。然而,VEGF-A的过表达和缺乏均可导致内皮细胞损伤和蛋白尿(51)。血管生成素2(Ang 2)主要由肾小球内皮细胞产生,具有拮抗作用。它通过Ang 1竞争性抑制Tie 2受体的激活,从而增加内皮细胞凋亡和渗透性(47)。此外,足细胞分泌的血管内皮生长因子B(VEGF-B)可作为致病因子,促进脂质蓄积和脂毒性,损伤内皮细胞表面,并通过与内皮细胞上的受体结合诱导蛋白尿(52)。
Glomerular endothelial cells secrete endothelin-1 (ET-1), a vasoconstrictive peptide with multifaceted effects. ET-1 exerts diverse physiological and pathophysiological roles by binding to ETAR or ETBR on the surface of endothelial cells, mesangial cells, or surrounding cells (53). While ETAR primarily mediates vasoconstriction, cell proliferation, fibrosis, podocyte injury, and inflammatory response (54), ETBR predominantly mediates vasodilation, anti-proliferation, and anti-fibrosis, thereby exerting a protective effect (55). These crosstalk mechanisms significantly contribute to the regulatory processes involved in glomerular injury and repair.
肾小球内皮细胞分泌内皮素-1(ET-1),一种具有多方面作用的血管收缩肽。ET-1通过与内皮细胞、系膜细胞或周围细胞表面上的ETAR或ETBR结合而发挥多种生理和病理生理作用(53)。ETAR主要介导血管收缩、细胞增殖、纤维化、足细胞损伤和炎症反应(54),ETBR主要介导血管舒张、抗增殖和抗纤维化,从而发挥保护作用(55)。这些相互作用机制对参与肾小球损伤和修复的调节过程有重要作用。
Furthermore, the transforming growth factor β (TGF-β) released by podocytes activates various downstream signals within the Smad signaling pathway by binding to distinct receptors. This activation promotes mesangial cell proliferation, differentiation, and matrix deposition, influencing the phenotype and function of endothelial cells, mesangial cells, and podocytes themselves, ultimately leading to glomerular fibrosis (56). Exosomes, small extracellular vesicles, possess the capability to transport numerous molecular components, including proteins, lipids, DNA, miRNA, and lncRNA (57). Recent evidence has shed light on the role of exosomes in compromising the structural integrity of glomeruli, renal tubules, and renal interstitium. Under hyperglycemic conditions, mesangial cell-derived exosomes can potentially alter the functionality of podocytes through the delivery of TGF-β1, thereby contributing to the pathogenesis of diabetic nephropathy (58, 59).
此外,足细胞释放的转化生长因子β(TGF-β)通过与不同的受体结合来激活Smad信号通路内的各种下游信号。这种激活促进肾小球系膜细胞增殖、分化和基质沉积,影响内皮细胞、系膜细胞和足细胞自身的表型和功能,最终导致肾小球纤维化(56)。外泌体,小的细胞外囊泡,具有运输多种分子组分的能力,包括蛋白质、脂质、DNA、miRNA和lncRNA(57)。最近的证据已经阐明了外泌体在损害肾小球、肾小管和肾小管的结构完整性中的作用。在高血糖条件下,系膜细胞来源的外泌体可通过TGF-β1的递送潜在地改变足细胞的功能,从而促进糖尿病肾病的发病机制(58,59)。
Cytokines, such as TNF-α and TGF-β, secreted by mesangial cells, are known to reduce the expression of podocyte-associated proteins, including nephrin and podocin. This reduction weakens or disrupts junctions within the slit diaphragm, subsequently increasing the permeability of the glomerular filtration membrane. Consequently, proteinuria and impaired renal function ensue (60). This mechanism may partially elucidate the pathogenesis of FSGS.
已知由系膜细胞分泌的细胞因子如TNF-α和TGF-β可降低足细胞相关蛋白的表达,包括nephrin和podocin。这种减少削弱或破坏狭缝隔膜内的连接,随后增加肾小球滤过膜的渗透性。因此,蛋白尿和肾功能受损(60)。这一机制可能部分阐明FSGS的发病机制。
The interplay between podocytes and other cells in glomeruli, as well as among podocytes themselves, plays a critical role in the pathogenesis of various kidney diseases, including glomerulonephritis, FSGS, and LN. This intricate process involves the complex interaction and regulation of various cytokines and receptors, ultimately contributing to the progression of glomerular damage and dysfunction. Understanding the molecular mechanisms underlying these crosstalks is instrumental in deciphering the pathogenesis of kidney diseases and serves as a foundation for the development of novel therapeutic strategies aimed at preserving glomerular function and ameliorating the progression of proteinuric glomerular diseases.
足细胞和肾小球中其他细胞之间以及足细胞本身之间的相互作用在各种肾脏疾病(包括肾小球肾炎、FSGS和LN)的发病机制中起关键作用。这个复杂的过程涉及各种细胞因子和受体的复杂相互作用和调节,最终导致肾小球损伤和功能障碍的进展。了解这些串扰背后的分子机制有助于解释肾脏疾病的发病机制,并为开发旨在保护肾小球功能和改善蛋白尿性肾小球疾病进展的新治疗策略奠定基础。
4 Common proteinuric glomerular diseases associated with immune responses in podocytes
4与足细胞免疫反应相关的常见蛋白尿性肾小球疾病
4.1 Minimal change disease
4.1微小病变
MCD is the predominant type of nephrotic syndrome in children and adolescents. However, in adults, MCD accounts for a smaller proportion, approximately 10% to 16% of nephrotic syndrome cases (61). Clinically, MCD is characterized by massive proteinuria, hypoalbuminemia, edema, and dyslipidemia. Kidney biopsy typically reveals predominantly normal glomeruli under light microscopy, with the only notable finding being the diffuse disappearance of podocyte foot processes observed under transmission electron microscopy (62). Although the precise pathogenesis of MCD remains elusive, numerous research findings suggest its association with immune system dysregulation.
MCD是儿童和青少年肾病综合征的主要类型。然而,在成人中,MCD所占比例较小,约占肾病综合征病例的10%至16%(61)。临床上,MCD以大量蛋白尿、低白蛋白血症、水肿和血脂异常为特征。肾活检通常在光镜下显示正常肾小球为主,唯一值得注意的发现是在透射电镜下观察到足细胞足突的弥漫性消失(62)。虽然MCD的确切发病机制仍然难以捉摸,但许多研究结果表明其与免疫系统失调有关。
Traditionally, it has been postulated that MCD is mediated by certain unidentified circulating factors, possibly released by T cells, which target podocytes directly, leading to their dysfunction and consequent proteinuria (63). Evidence indicates that the circulating factors responsible for MCD may include specific cytokines, primarily belonging to the T helper 2 cell (Th2) subgroup, such as interleukin-13 (IL-13), interleukin-8 (IL-8), interleukin-4 (IL-4), among others (64–67). On one hand, T cell effector factors serve as the source of the aforementioned pro-inflammatory cytokines and others, which can directly or indirectly impair podocytes, resulting in proteinuria. On the other hand, regulatory T cells (Treg cells) maintain immune balance by binding to CD80 on antigen-presenting cells (APCs) through the expression of Cytotoxic T lymphocyte associate protein-4 (CTLA-4) (68). The reduced number and function of Treg cells in MCD patients may lead to immune dysregulation and an inflammatory response (69). Additionally, MCD has been associated with certain Treg cell-related genetic or acquired immune deficiency syndromes (70).
传统上认为MCD是由某些未知的循环因子介导的,可能由T细胞释放,直接靶向足细胞,导致其功能障碍和随后的蛋白尿(63)。有证据表明,导致MCD的循环因子可能包括特异性细胞因子,主要属于辅助性T细胞2(Th 2)亚群,如白介素-13(IL-13)、白介素-8(IL-8)、白介素-4(IL-4)等(64- 67)。一方面,T细胞效应因子作为上述促炎细胞因子和其他因子的来源,其可直接或间接损害足细胞,导致蛋白尿。另一方面,调节性T细胞(Treg细胞)通过表达细胞毒性T淋巴细胞相关蛋白-4(CTLA-4)与抗原呈递细胞(APC)上的CD 80结合来维持免疫平衡(68)。 MCD患者中Treg细胞数量和功能的减少可能导致免疫失调和炎症反应(69)。此外,MCD与某些Treg细胞相关的遗传或获得性免疫缺陷综合征相关(70)。
While much of the research on MCD has traditionally focused on T cell-related cytokines, recent studies have shed light on the involvement of B cells and specific autoantibodies, such as those targeting nephrin, a critical podocyte protein (71). The role of B cells in MCD pathogenesis has gained increased attention in recent years, particularly following the use of rituximab (RTX), a monoclonal antibody targeting the pan-B-cell marker CD20, which has shown efficacy in reducing recurrence rates (72, 73). In the process of antibody synthesis, B cells are capable of exerting a direct impact on the structural integrity and functional capacities of podocytes through the release of particular cytokines. Additionally, B cells can modulate T cell responses, thereby contributing to the pathogenesis of diseases. This dual capacity underscores the complex and integral participation of B cells in disease pathology, extending their influence beyond the classical role of antibody generation (74). Some studies suggest that B cells may contribute to MCD development by promoting T cell responses or producing autoantibodies. For instance, elevated levels of B cell-activating factor in the serum of MCD patients have been observed, which may drive T cell activation in MCD (75). Research has also revealed increased levels of T cells bearing immunoglobulin M (IgM) on their surface in patients with idiopathic nephrotic syndrome (INS) who exhibit poor treatment responses. In vitro experiments have demonstrated that these T cells can induce rearrangement of the podocyte cytoskeleton, indicating that the interplay between B cells and T cells may play a crucial role in MCD pathogenesis (76). Furthermore, IgM can trigger the classical complement activation pathway in the glomeruli of INS patients (77). These studies suggest that B cells may contribute to MCD through various mechanisms, but further research is required to elucidate their exact roles. In addition, certain autoantibodies have been considered mediators of MCD. Studies have shown that around 50% of children with relapsing hormone-sensitive nephrotic syndrome have elevated plasma anti-UCHL1 (ubiquitin C-terminal hydrolase-L1) antibody levels. However, in adult MCD patients, no elevation of anti-UCHL1 antibody levels was observed (78). This suggests the existence of different immune mechanisms in children and adults with MCD.
虽然传统上对MCD的研究主要集中在T细胞相关的细胞因子上,但最近的研究揭示了B细胞和特异性自身抗体的参与,例如靶向nephrin(一种关键的足细胞蛋白)的抗体(71)。近年来,B细胞在MCD发病机制中的作用受到越来越多的关注,特别是在使用利妥昔单抗(RTX)后,利妥昔单抗是一种靶向泛B细胞标志物CD 20的单克隆抗体,已显示出降低复发率的疗效(72,73)。在抗体合成过程中,B细胞能够通过释放特定的细胞因子对足细胞的结构完整性和功能能力产生直接影响。此外,B细胞可以调节T细胞应答,从而有助于疾病的发病机制。这种双重能力强调了B细胞在疾病病理学中的复杂和整体参与,将其影响力扩展到抗体生成的经典作用之外(74)。 一些研究表明,B细胞可能通过促进T细胞应答或产生自身抗体而促进MCD的发展。例如,已观察到MCD患者血清中B细胞活化因子水平升高,这可能驱动MCD中的T细胞活化(75)。研究还显示,在表现出不良治疗反应的特发性肾病综合征(INS)患者中,其表面上携带免疫球蛋白M(IgM)的T细胞水平增加。体外实验表明,这些T细胞可诱导足细胞骨架重排,表明B细胞和T细胞之间的相互作用可能在MCD发病机制中起关键作用(76)。此外,IgM可触发INS患者肾小球中的经典补体激活途径(77)。这些研究表明,B细胞可能通过各种机制参与MCD,但需要进一步研究来阐明其确切作用。 此外,某些自身抗体被认为是MCD的介质。研究表明,约50%的复发性肾病综合征患儿血浆抗UCHL 1(泛素C末端水解酶-L1)抗体水平升高。然而,在成人MCD患者中,未观察到抗UCHL 1抗体水平升高(78)。这表明MCD儿童和成人存在不同的免疫机制。
In summary, the pathogenesis of MCD involves complex interactions among the immune system, glomerular cells, and genetics, with multiple potential pathogenic factors and mechanisms at play. MCD is not a straightforward immune or podocyte disease. Heterogeneity and the absence of reliable animal models pose challenges to our understanding of MCD. Therefore, future research should focus on developing new experimental models, screening for new autoantibodies, and improving our understanding of cell-cell interactions in this disease.
总之,MCD的发病机制涉及免疫系统、肾小球细胞和遗传学之间复杂的相互作用,多种潜在的致病因素和机制在起作用。MCD不是一种简单的免疫或足细胞疾病。异质性和缺乏可靠的动物模型对我们理解MCD提出了挑战。因此,未来的研究应该集中在开发新的实验模型,筛选新的自身抗体,并提高我们对这种疾病中细胞-细胞相互作用的理解。
4.2 Focal segmental glomerulosclerosis
4.2局灶节段性肾小球硬化
FSGS is a group of glomerular lesions that were once considered to have a low incidence but have shown a gradual increase in global prevalence in recent decades. Reports suggest that the annual incidence of FSGS ranges from 0.2 to 1.8 cases per 100,000 people (79). Histopathologically, FSGS is characterized by partial sclerosis of some glomeruli, with each affected glomerulus showing only segmental involvement. Clinically, FSGS commonly presents as proteinuria, nephrotic syndrome, with or without kidney function impairment (80). While the precise mechanisms underlying the development of FSGS remain incompletely understood, emerging evidence suggests significant involvement of the immune system in the pathogenesis, with podocyte injury considered to be at the core of the disease.
FSGS是一组肾小球病变,曾被认为发病率低,但近几十年来全球患病率逐渐增加。报告显示,FSGS的年发病率范围为每10万人0.2至1.8例(79)。组织学上,FSGS的特征是部分肾小球的部分硬化,每个受累的肾小球仅显示节段性受累。临床上,FSGS通常表现为蛋白尿、肾病综合征,伴或不伴肾功能损害(80)。虽然FSGS发展的确切机制仍不完全清楚,但新出现的证据表明免疫系统在发病机制中有重要作用,足细胞损伤被认为是疾病的核心。
FSGS has been linked to various immune-related factors, including infections, tumors, drugs, and genetic mutations, which can impact the structure and function of podocytes, leading to proteinuria and a decline in kidney function (81–85). T cells, a crucial component of the adaptive immune system, secrete various cytokines that regulate glomerular permeability and inflammatory responses. As early as 1974, Shalhoub proposed the hypothesis that FSGS is a T-cell-mediated disease (86). Subsequent research has identified abnormalities in different subtypes and functional states of T cells in FSGS patients. Some studies have reported a progressive decrease in the relative numbers of CD4 and CD8 T cells with disease progression (87). Reports have also demonstrated an increase in Th17 cells and a decrease in Treg cells among CD4 T cells in some adult patients with INS (88). Furthermore, various observations have associated the levels of certain cytokines secreted by T cells with glomerulosclerosis in FSGS patients. For example, interleukin-17 (IL-17), produced by the Th17 subgroup, has been linked to glomerulosclerosis in FSGS patients (89). Other studies have revealed that FSGS is related to the activation of the tumor necrosis factor-α (TNF-α) pathway in podocytes (90, 91). These cytokines may induce morphological changes, dedifferentiation, and apoptosis in podocytes by directly or indirectly affecting them. Podocytes express various TLRs involved in innate immunity, capable of activating signaling pathways that lead to morphological changes in podocytes and induce the expression of chemokines (92, 93). Moreover, TLR signaling can enhance the expression of CD80 on podocytes, a molecule that stimulates T cells (93, 94). Therefore, TLRs may also modulate the interaction between podocytes and T cells.
FSGS与各种免疫相关因素有关,包括感染、肿瘤、药物和基因突变,这些因素会影响足细胞的结构和功能,导致蛋白尿和肾功能下降(81- 85)。T细胞是适应性免疫系统的重要组成部分,分泌各种细胞因子,调节肾小球通透性和炎症反应。早在1974年,Shalhoub就提出了FSGS是一种T细胞介导的疾病的假设(86)。随后的研究发现FSGS患者T细胞的不同亚型和功能状态异常。一些研究报告称,随着疾病进展,CD 4和CD 8 T细胞的相对数量逐渐减少(87)。报告还表明,在一些成人INS患者中,CD 4 T细胞中Th 17细胞增加,Treg细胞减少(88)。 此外,各种观察结果表明,FSGS患者中T细胞分泌的某些细胞因子水平与肾小球硬化症相关。例如,Th 17亚群产生的白细胞介素-17(IL-17)与FSGS患者的肾小球硬化症有关(89)。其他研究表明FSGS与足细胞中肿瘤坏死因子-α(TNF-α)通路的激活有关(90,91)。这些细胞因子可以通过直接或间接地影响足细胞而诱导足细胞的形态学改变、去分化和凋亡。足细胞表达参与先天免疫的各种TLR,能够激活导致足细胞形态变化的信号传导途径并诱导趋化因子的表达(92,93)。此外,TLR信号传导可增强足细胞上的CD 80(刺激T细胞的分子)的表达(93,94)。因此,TLR也可以调节足细胞和T细胞之间的相互作用。
B cells are another crucial component of the adaptive immune system, primarily responsible for mediating humoral immune responses through the production of antibodies. Additionally, they perform various other immune functions. B cells function as efficient APCs, processing and presenting antigens to T cells, thereby activating T cell responses to antigens. Moreover, B cells secrete various cytokines, including both pro-inflammatory and anti-inflammatory factors, to regulate the balance of immune responses (95). While FSGS is not typically considered a typical immune complex-mediated disease, increasing evidence suggests that B cells also play a significant role in FSGS. Several potential autoantigens and antibodies associated with B cells have been identified in FSGS. For instance, annexin A2, a protein involved in apoptosis and signal transduction, has been identified as a potential autoantigen through mass spectrometry analysis (96). Another study found that 29% of INS patients had anti-nephrin immunoglobulin G (IgG) antibodies in their plasma, which decreased with treatment and disappeared in patients with complete remission (97). A recent report by Dr. Hattori and colleagues showed that circulating anti-nephrin autoantibodies detected in plasma samples from a recurrent FSGS patient after kidney transplantation results in the tyrosine phosphorylation of nephrin, leading to alterations in nephrin distribution and podocyte foot process effacement. It was indicated that circulating anti-nephrin autoantibodies could be a possible pathogenic candidate for circulating factors in the posttransplant recurrence of primary FSGS (97). Furthermore, there is evidence indicating a higher frequency of the human leukocyte antigen (HLA)-A30 antigen in primary FSGS patients, with HLA-A30 being associated with FSGS recurrence (98).
B细胞是适应性免疫系统的另一个重要组成部分,主要负责通过产生抗体介导体液免疫应答。此外,它们还执行各种其他免疫功能。B细胞充当有效的APC,加工抗原并将抗原呈递给T细胞,从而激活T细胞对抗原的应答。此外,B细胞分泌各种细胞因子,包括促炎因子和抗炎因子,以调节免疫应答的平衡(95)。虽然FSGS通常不被认为是典型的免疫复合物介导的疾病,但越来越多的证据表明B细胞在FSGS中也起重要作用。已经在FSGS中鉴定出几种与B细胞相关的潜在自身抗原和抗体。例如,膜联蛋白A2(一种参与细胞凋亡和信号转导的蛋白质)已通过质谱分析被鉴定为潜在的自身抗原(96)。 另一项研究发现,29%的INS患者血浆中存在抗nephrin免疫球蛋白G(IgG)抗体,该抗体随着治疗而降低,在完全缓解患者中消失(97)。Hattori博士及其同事最近的一份报告显示,在肾移植后复发性FSGS患者的血浆样本中检测到的循环抗nephrin自身抗体导致nephrin的酪氨酸磷酸化,导致nephrin分布改变和足细胞足突消失。研究表明,循环中的抗nephrin自身抗体可能是原发性FSGS移植后复发循环因素的致病候选者(97)。此外,有证据表明原发性FSGS患者中人类白细胞抗原(HLA)-A30抗原的频率较高,HLA-A30与FSGS复发相关(98)。
The Src homology 3 domain-binding protein 2 (SH3BP2) is an adaptor protein ubiquitously expressed in immune cells, governing signaling pathways within the cell (99). Kidney biopsy samples from patients with these conditions consistently exhibit upregulation of the SH3BP2 signaling complex, suggesting its potential involvement in immune activation—a prominent feature of these podocytopathies (100). Research led by Tarak Srivastava and colleagues, using murine models, has further underscored the significance of SH3BP2 in podocyte injury. Transgenic mice with induced mutations in the SH3BP2 gene displayed features indicative of podocyte dysfunction, including elevated urinary albumin levels, a decrease in serum albumin, and an increase in mesangial cell proliferation (100). Collectively, these findings position SH3BP2 and its associated proteins at the forefront of immunological processes that contribute to podocyte injury.
Src同源性3结构域结合蛋白2(SH 3BP 2)是一种在免疫细胞中普遍表达的衔接蛋白,控制细胞内的信号传导途径(99)。这些疾病患者的肾活检样本始终显示SH 3BP 2信号复合物的上调,提示其可能参与免疫激活-这是足细胞病变的一个显著特征(100)。由Tarak Srivastava及其同事领导的研究,使用小鼠模型,进一步强调了SH 3BP 2在足细胞损伤中的重要性。SH 3BP 2基因突变的转基因小鼠表现出足细胞功能障碍的特征,包括尿白蛋白水平升高、血清白蛋白降低和系膜细胞增殖增加(100)。总的来说,这些发现将SH 3BP 2及其相关蛋白定位在导致足细胞损伤的免疫过程的最前沿。
Despite the unclear pathogenesis of FSGS, comprehensively studying the structure and function of proteins related to podocytes, as well as the mechanisms underlying podocyte injury in FSGS, offers essential insights for deciphering its pathophysiology. These investigations could reveal novel avenues for clinical treatment, potentially resulting in the effective reduction or deceleration of the glomerulosclerosis process and postponing the onset of end-stage kidney failure.
尽管FSGS的发病机制尚不清楚,但全面研究足细胞相关蛋白的结构和功能,以及FSGS中足细胞损伤的机制,为破译其病理生理学提供了必要的见解。这些研究可能揭示临床治疗的新途径,可能导致肾小球硬化过程的有效减少或减速,并推迟终末期肾衰竭的发作。
4.3 Primary membranous nephropathy
4.3原发性膜性肾病
Membranous nephropathy (MN) is a common kidney disease affecting individuals across different age groups, particularly adults. Pathologically, it involves the deposition of immune complexes on the outer side of the GBM located beneath the podocyte, accompanied by diffuse GBM thickening. Approximately 20% to 30% of MN cases are associated with secondary factors such as systemic autoimmune diseases, infections, drugs, malignancy, or hematopoietic stem cell transplantation, known as secondary MN. In cases where no clear causal relationship or recognizable systemic disease, infection, or drug exposure is identified, the condition is termed primary membranous nephropathy (pMN), often perceived as a kidney-specific autoimmune disease (101, 102). pMN is the most common cause of primary nephrotic syndrome in non-diabetic patients, accounting for approximately 30% of adult non-diabetic nephrotic syndrome cases. It is characterized by massive proteinuria, hypoalbuminemia, dyslipidemia, and edema, while a small number of patients present with asymptomatic proteinuria (103). The typical microscopic pathological features of pMN include GBM thickening, podocyte foot process effacement, and spike-like electron-dense deposits under the glomerular podocytes. Immunofluorescence analysis typically shows granular deposits of IgG and C3 along the capillary wall, with IgG4 being the dominant IgG subtype in pMN (104).
膜性肾病(MN)是一种常见的肾脏疾病,影响不同年龄组的个体,特别是成年人。在病理学上,它涉及免疫复合物在位于足细胞下方的GBM外侧的沉积,伴有弥漫性GBM增厚。大约20%至30%的MN病例与继发性因素有关,如系统性自身免疫性疾病、感染、药物、恶性肿瘤或造血干细胞移植,称为继发性MN。在没有明确的因果关系或可识别的全身性疾病、感染或药物暴露的情况下,这种情况被称为原发性膜性肾病(pMN),通常被认为是肾脏特异性自身免疫性疾病(101,102)。pMN是非糖尿病患者中原发性肾病综合征的最常见原因,约占成人非糖尿病肾病综合征病例的30%。 其特征为大量蛋白尿、低白蛋白血症、血脂异常和水肿,少数患者表现为无症状蛋白尿(103)。pMN的典型显微病理特征包括肾小球基底膜增厚、足细胞足突消失和肾小球足细胞下的棘状电子致密沉积物。免疫荧光分析通常显示IgG和C3沿着毛细血管壁的颗粒状沉积物,其中IgG 4是pMN中的主要IgG亚型(104)。
The pathogenesis of pMN is not yet fully elucidated. According to the classical theory, antigens on the podocytes bind to specific antibodies, forming in situ immune complexes that deposit at the GBM. This deposition activates complement and creates membrane attack complexes, leading to damage to the glomerular filtration barrier and subsequent proteinuria (105). Recent progress in understanding the pathogenesis of pMN has been driven by the discovery of two key podocyte antigens: phospholipase A2 receptor (PLA2R) and thrombospondin type-1 domain-containing 7A (THSD7A), which serve as antibody markers in 70% and 2-3% of pMN patients, respectively (106). Animal models have validated the pathogenicity of human PLA2R and THSD7A antibodies. Both PLA2R and THSD7A are transmembrane proteins expressed by podocytes that can bind to circulating IgG4 subtype autoantibodies, forming subepithelial immune complexes (107, 108). Tomas NM and colleagues engineered a transgenic mouse model that specifically expresses human phospholipase A2 receptor 1 (hPLA2R1) in podocytes. These mice spontaneously generate antibodies against hPLA2R1, resulting in the development of nephrotic syndrome and demonstrating classical histological features of MN (109). This rodent model faithfully recapitulates key aspects of human MN, providing a valuable tool for probing the pathophysiological underpinnings of the disease and for the assessment of targeted therapeutic interventions. Additionally, several other podocyte-intrinsic antigens, such as high-temperature requirement A1 (HTRA1), netrin G1 (NTNG1), contactin-1 (CNTN1), and Semaphorin 3B (SEMA3B), have been identified, although the pathogenicity of these antigens in MN remains to be confirmed (110–113).
pMN的发病机制尚未完全阐明。根据经典理论,足细胞上的抗原与特异性抗体结合,形成原位免疫复合物,存款在GBM。这种沉积激活补体并产生膜攻击复合物,导致肾小球滤过屏障损伤和随后的蛋白尿(105)。最近对pMN发病机制的理解进展是由于发现了两种关键的足细胞抗原:磷脂酶A2受体(PLA 2 R)和含1型血小板反应蛋白结构域的7A(THSD 7A),这两种抗原分别在70%和2-3%的pMN患者中作为抗体标记物(106)。动物模型已经验证了人PLA 2 R和THSD 7A抗体的致病性。PLA 2 R和THSD 7A均为足细胞表达的跨膜蛋白,可与循环中的IgG 4亚型自身抗体结合,形成上皮下免疫复合物(107,108)。 Tomas NM及其同事设计了一种转基因小鼠模型,该模型在足细胞中特异性表达人磷脂酶A2受体1(hPLA 2 R1)。这些小鼠自发产生抗hPLA 2 R1的抗体,导致肾病综合征的发生,并显示MN的典型组织学特征(109)。这种啮齿动物模型忠实地概括了人类MN的关键方面,为探索疾病的病理生理学基础和评估靶向治疗干预提供了有价值的工具。此外,已经鉴定了几种其他足细胞内在抗原,如高温要求A1(HTRA 1)、netrin G1(NTNG 1)、接触蛋白-1(CNTN 1)和脑信号蛋白3B(SEMA 3B),尽管这些抗原在MN中的致病性仍有待证实(110- 113)。
Furthermore, some potential antigens that are not expressed by podocytes have been associated with pMN. Studies have shown that certain pMN cases are linked to the accumulation of exostosin glycosyltransferase 1 (EXT1) and exostosin glycosyltransferase 2 (EXT2). In a study of 48 PLA2R-negative pMN cases, EXT1 and EXT2 were detected in 21 of them but not in PLA2R-related pMN cases or control cases (114). Additionally, a mass spectrometry study conducted on 126 PLA2R-negative MN cases revealed that 29 cases were positive for neural epidermal growth factor-like 1 protein (NELL-1) (115). The clinical and kidney biopsy results of NELL-1-positive MN exhibited characteristics of pMN. In 5.7% of PLA2R-negative pMN cases, a unique protein called protocadherin 7 (PCDH7) was detected, and notably, all other antigens were negative in these cases (116). PCDH7-associated pMN appears to be a distinct, previously unidentified type of MN. Additionally, researchers have identified a novel antigen, neural cell adhesion molecule 1 (NCAM1), in rare pMN cases, with a prevalence of 2.0% in pMN cases (117). These antigens may be neo- or allo-antigens that deposit in the subepithelial space and bind to IgG1 or IgG3 subtype autoantibodies. The mechanism of action of these antigens in MN is unclear, and their potential associations with other diseases remain uncertain (102).
此外,一些不被足细胞表达的潜在抗原已经与pMN相关。研究表明,某些pMN病例与外生骨素糖基转移酶1(EXT 1)和外生骨素糖基转移酶2(EXT 2)的积累有关。在48例PLA 2 R阴性pMN病例的研究中,在其中21例中检测到EXT 1和EXT 2,但在PLA 2 R相关pMN病例或对照病例中未检测到EXT 1和EXT 2(114)。此外,对126例PLA 2 R阴性MN病例进行的质谱研究显示,29例病例为神经表皮生长因子样1蛋白(NELL-1)阳性(115)。NELL-1阳性MN的临床和肾活检结果显示出pMN的特征。在5.7%的PLA 2 R阴性pMN病例中,检测到一种称为原钙粘蛋白7(PCDH 7)的独特蛋白,值得注意的是,在这些病例中所有其他抗原均为阴性(116)。PCDH 7相关的pMN似乎是一种独特的,以前未识别的类型的MN。 此外,研究人员在罕见的pMN病例中发现了一种新的抗原,即神经细胞粘附分子1(NCAM 1),其患病率为2.0%(117)。这些抗原可以是新抗原或同种异体抗原,其存款在上皮下空间中并与IgG 1或IgG 3亚型自身抗体结合。这些抗原在MN中的作用机制尚不清楚,它们与其他疾病的潜在关联仍不确定(102)。
The pathogenesis of pMN is highly complex and results from the interaction of multiple factors. As our understanding of the molecular structure and gene loci of target antigens continues to grow, different target antigens and gene loci may provide new insights for the diagnosis and treatment of this disease. Further exploration of the mechanism of podocyte immunity in MN and the discovery of more effective and safe therapeutic targets are crucial directions for future pMN research.
pMN的发病机制非常复杂,是多种因素相互作用的结果。随着我们对靶抗原的分子结构和基因位点的了解不断加深,不同的靶抗原和基因位点可能会为这种疾病的诊断和治疗提供新的见解。进一步探索足细胞免疫在MN中的作用机制,发现更有效、更安全的治疗靶点是pMN研究的重要方向。
4.4 Lupus nephritis with podocyte injury
4.4狼疮性肾炎伴足细胞损伤
Systemic lupus erythematosus (SLE) is a complex autoimmune disease that affects multiple systems and organs throughout the body. It’s estimated that up to 40% of SLE patients will develop lupus nephritis (LN) (118). LN is a common and serious complication of SLE, significantly impacting the long-term prognosis of patients. The hallmark histopathological feature of LN is the deposition of immune complexes and inflammatory reactions in the kidneys. This ultimately leads to the destruction of kidney parenchyma and a clinical decline in kidney function (119). The most internationally recognized pathological classification of LN is the 2003 classification of lupus nephritis by the International Society of Nephrology (ISN) and the Renal Pathology Society (RPS), which divides LN into types I-VI (120). However, this classification system does not encompass all types of LN pathology. Some LN patients may have kidney biopsy results that do not align with their clinical manifestations, such as having massive proteinuria with relatively normal glomeruli or only mild glomerular mesangial proliferation. These patients may have a unique subtype of LN known as LN with podocyte lesions (119).
系统性红斑狼疮(SLE)是一种复杂的自身免疫性疾病,影响全身多个系统和器官。据估计,高达40%的SLE患者会发展为狼疮性肾炎(LN)(118)。LN是SLE常见而严重的并发症,严重影响患者的远期预后。LN的标志性组织病理学特征是免疫复合物和炎症反应在肾脏中的沉积。这最终导致肾实质的破坏和肾功能的临床下降(119)。国际上最公认的LN病理分类是国际肾病学会(ISN)和肾脏病理学会(RPS)于2003年对狼疮性肾炎进行的分类,该分类将LN分为I-VI型(120)。然而,这种分类系统并不包括所有类型的LN病理。 一些LN患者的肾活检结果可能与他们的临床表现不一致,如大量蛋白尿伴相对正常的肾小球或仅轻度肾小球系膜增生。这些患者可能有一种独特的LN亚型,即伴有足细胞病变的LN(119)。
The pathogenesis of LN is not completely understood, but the prevailing view is that LN is characterized by the deposition of immune complexes (IC) formed by various autoantibodies, such as anti-double-stranded DNA (ds-DNA) antibodies and antigens. Infiltration of inflammatory cells occurs, with podocytes being one of the primary targets of IC attacks (121). Anti-dsDNA antibodies can recognize various DNA structures and also cross-react with various non-DNA molecules present in the renal matrix or on the surfaces of endogenous renal cells, such as α-actinin, annexin II, collagen III/IV, and others (122–124). Additionally, the degree of glycosylation of anti-dsDNA antibodies can affect their pathogenicity, with α-2,6-sialylation attenuating their effects and fructosylation exacerbating their nephritogenic activity (125). The deposition of IC can activate the complement system, leading to the production of various complement components, such as C3, C4, C5a, and more. These complement components can attract and activate inflammatory cells like neutrophils, monocytes, and macrophages, which in turn release podocyte-toxic substances, resulting in direct damage or apoptosis of podocytes (126). An alternative hypothesis posits that the interplay of podocytes and TLRs could be a contributing factor in the pathogenesis of LN. The overexpression of TLR8 and TLR9 plays a key role in stimulating the NF-κB pathway, which results in the generation of specific inflammatory cytokines, namely IL1b, IL6, IFN-g, and TNF-α (127). This, in turn, intensifies the inflammatory response and contributes to glomerular damage. Significantly, an increase in TLR9 expression has been robustly associated with podocyte impairment. This is evident in the decline in podocyte numbers, loss of foot processes, and emergence of microvillous protrusions, along with the thickening and subsequent wrinkling of the basal membrane (14).
LN的发病机制尚不完全清楚,但流行的观点是LN的特征是由各种自身抗体形成的免疫复合物(IC)沉积,如抗双链DNA(ds-DNA)抗体和抗原。足细胞是IC攻击的主要目标之一(121)。抗dsDNA抗体可识别各种DNA结构,也可与肾基质或内源性肾细胞表面的各种非DNA分子发生交叉反应,如α-辅肌动蛋白、膜联蛋白II、胶原蛋白III/IV等(122- 124)。此外,抗dsDNA抗体的糖基化程度可影响其致病性,α-2,6-唾液酸化可减弱其作用,果糖基化可加重其致肾炎活性(125)。IC的沉积可以激活补体系统,导致产生各种补体成分,如C3、C4、C5 a等。 这些补体成分可以吸引并激活炎性细胞,如中性粒细胞、单核细胞和巨噬细胞,进而释放足细胞毒性物质,导致足细胞的直接损伤或凋亡(126)。另一种假设认为足细胞和TLR的相互作用可能是LN发病机制的一个促成因素。TLR 8和TLR 9的过表达在刺激NF-κB通路中起关键作用,导致产生特异性炎性细胞因子,即IL 1 B、IL 6、IFN-g和TNF-α(127)。这反过来又加剧了炎症反应并导致肾小球损伤。值得注意的是,TLR 9表达的增加与足细胞损伤密切相关。足细胞数量减少、足突消失、微绒毛突起出现、沿着基底膜增厚和随后的褶皱都是明显的(14)。
Recent research has revealed that, in addition to the involvement of relevant aberrant autoantibodies in antigen-antibody reactions contributing to the pathogenesis of LN, renal cells actively participate in the inflammation and immune response of LN. Kidneys harbor tertiary lymphoid structures (TLSs), resembling lymph node-like structures housing immune cells such as T cells, B cells, dendritic cells, macrophages, and more. TLSs serve as local immunity structures that promote adaptive immunity; their unencapsulated structure enables direct exposure to diverse stimuli from an inflamed environment, including CKD. These immune cells proliferate, differentiate, activate, and form memory within TLSs, actively engaging in the production of autoantibodies and pro-inflammatory factors, ultimately causing kidney tissue damage. Functional characterization of TLSs in CKD and the development of interventions to regulate kidney TLSs potentially lead to promising therapeutic avenues (128, 129). Notably, the preferential expression of TCR Vβ gene expression in intrarenal T cells, driven by antigen stimulation compared to peripheral blood lymphocytes, and the high expression of IL-4 and IL-10 on intrarenal T cells from LN patients, suggest that intrarenal T cells potentially play a critical role in the pathogenesis of LN (130). Furthermore, CD8+ T cells can induce podocyte injury, directly or indirectly, leading to crescent formation (131). Macrophages play a pivotal role in the pathogenesis of LN by mediating impaired kidney repair mechanisms in lupus-susceptible mice. Studies have revealed that these aberrant macrophages facilitate suboptimal kidney repair, precipitating the onset of LN in such mice (132). Similarly, dendritic cells, renowned as the most powerful APCs, are integral to bridging innate and adaptive immunity. Their role in engulfing apoptotic blebs followed by the promotion of Th17 cell differentiation is pivotal, leading to the maladaptive immune responses and the breakdown of self-tolerance that are hallmarks of SLE (133). Furthermore, podocytes are not only structural components of the glomerulus but also actively contribute to immune responses. These cells have been increasingly recognized for their significant role in the pathogenic mechanisms underlying LN. Dysregulated autophagy in podocytes is posited to be a contributory factor in the pathogenesis of SLE (134). Observations indicate that LN stimulates Cyclooxygenase-2(COX-2) in the endoplasmic reticulum stress pathway and activates Activating Transcription Factor 4(ATF4) in podocytes, while inhibition of COX-2 reduces LN-induced autophagy in these cells, highlighting COX-2 as a viable therapeutic target for LN (135). TLR9 has been identified as playing a significant role in the pathophysiology of LN, evidenced by its substantial up-regulation within glomerular structures (136). The interaction between TLR9 and IgG originating from patients is a critical aspect of the disease mechanism. These IgG molecules infiltrate podocytes, eliciting an enhanced expression of calcium/calmodulin-dependent protein kinase IV (CaMK4) (32). This increase in CaMK4 expression subsequently leads to the up-regulation of genes involved in podocyte damage and T-cell activation (137). Moreover, there is a discernible expression and activation of Nod-like receptor protein 3 (NLRP3) inflammasomes within the podocytes in instances of LN, as observed in lupus-susceptible murine models (38). Importantly, the pharmacological inhibition of the NLRP3 inflammasome has been shown to mitigate proteinuria and diminish both the histological deterioration of kidney tissue and the effacement of podocyte foot processes (138). These findings suggest that the activation of NLRP3 plays a crucial role in the advancement of podocyte injury and the ensuing proteinuria, comprising a significant aspect of the pathogenesis of LN.
最近的研究表明,除了相关异常自身抗体参与导致LN发病的抗原抗体反应外,肾细胞积极参与LN的炎症和免疫反应。肾脏具有三级淋巴样结构(TLS),类似于容纳免疫细胞如T细胞、B细胞、树突细胞、巨噬细胞等的淋巴结样结构。TLSs作为促进适应性免疫的局部免疫结构;其未封装的结构使其能够直接暴露于炎症环境(包括CKD)的各种刺激。这些免疫细胞增殖、分化、激活并在TLS内形成记忆,积极参与自身抗体和促炎因子的产生,最终导致肾组织损伤。CKD中TLS的功能表征和调节肾脏TLS的干预措施的开发可能会带来有希望的治疗途径(128,129)。 值得注意的是,与外周血淋巴细胞相比,肾内T细胞中TCR Vβ基因表达的优先表达(由抗原刺激驱动),以及LN患者肾内T细胞上IL-4和IL-10的高表达,表明肾内T细胞可能在LN的发病机制中发挥关键作用(130)。此外,CD8 + T细胞可直接或间接诱导足细胞损伤,导致新月体形成(131)。巨噬细胞通过介导狼疮易感小鼠受损的肾脏修复机制在LN的发病机制中发挥关键作用。研究表明,这些异常的巨噬细胞促进了次优的肾脏修复,促使这些小鼠发生LN(132)。同样,树突状细胞,作为最强大的APC而闻名,是连接先天性免疫和适应性免疫的组成部分。 它们在吞噬凋亡性大疱后促进Th 17细胞分化中的作用至关重要,导致适应不良免疫应答和自身耐受性的破坏,这是SLE的标志(133)。此外,足细胞不仅是肾小球的结构组成部分,而且还积极促进免疫反应。这些细胞在LN的致病机制中的重要作用越来越被人们所认识。足细胞自噬失调被认为是SLE发病机制的一个促成因素(134)。观察结果表明LN刺激内质网应激途径中的环氧合酶-2(考克斯-2)并激活足细胞中的激活转录因子4(ATF 4),而抑制考克斯-2可减少这些细胞中LN诱导的自噬,这突出表明考克斯-2是LN的可行治疗靶点(135)。 TLR 9在肾小球结构中的显著上调证实了其在LN的病理生理学中起重要作用(136)。TLR 9与患者IgG之间的相互作用是疾病机制的关键方面。这些IgG分子浸润足细胞,引起钙/钙调蛋白依赖性蛋白激酶IV(CaMK 4)的表达增强(32)。CaMK 4表达的增加随后导致足细胞损伤和T细胞活化相关基因的上调(137)。此外,如在狼疮易感小鼠模型中所观察到的,在LN的情况下,足细胞内存在可辨别的Nod样受体蛋白3(NLRP 3)炎性体的表达和活化(38)。重要的是,药理学抑制NLRP 3炎性小体可减轻蛋白尿,减少肾组织的组织学恶化和足细胞足突消失(138)。 这些发现表明NLRP 3的激活在足细胞损伤的进展和随后的蛋白尿中起着至关重要的作用,包括LN发病机制的重要方面。
In recent years, significant progress has been made in understanding the pathogenesis, diagnosis, and treatment of podocyte immune injury in LN. However, there is still a significant research agenda focused on exploring various pathogenic mechanisms and identifying biomarkers associated with this condition. Current research aims to uncover new diagnostic methods and biomarkers to gain a deeper understanding of the underlying processes causing podocyte immune injury in LN. Moreover, identifying potential therapeutic targets is crucial for the development of more effective treatments for this severe complication of SLE.
近年来,对LN足细胞免疫损伤的发病机制、诊断和治疗的认识取得了重大进展。然而,仍然有一个重要的研究议程集中在探索各种致病机制和识别与这种疾病相关的生物标志物上。当前的研究旨在发现新的诊断方法和生物标志物,以更深入地了解导致LN足细胞免疫损伤的潜在过程。此外,确定潜在的治疗靶点对于开发更有效的治疗方法来治疗这种严重的系统性红斑狼疮并发症至关重要。
5 Therapeutic strategies targeting immunity in podocyte injury
足细胞损伤的5种靶向免疫治疗策略
5.1 Current standard therapies for podocyte-related proteinuric glomerular diseases
5.1足细胞相关蛋白尿性肾小球疾病的当前标准治疗
The clinical manifestations of podocyte-related kidney diseases often present as proteinuria. Most of the diseases are widely acknowledged as immune-mediated kidney disorders. As a result, the standard approach to treating proteinuric glomerular kidney diseases associated with podocyte injury primarily revolves around reducing proteinuria and moderating hyperactive immune responses through the application of immunosuppressive therapy (139). Aside from the commonly used drugs like renin-angiotensin-aldosterone inhibitors, sodium-glucose cotransporter-1 inhibitors, glucagon-like peptide-1 agonists, and mineralocorticoid receptor antagonists, which reduce proteinuria and protect kidney functions through non-immunological mechanisms of action (140), we will focus on the immune-related drugs for podocyte-related proteinuric kidney diseases below.
足细胞相关性肾脏疾病的临床表现常以蛋白尿为主。大多数疾病被广泛认为是免疫介导的肾脏疾病。因此,治疗与足细胞损伤相关的蛋白尿性肾小球性肾病的标准方法主要是通过应用免疫抑制治疗减少蛋白尿和调节过度活跃的免疫反应(139)。除了常用的药物如肾素-血管紧张素-醛固酮抑制剂、钠-葡萄糖协同转运蛋白-1抑制剂、胰高血糖素样肽-1激动剂和盐皮质激素受体拮抗剂通过非免疫作用机制减少蛋白尿和保护肾功能外(140),我们将重点关注以下足细胞相关蛋白尿性肾病的免疫相关药物。
5.1.1 Immunosuppressive drugs
5.1.1免疫抑制药物
Glucocorticoids (GCs) have been the mainstay in the treatment of podocyte-related proteinuric kidney diseases for several decades (141). These agents primarily exert their effects by binding to the glucocorticoid receptor (GR) in podocytes, thereby modulating gene expression and influencing the structure and function of these cells (142). For instance, GCs are capable of suppressing the secretion of various pro-inflammatory cytokines, such as interleukins (IL), TGF-β, and tumor necrosis factor (TNF), thereby attenuating the inflammatory response in the glomerulus (143). Furthermore, GCs activate the gene promoter of nephrin, a critical protein in the kidney’s slit diaphragm, thereby facilitating its proper glycosylation and phosphorylation, and reinforcing its connection with the actin cytoskeleton, ultimately maintaining the integrity and stability of the slit diaphragm (144–147). The KDIGO (Kidney Disease: Improving Global Outcomes) 2012 guidelines classified nephrotic syndrome into different types based on the response to glucocorticoid therapy, namely, glucocorticoid-sensitive and glucocorticoid-resistant nephrotic syndrome (148). It has been observed that oral glucocorticoid therapy in children with nephrotic syndrome may encounter issues of resistance, with approximately 50% of affected children eventually progressing to end-stage kidney disease if unresponsive after five years of treatment (149). Consequently, the implementation of specific immunosuppressive therapies, including drugs such as cyclophosphamide, has become necessary (150). The combination of alkylating agents with steroids has demonstrated high efficacy in managing high-risk podocyte-associated proteinuric kidney diseases such as MN (151). Studies have indicated the effectiveness of the combination of cyclophosphamide with prednisone for various high-risk MN patients (152). Research indicates that high-dose cyclophosphamide suppresses CD103+ dendritic cells in a rat model, resulting in changes in their frequency, surface molecule expression, and antigen-capturing ability. This alteration enhances CD4+ T cell activation, modulates the TLR/MyD88/MAPK pathway, and results in increased Treg levels while reducing Th1/Th2 differentiation and Th17 generation (153). In a ten-year multicenter retrospective study involving 752 pMN patients, cyclophosphamide demonstrated superior performance compared to calcineurin inhibitors. Cyclophosphamide showed statistically significant improvements in treatment response, kidney function preservation, and a lower recurrence rate (154). Despite the fact that calcineurin inhibitors are less effective compared to cyclophosphamide in the management of PMN, they maintain a crucial role in the treatment of podocyte immunological injury.
几十年来,糖皮质激素(GC)一直是治疗足细胞相关蛋白尿性肾病的主要药物(141)。这些药物主要通过与足细胞中的糖皮质激素受体(GR)结合发挥作用,从而调节基因表达并影响这些细胞的结构和功能(142)。例如,GC能够抑制多种促炎细胞因子的分泌,如白细胞介素(IL)、TGF-β和肿瘤坏死因子(TNF),从而减轻肾小球中的炎症反应(143)。此外,GC激活肾裂膜中的关键蛋白nephrin的基因启动子,从而促进其适当的糖基化和磷酸化,并加强其与肌动蛋白细胞骨架的连接,最终保持裂膜的完整性和稳定性(144- 147)。 KDIGO(肾脏疾病:改善全球结局)2012年指南根据对糖皮质激素治疗的反应将肾病综合征分为不同类型,即糖皮质激素敏感型和糖皮质激素耐药型肾病综合征(148)。据观察,肾病综合征患儿口服糖皮质激素治疗可能会遇到耐药问题,约50%的患儿在治疗5年后无反应,最终进展为终末期肾病(149)。因此,实施特定的免疫抑制疗法,包括环磷酰胺等药物,已成为必要的(150)。烷化剂与类固醇的联合应用在治疗高危足细胞相关蛋白尿性肾病如MN方面显示出较高的疗效(151)。研究表明环磷酰胺联合泼尼松治疗各种高危MN患者的有效性(152)。 研究表明,高剂量环磷酰胺抑制大鼠模型中的CD 103 + 树突状细胞,导致其频率、表面分子表达和抗原捕获能力发生变化。这种改变增强了CD4 + T细胞活化,调节TLR/MyD 88/MAPK通路,导致Treg水平升高,同时减少Th 1/Th 2分化和Th 17生成(153)。在一项涉及752例pMN患者的10年多中心回顾性研究中,环磷酰胺表现出比钙调磷酸酶抑制剂更好的上级性能。环磷酰胺在治疗反应、肾功能保护和较低复发率方面显示出统计学显著改善(154)。尽管钙调神经磷酸酶抑制剂在PMN的管理中不如环磷酰胺有效,但它们在足细胞免疫损伤的治疗中仍然发挥着至关重要的作用。
Calcineurin inhibitors, such as cyclosporine A, tacrolimus, and voclosporin, belong to a class of immunosuppressants that inhibit T cell activation and regulate the Th17 immune response, thereby modulating the occurrence and development of podocyte-related proteinuric kidney diseases (155, 156). Cyclosporine A is of vital importance for the stabilization and protection of podocytes. Evidence has shown that it directly stabilizes the actin cytoskeleton in podocytes, thus helping to maintain the integrity of the glomerular filtration barrier, which is essential for proper podocyte functioning (157). Moreover, research has revealed that Cyclosporine A indirectly safeguards podocytes by regulating the phosphorylation of proteins implicated in the control of actin dynamics, such as WAVE1 (158), and by stabilizing the expression of cofilin-1 (159). Furthermore, Cyclosporine A has proven to be efficacious in addressing specific genetic podocyte damage (160). Tacrolimus is a substance that has been conclusively shown to offer protection to podocytes via mechanisms that include cytoskeleton stabilization, cell apoptosis inhibition, and damage repair (161). Various rodent models of kidney injury have highlighted the ability of Tacrolimus to rejuvenate impaired podocytes and maintain the expression level of Calcineurin Binding Protein 1 (Cabin1) (162, 163). In the context of diabetic nephropathy, Tacrolimus confers protection to podocytes from apoptotic death through downregulating Transient Receptor Potential Channel 6 (TRPC6) (164). Moreover, Tacrolimus aids in mitigating podocytic injury by reinstating FK506 Binding Protein 12 (FKBP12) on the actin cytoskeleton (165). Despite the rapid reduction in proteinuria in pMN upon treatment with calcineurin inhibitors, their long-term effects tend to be unstable, leading to relapses upon discontinuation, and are associated with certain toxic side effects (166). Therefore, the KDIGO 2021 guidelines suggest the use of calcineurin inhibitors in patients with normal kidney function and a moderate risk of disease progression to shorten the duration of proteinuria (139). Voclosporin, a newly developed calcineurin inhibitor, has been licensed in a multitude of countries for the management of lupus nephritis (167). Besides its recognized T-cell immunosuppressive capabilities, Voclosporin serves a dual role in the stabilization of podocytes and exhibiting anti-proteinuric traits. Empirical evidence suggests its superior efficacy in proteinuria reduction, outpacing both cyclosporine A and Tacrolimus (168).
钙调磷酸酶抑制剂,如环孢素A、他克莫司和voclosporin,属于一类免疫抑制剂,可抑制T细胞活化并调节Th 17免疫应答,从而调节足细胞相关蛋白尿性肾病的发生和发展(155,156)。环孢素A对足细胞的稳定和保护至关重要。有证据表明,它直接稳定足细胞中的肌动蛋白细胞骨架,从而有助于维持肾小球滤过屏障的完整性,这对足细胞的正常功能至关重要(157)。此外,研究表明,环孢菌素A通过调节与肌动蛋白动力学控制有关的蛋白质(如WAVE 1)的磷酸化,以及稳定cofilin-1的表达,间接保护足细胞(159)。此外,环孢菌素A已被证明可有效解决特定遗传足细胞损伤(160)。 他克莫司是一种已被证实可通过细胞骨架稳定、细胞凋亡抑制和损伤修复等机制保护足细胞的物质(161)。各种啮齿动物肾损伤模型都强调了他克莫司使受损足细胞恢复活力并维持钙调磷酸酶结合蛋白1(Cabin1)表达水平的能力(162,163)。在糖尿病肾病的背景下,他克莫司通过下调瞬时受体电位通道6(TRPC 6)保护足细胞免于凋亡(164)。此外,他克莫司通过恢复肌动蛋白细胞骨架上的FK506结合蛋白12(FKBP12)来帮助减轻足细胞损伤(165)。尽管钙调磷酸酶抑制剂治疗后pMN患者蛋白尿迅速减少,但其长期作用往往不稳定,导致停药后复发,并伴有某些毒副作用(166)。 因此,KDIGO 2021指南建议在肾功能正常和中度疾病进展风险的患者中使用钙调磷酸酶抑制剂,以缩短蛋白尿的持续时间(139)。Voclosporin是一种新开发的钙调磷酸酶抑制剂,已在许多国家获得许可用于治疗狼疮性肾炎(167)。除了其公认的T细胞免疫抑制能力外,Voclosporin在稳定足细胞和显示抗蛋白尿特性方面具有双重作用。经验证据表明其在减少蛋白尿方面的上级疗效优于环孢素A和他克莫司(168)。
While mycophenolate mofetil (MMF) is well-established as an immunosuppressant for renal immune diseases, the intricacies of its mechanistic effect on podocytes remain to be fully elucidated. Nevertheless, several lines of research provide intriguing insights into potential modes of action. It has been suggested that one facet of MMF’s protective effect against podocyte injury could be attributed to its role in restoring the integrity of the cytoskeletal axis of actin filaments (169, 170). Furthermore, experiments utilizing diabetic mouse models treated with MMF showed a notable reduction in the number of infiltrated CD4 and CD8 T cells within the kidneys. More interestingly, the previous downward trajectory of nephrin and WT1 expression in the glomeruli appeared to be successfully arrested and reversed (171).
虽然霉酚酸酯(MMF)是一种公认的肾脏免疫性疾病的免疫抑制剂,但其对足细胞的复杂机制仍有待充分阐明。尽管如此,几条研究路线为潜在的行动模式提供了有趣的见解。研究表明,霉酚酸酯对足细胞损伤的保护作用的一个方面可能是其在恢复肌动蛋白丝细胞骨架轴完整性方面的作用(169,170)。此外,利用用MMF治疗的糖尿病小鼠模型的实验显示肾脏内浸润的CD4和CD8 T细胞的数量显著减少。更有趣的是,之前肾小球中nephrin和WT 1表达的下降轨迹似乎被成功阻止和逆转(171)。
While immunosuppressive drugs primarily target immune-mediated glomerular diseases, their use may lead to serious side effects, especially after prolonged use, such as infections, osteoporosis, and hypertension. Moreover, these drugs may not be effective or have limited efficacy in some non-immune proteinuric glomerular diseases, including genetic or metabolic glomerular diseases. Consequently, the development of novel drugs to manage various types of podocyte immune injury is urgently needed.
虽然免疫抑制药物主要针对免疫介导的肾小球疾病,但它们的使用可能导致严重的副作用,特别是在长期使用后,如感染,骨质疏松症和高血压。此外,这些药物在一些非免疫性蛋白尿性肾小球疾病中可能无效或疗效有限,包括遗传性或代谢性肾小球疾病。因此,迫切需要开发新的药物来管理各种类型的足细胞免疫损伤。
5.1.2 Biological agents 5.1.2生物制剂
In recent years, our understanding of the molecular mechanisms and immune targets involved in podocyte injury has advanced, leading to the continuous exploration and development of novel immune-targeted therapeutic strategies (Table 2). Biologic agents have emerged as a pivotal treatment option for immune-related diseases and hold significant promise for addressing podocyte-related conditions. RTX, a human-mouse chimeric CD20 monoclonal antibody, disrupts the interaction between B cells and T cells by specifically binding to CD20, inducing CD20+ B cell depletion through antibody-dependent and complement-dependent cytotoxic effects (186, 187). In podocytes, RTX also plays a protective role by preserving the actin cytoskeleton, preventing the downregulation of SMPDL-3b, and inhibiting actin cytoskeleton remodeling, ultimately reducing proteinuria (188). A combined treatment approach involving RTX, initial short-term low-dose oral cyclophosphamide, and a rapid tapering of prednisone has shown encouraging results. After a follow-up period of 25 to 62 months for 60 patients with pMN receiving this combined treatment, the study by Zonozi et al. reported that 100% of patients achieved partial remission, with 90% achieving lasting complete remission, while maintaining an acceptable safety profile (189). Ofatumumab (OFA), a new generation of anti-CD20 IgG1κ human monoclonal antibodies, offers an excellent alternative for patients resistant to or sensitized against RTX. OFA is a well-tolerated and safe treatment with longer intervals between doses, which can effectively replace RTX (190). Some studies even suggest that low-dose OFA can induce remission of proteinuria in children with long-term treatment-resistant INS (191). RTX can also be used in combination with calcineurin inhibitors to enhance therapeutic efficacy (192).
近年来,我们对足细胞损伤的分子机制和免疫靶点的理解有所进展,从而不断探索和开发新的免疫靶向治疗策略(表2)。生物制剂已成为免疫相关疾病的关键治疗选择,并有望解决足细胞相关疾病。RTX是一种人-鼠嵌合CD 20单克隆抗体,通过特异性结合CD 20破坏B细胞和T细胞之间的相互作用,通过抗体依赖性和补体依赖性细胞毒性效应诱导CD 20 + B细胞耗竭(186,187)。在足细胞中,RTX还通过保护肌动蛋白细胞骨架,防止SMPDL-3b下调,抑制肌动蛋白细胞骨架重塑,最终减少蛋白尿而发挥保护作用(188)。 一种包括RTX、初始短期低剂量口服环磷酰胺和泼尼松快速减量的联合治疗方法显示出令人鼓舞的结果。Zonozi等人的研究对60例接受该联合治疗的pMN患者进行了25 - 62个月的随访,结果显示100%的患者获得部分缓解,90%的患者获得持续完全缓解,同时保持了可接受的安全性(189)。奥法木单抗(OFA)是新一代抗CD 20 IgG 1 κ人源单克隆抗体,为对RTX耐药或致敏的患者提供了极好的替代方案。OFA是一种耐受性良好且安全的治疗方法,给药间隔较长,可有效替代RTX(190)。一些研究甚至表明,低剂量OFA可以缓解长期治疗抵抗性INS患儿的蛋白尿(191)。RTX也可以与钙调磷酸酶抑制剂联合使用,以提高治疗效果(192)。
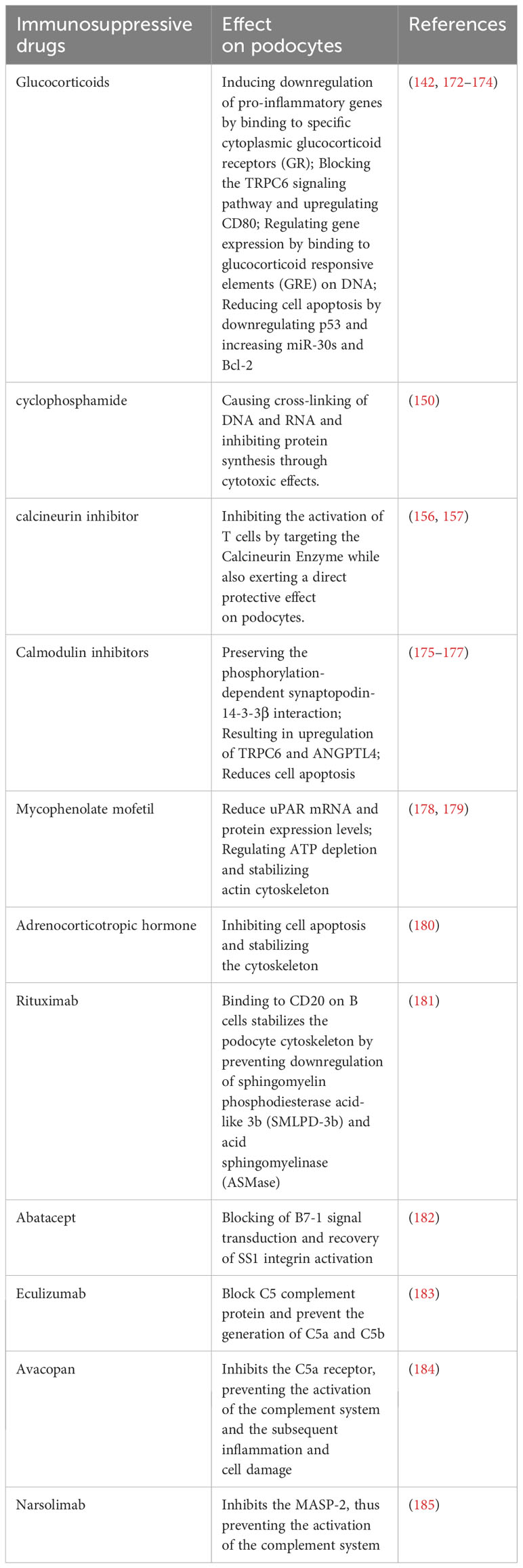
Table 2 Commonly used immunotherapy drugs for podocyte-related proteinuric kidney disease.
表2足细胞相关蛋白尿肾病常用免疫治疗药物。
Abatacept, categorized as an immunomodulatory drug, is formed by fusing cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), a surface protein, with the Fc segment of human immunoglobulin. External factors like viral infections and Treg function can impact the expression of B7-1 (CD80) on podocytes, potentially causing podocyte cytoskeletal disarray and severe proteinuria (193). CTLA-4, a critical co-factor expressed on podocytes and Tregs, binds to B7-1 (CD80), inhibits T cell activation, and reinstates SS1 integrin activation, thereby alleviating podocyte injury and proteinuria (194). Contrary to conventional wisdom, recent research challenges the notion of increased B7-1 expression within the podocytes of individuals with proteinuria. Studies investigating minimal change disease (MCD) and focal segmental glomerulosclerosis (FSGS) utilizing multiple antibodies and assays found no significant upregulation of podocyte B7-1 in these conditions, casting doubt on the efficacy of B7-1 inhibitory treatment (195). Moreover, B7-1 is not induced in podocytes of patients with diabetic nephropathy (DN) or in BTBR ob/ob mice, a type 2 diabetes model (196). A recent study exploring abatacept’s efficacy in post-kidney transplant FSGS patients who developed FSGS after transplantation and failed conventional therapy revealed that responders to abatacept were B7-1 positive. This suggests that podocyte B7-1 staining in kidney transplant biopsies might identify patients benefiting from abatacept (197). Researchers propose that the mechanism behind the anti-proteinuric effects of B7-1 inhibitors may involve the suppression of immune cell activation rather than direct effects on podocytes (195). Furthermore, some evidence suggests a direct podocyte protective role of abatacept (198). Consequently, further research is crucial to elucidate the association between B7-1 and podocyte damage in different kidney conditions.
阿巴西普是一种免疫调节药物,通过将细胞毒性T淋巴细胞相关抗原4(CTLA-4)(一种表面蛋白)与人免疫球蛋白的Fc段融合而形成。病毒感染和Treg功能等外部因素可影响足细胞上B7-1(CD 80)的表达,可能导致足细胞骨架紊乱和严重蛋白尿(193)。CTLA-4是一种在足细胞和T细胞上表达的关键辅因子,可与B7-1(CD 80)结合,抑制T细胞活化,并恢复SS 1整联蛋白活化,从而减轻足细胞损伤和蛋白尿(194)。与传统观点相反,最近的研究挑战了蛋白尿个体足细胞内B7-1表达增加的概念。 利用多种抗体和检测方法研究微小病变病(MCD)和局灶节段性肾小球硬化症(FSGS)的研究发现,在这些疾病中,足细胞B7-1没有显著上调,这对B7-1抑制治疗的疗效产生了怀疑(195)。此外,B7-1在糖尿病肾病(DN)患者或BTBR ob/ob小鼠(一种2型糖尿病模型)的足细胞中未被诱导(196)。最近的一项研究探讨了阿巴西普在肾移植后FSGS患者中的疗效,这些患者在移植后发展为FSGS并且常规治疗失败,该研究显示阿巴西普的应答者是B7-1阳性。这表明肾移植活检组织中的足细胞B7-1染色可能识别出受益于阿巴西普的患者(197)。研究人员提出,B7-1抑制剂抗蛋白尿作用的机制可能涉及抑制免疫细胞活化,而不是直接作用于足细胞(195)。 此外,一些证据表明阿巴西普具有直接的足细胞保护作用(198)。因此,进一步的研究对于阐明B7-1与不同肾脏条件下足细胞损伤之间的关联至关重要。
Immunotherapy, which leverages the immune system to intervene in podocyte damage, is a promising approach known for its high specificity, minimal side effects, and durable benefits. It can be applied to treat various types of podocyte damage. Future research should prioritize a deeper understanding of the mechanisms involved and the optimization of parameters for immunotherapy to improve its clinical applicability and effectiveness.
免疫疗法利用免疫系统干预足细胞损伤,是一种有前途的方法,以其高特异性,最小的副作用和持久的益处而闻名。它可用于治疗各种类型的足细胞损伤。未来的研究应该优先考虑更深入地了解所涉及的机制和免疫治疗参数的优化,以提高其临床适用性和有效性。
5.2 Novel therapeutic approaches
5.2新的治疗方法
In recent years, as our understanding of the immune mechanisms underlying podocyte injury in proteinuric kidney diseases has deepened, new prospects for immunotherapy in managing podocyte diseases have emerged. The involvement of the complement system in the pathogenesis of podocyte injury has been highlighted (199). C5b is known to induce osmotic lysis of podocytes, leading to alterations in the actin cytoskeleton and the podocyte slit diaphragm, as well as limiting the proliferation of podocytes (200). Therefore, the utilization of the complement inhibitor Eculizumab to impede the activation of the complement cascade and the subsequent generation of C5b constitutes an effective strategy for intervention (183). Avacopan, as a selective inhibitor of the C5a receptor, orchestrates a blockade against C5a activity (201). By attenuating the complement system’s activation and thereby abating subsequent inflammatory responses and cellular damage, this inhibition facilitates an indirect protective effect on podocytes through the modulation of the inflammatory environment (184). Narsolimab, or OMS721, is a fully human monoclonal antibody that specifically targets and inhibits mannan-binding lectin-associated serine protease-2 (MASP-2) (202). MASP-2 serves as the effector enzyme within the lectin pathway of the complement system, which is regarded as one of the principal routes of complement activation (203). By effectively inhibiting MASP-2, narsolimab disrupts the activation of the lectin pathway, thereby mitigating complement-mediated inflammation and minimizing endothelial damage (204). In a recent study, the administration of narsolimab demonstrated clinically significant reductions in proteinuria and sustained stability in estimated glomerular filtration rates (185).
近年来,随着我们对蛋白尿肾病中足细胞损伤的免疫机制的理解不断加深,免疫治疗在管理足细胞疾病中的新前景已经出现。补体系统参与足细胞损伤的发病机制已被强调(199)。已知C5b可诱导足细胞的渗透溶解,导致肌动蛋白细胞骨架和足细胞狭缝隔膜的改变,并限制足细胞的增殖(200)。因此,使用补体抑制剂依库珠单抗来阻止补体级联的激活和随后C5b的产生构成了一种有效的干预策略(183)。Avacopan作为C5a受体的选择性抑制剂,可阻断C5a活性(201)。 通过减弱补体系统的激活,从而减轻随后的炎症反应和细胞损伤,这种抑制通过调节炎症环境促进对足细胞的间接保护作用(184)。Narsolimab或OMS 721是一种全人源单克隆抗体,可特异性靶向并抑制甘露聚糖结合凝集素相关丝氨酸蛋白酶-2(MASP-2)(202)。MASP-2作为补体系统凝集素途径内的效应酶,其被认为是补体活化的主要途径之一(203)。通过有效抑制MASP-2,narsolimab可破坏凝集素途径的激活,从而减轻补体介导的炎症并最大限度地减少内皮损伤(204)。在最近的一项研究中,narsolimab的给药显示出蛋白尿的临床显著减少和估计的肾小球滤过率的持续稳定性(185)。
In the case of patients with MCD, podocytes express angiopoietin-like 4 (Angptl4), with one of its forms being hyposialylated Angptl4, which binds to the glomerular basement membrane and endothelial cells, leading to proteinuria (205). Utilizing N-acetyl-D-mannosamine has shown promise in converting hyposialylated Angptl4 to its sialylated form, which can be taken up and stored by podocytes, thereby reducing the production of proteinuria (206). Although this appears to be a potential treatment option, relevant clinical data remains limited.
在MCD患者中,足细胞表达血管生成素样4(Angptl 4),其中一种形式为低唾液酸化Angptl 4,与肾小球基底膜和内皮细胞结合,导致蛋白尿(205)。利用N-乙酰基-D-甘露糖胺已显示出将低唾液酸化的Angptl 4转化为其唾液酸化形式的前景,其可被足细胞吸收和储存,从而减少蛋白尿的产生(206)。虽然这似乎是一种潜在的治疗选择,但相关的临床数据仍然有限。
The field of immunotherapy for podocyte preservation is in a state of constant advancement, highlighted by the emergence of various natural compounds and small-molecule inhibitors known for their podocyte-protective properties. For example, curcumin, derived from turmeric, has been recognized for effectively reducing reactive oxygen species (ROS) and RIPK3 expression, thereby establishing itself as a potential therapeutic agent for combating diabetic nephropathy (207). Additionally, luteolin exerts a protective effect against glucose-induced podocyte stress by inhibiting the NLRP3 inflammasome, a critical component of the immune system and inflammatory response (208). Expanding the scope, Astragaloside IV is acknowledged for its comprehensive protective capabilities, which include mitigating endoplasmic reticulum stress, promoting autophagy, and improving mitochondrial function (209). This multitargeted approach involves interaction with several signaling pathways such as SERCA2, AMPKα, and Nrf2-ARE/TFAM, highlighting the molecule’s potential in supporting podocyte integrity (210, 211). In a similar vein, hyperoside presents a protective strategy by modulating mitochondrial dynamics. It specifically inhibits mitochondrial fission, which has been observed to diminish albuminuria and renal damage in experimental models, further emphasizing its therapeutic potential (212).
用于足细胞保护的免疫治疗领域处于不断发展的状态,突出表现为各种天然化合物和小分子抑制剂的出现,这些化合物和小分子抑制剂以其足细胞保护特性而闻名。例如,从姜黄中提取的姜黄素被认为可以有效降低活性氧(ROS)和RIPK3的表达,从而成为治疗糖尿病肾病的潜在治疗药物(207)。此外,毛地黄黄酮通过抑制NLRP 3炎性小体(免疫系统和炎症反应的关键成分),对葡萄糖诱导的足细胞应激发挥保护作用(208)。扩大范围,黄芪甲苷四是公认的全面保护能力,其中包括减轻内质网应激,促进自噬,并改善线粒体功能(209)。 这种多靶点方法涉及与SERCA 2、AMPKα和Nrf 2-ARE/TFAM等几种信号通路的相互作用,突出了该分子支持足细胞完整性的潜力(210,211)。类似地,金丝桃苷通过调节线粒体动力学来提供保护策略。它特异性抑制线粒体分裂,在实验模型中观察到线粒体分裂可减少蛋白尿和肾损伤,进一步强调了其治疗潜力(212)。
Within the realm of metabolic regulation, the Rho-associated coiled-coil-containing protein kinase 2 (ROCK2) signaling pathway has been identified as a crucial regulator with significant implications for podocyte health. The ROCK2 signaling pathway plays an instrumental role in numerous metabolic functions critical to podocyte health, notably the initiation of apoptosis (213). Additionally, it disrupts fatty acid oxidation through the downregulation of peroxisome proliferator-activated receptor alpha (PPARα), thus underlining the pathway’s fundamental influence on cellular metabolism and the podocyte’s adaptability to metabolic stress (214). Additionally, in the intricate web of intracellular signaling, the mechanistic target of the rapamycin (mTOR) pathway stands out, particularly through its two distinct complexes, mTORC1, and mTORC2, as a vital target for immunotherapeutic intervention (215). Inhibiting these complexes has been shown to offer reprieve to stressed podocytes, principally by counteracting the adverse effects of hyperglycemia (216). These effects extend to safeguarding cell viability, reducing apoptosis, and maintaining the stability of structural proteins, which are fundamental to podocyte function. Moreover, contemporary research has shed light on the utility of angiotensin II inhibition in podocyte protection and the retardation of glomerulosclerosis progression (217). The benefits of this inhibition extend beyond the direct antagonism of angiotensin II type 1 (AT1) receptors on the podocytes themselves (218). Emerging evidence suggests additional protective mechanisms that may involve crosstalk with other cellular entities or alternative molecular pathways, presenting potential avenues for therapeutic interventions (Table 3).
在代谢调节领域,Rho相关的含有卷曲螺旋的蛋白激酶2(ROCK2)信号通路已被鉴定为对足细胞健康具有重要意义的关键调节剂。ROCK2信号通路在许多对足细胞健康至关重要的代谢功能中起着重要作用,特别是细胞凋亡的启动(213)。此外,它通过下调过氧化物酶体增殖物激活受体α(PPARα)破坏脂肪酸氧化,从而强调了该途径对细胞代谢和足细胞对代谢应激的适应性的根本影响(214)。此外,在错综复杂的细胞内信号网络中,雷帕霉素(mTOR)通路的机制靶点突出,特别是通过其两种不同的复合物mTORC 1和mTORC 2,作为免疫干预的重要靶点(215)。 抑制这些复合物已被证明可以缓解应激足细胞,主要是通过抵消高血糖的不良影响(216)。这些作用延伸到保护细胞活力,减少凋亡,并保持结构蛋白的稳定性,这是足细胞功能的基础。此外,当代研究揭示了血管紧张素II抑制剂在足细胞保护和肾小球硬化进展延迟中的作用(217)。这种抑制作用的益处超出了对足细胞本身血管紧张素II 1型(AT1)受体的直接拮抗作用(218)。新出现的证据表明,可能涉及与其他细胞实体或替代分子途径的串扰的其他保护机制,为治疗干预提供了潜在途径(表3)。
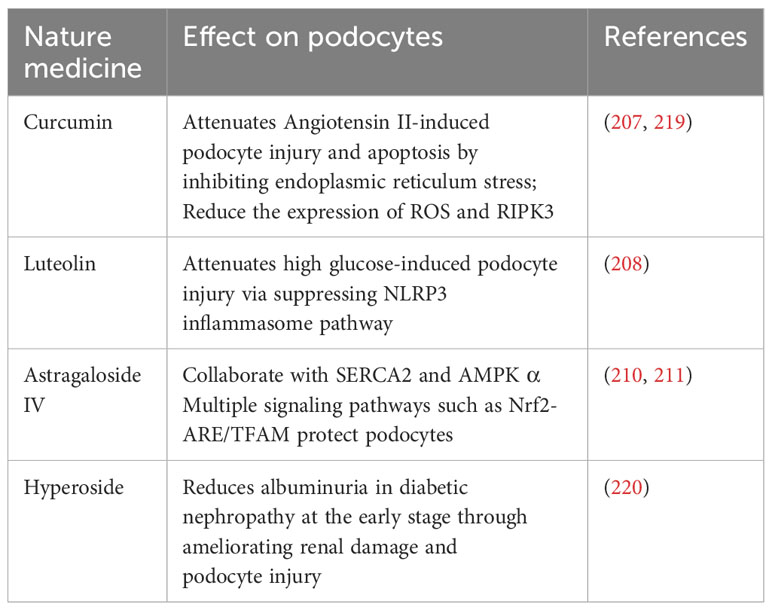
Table 3 Some natural medications with immunotherapeutic effects on podocyte injury.
表3部分天然药物对足细胞损伤有免疫调节作用。
Collectively, these diverse strategies underscore the rapid advancement in the realm of podocyte immunotherapy, paving the way for novel, tailored treatment options aimed at reducing the burden of podocyte injury and related kidney diseases. Each compound or pathway presents its mechanism of action, which when considered in conjunction, could offer a comprehensive, multi-targeted approach to combat renal pathologies associated with podocyte damage.
总的来说,这些不同的策略强调了足细胞免疫治疗领域的快速发展,为旨在减轻足细胞损伤和相关肾脏疾病负担的新型、量身定制的治疗方案铺平了道路。每种化合物或途径都有其作用机制,结合考虑,可以提供一种全面的、多靶点的方法来对抗与足细胞损伤相关的肾脏病理。
6 Conclusion remarks 6结论
Podocytes play a crucial role in both renal physiology and pathology, providing valuable insights into kidney function. While prior research has highlighted tubulointerstitial fibrosis as a key pathological mechanism in the progression and kidney failure of CKD, recent studies have also underscored the connection between the dysfunction and death of podocytes and the advancement of CKD. The immune response of podocytes serves as a pivotal factor in the development and progression of various proteinuric kidney diseases and presents itself as a promising therapeutic target. Despite advancements, numerous aspects of the mechanisms governing podocyte immune responses remain uncharted, emphasizing the need for further exploration. Subsequent research endeavors should aim to identify novel podocyte-related antigens and antibodies, unravel the intricacies of the regulatory mechanisms governing the interaction between podocytes and other immune cells, and develop innovative therapeutic strategies that specifically target podocyte immune responses. These efforts are essential for enhancing the prognosis and overall quality of life for patients affected by kidney disease and for addressing the current unmet medical needs in this area.
足细胞在肾脏生理和病理中起着至关重要的作用,为了解肾脏功能提供了有价值的见解。虽然先前的研究已经强调肾小管间质纤维化是CKD进展和肾衰竭的关键病理机制,但最近的研究也强调了足细胞功能障碍和死亡与CKD进展之间的联系。足细胞的免疫应答在各种蛋白尿性肾脏疾病的发生和发展中起着关键作用,并将其本身作为一个有前途的治疗靶点。尽管取得了进展,但足细胞免疫反应机制的许多方面仍然未知,强调需要进一步探索。 后续的研究工作应旨在确定新的足细胞相关抗原和抗体,解开错综复杂的监管机制之间的相互作用足细胞和其他免疫细胞,并开发创新的治疗策略,专门针对足细胞免疫反应。这些努力对于改善肾病患者的预后和整体生活质量以及解决该领域目前未满足的医疗需求至关重要。
Author contributions 作者贡献
HJ: Conceptualization, Supervision, Writing – review & editing. ZS: Investigation, Writing – original draft. JZ: Investigation, Writing – review & editing. CL: Funding acquisition, Writing – review & editing. YQ: Writing – review & editing. CX: Software, Writing – review & editing. SY: Writing – review & editing. XT: Conceptualization, Supervision, Writing – review & editing.
概念化、监督、写作- ZS:调查,写作-原始草案。JZ:调查,写作-审查和编辑。CL:资金获取,写作-审查和编辑。YQ:写作-审查和编辑。CX:软件,写作-审查和编辑。SY:写作-审查和编辑。XT:概念化,监督,写作-审查和编辑。
Funding 资金
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by a grant from the Central government guide local science and technology development special fund project: Xinjiang kidney disease big data smart medical innovation research and application base construction (grant number ZYYD2022C18) to CL. It was also supported by the Tianshan Talents - High-level Leading Talents Project (Department of Science and Technology of Xinjiang Uygur Autonomous Region): Construction and expansion of precision diagnosis and treatment system of chronic kidney disease based on clinical pathway and DRG (grant number 2022TSYCLJ0022) to CL.
作者声明本文的研究、作者身份和/或出版获得了财政支持。这项工作得到了中央政府引导地方科技发展专项资金项目:新疆肾脏病大数据智慧医疗创新研究与应用基地建设(批准号ZYYD 2022 C18)的资助。还获得天山英才-高层次领军人才项目(新疆维吾尔自治区科技厅):基于临床路径和DRG的慢性肾脏病精准诊疗体系建设与拓展(资助号2022 TSYCLJ 0022)对CL的支持。
Acknowledgments 致谢
We thank the Clinical Research Center of Kidney Disease of Xinjiang Uygur Autonomous Region for providing technical support. We also thank the Figdraw (www.figdraw.com) for their support in drawing the figures.
感谢新疆维吾尔自治区肾脏病临床研究中心提供的技术支持。我们也感谢Figdraw(www.figdraw.com)在绘制这些图时给予的支持。
Conflict of interest 利益冲突
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
作者声明,本研究是在不存在任何可能被视为潜在利益冲突的商业或财务关系的情况下进行的。
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
提交人宣称,提交时他们是《前沿》的编辑委员会成员。这对同行审查进程和最后决定没有影响。
Publisher’s note 出版者的笔记
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
本文中表达的所有声明仅为作者的声明,不一定代表其附属组织或出版商、编辑和审稿人的声明。本文中可能评估的任何产品,或其制造商可能提出的任何声明,均不受出版商的担保或认可。
References 引用
1. Hausmann R, Grepl M, Knecht V, Moeller MJ. The glomerular filtration barrier function. Curr Opin Nephrol Hypertension (2012) 21:441–9. doi: 10.1097/MNH.0b013e328354a28e
1.豪斯曼R,Grepl M,Knecht V,Moeller MJ.肾小球滤过屏障功能。Curr Opin Nephrol Hypertension(2012)21:441-9. doi:10.1097/MNH.0b013e328354a28e
CrossRef Full Text | Google Scholar
交叉引用全文|Google Scholar
2. Medina Rangel PX, Priyadarshini A, Tian X. New insights into the immunity and podocyte in glomerular health and disease: from pathogenesis to therapy in proteinuric kidney disease. Integr Med Nephrol Androl (2021) 8:5. doi: 10.4103/imna.imna_26_21
2.作者:王晓,王晓,王晓.肾小球健康和疾病中免疫和足细胞的新见解:从蛋白尿肾病的发病机制到治疗。Integr Med Nephrol Androl(2021)8:5. doi:10.4103/imna.imna_26_21
CrossRef Full Text | Google Scholar
交叉引用全文|Google Scholar
3. Garg P. A review of podocyte biology. Am J Nephrol (2018) 47:3–13. doi: 10.1159/000481633
3. Garg P.足细胞生物学综述。Am J Nephrol(2018)47:3-13。doi:10.1159/000481633
PubMed Abstract | CrossRef Full Text | Google Scholar
PubMed摘要|交叉引用全文|Google Scholar
4. Schell C, Huber TB. The evolving complexity of the podocyte cytoskeleton. J Am Soc Nephrol (2017) 28:3166–74. doi: 10.1681/asn.2017020143
4. Schell C,Huber TB.足细胞骨架的复杂性。J Am Soc Nephrol(2017)28:3166-74。doi:10.1681/asn.2017020143
PubMed Abstract | CrossRef Full Text | Google Scholar
PubMed摘要|交叉引用全文|Google Scholar
5. Kocylowski MK, Aypek H, Bildl W, Helmstädter M, Trachte P, Dumoulin B, et al. A slit-diaphragm-associated protein network for dynamic control of renal filtration. Nat Commun (2022) 13:6446. doi: 10.1038/s41467-022-33748-1
5. Kocylowski MK,Aypek H,Bildl W,Helmstädter M,Trachte P,Dumoulin B,et al. A slit-diaphragm-associated protein network for dynamic control of renal filtration. Nat Commun(2022)13:6446. doi:10.1038/s41467-022-33748-1
PubMed Abstract | CrossRef Full Text | Google Scholar
PubMed摘要|交叉引用全文|Google Scholar
6. Bhargava R, Tsokos GC. The immune podocyte. Curr Opin Rheumatol (2019) 31:167–74. doi: 10.1097/bor.0000000000000578
6. Bhargava R,Tsokos GC.免疫足细胞。Curr Opin Rheumiol(2019)31:167-74. doi:10.1097/bor.00000000000578
PubMed Abstract | CrossRef Full Text | Google Scholar
PubMed摘要|交叉引用全文|Google Scholar
7. Banas MC, Banas B, Hudkins KL, Wietecha TA, Iyoda M, Bock E, et al. TLR4 links podocytes with the innate immune system to mediate glomerular injury. J Am Soc Nephrol (2008) 19:704–13. doi: 10.1681/asn.2007040395
7. Banas MC,Banas B,Hudkins KL,Wietecha TA,Iyoda M,Bock E,et al. TLR4 links podocytes with the innate immune system to mediate glomerular injury. J Am Soc Nephrol(2008)19:704-13. doi:10.1681/asn.2007040395
PubMed Abstract | CrossRef Full Text | Google Scholar
PubMed摘要|交叉引用全文|Google Scholar
8. Mazgaeen L, Gurung P. Recent advances in lipopolysaccharide recognition systems. Int J Mol Sci (2020) 21:379. doi: 10.3390/ijms21020379
8. Mazgaeen L,Gurung P.脂多糖识别系统的最新进展。Int J Mol Sci(2020)21:379. doi:10.3390/ijms 21020379
PubMed Abstract | CrossRef Full Text | Google Scholar
PubMed摘要|交叉引用全文|Google Scholar
9. Ma J, Chadban SJ, Zhao CY, Chen X, Kwan T, Panchapakesan U, et al. TLR4 activation promotes podocyte injury and interstitial fibrosis in diabetic nephropathy. PloS One (2014) 9:e97985. doi: 10.1371/journal.pone.0097985
9. Ma J,Chadban SJ,Zhao CY,Chen X,Kwan T,Panchapakesan U,et al. TLR4 activation promotes podocyte injury and interstitial fibrosis in diabetic nephropathy. PloS One(2014)9:e97985. doi:10.1371/journal.pone.0097985
PubMed Abstract | CrossRef Full Text | Google Scholar
PubMed摘要|交叉引用全文|Google Scholar
10. Behzadi P, García-Perdomo HA, Karpiński TM, Niedźwiedzka-Rystwej P. Toll-like receptors: general molecular and structural biology. J Immunol Res (2021) 2021:1–21. doi: 10.1155/2021/9914854
10. Behzadi P,García-Perdomo HA,Karpienski TM,Niedzwiedzka-Rystwej P. Toll样受体:一般分子和结构生物学。J Immunol Res(2021)2021:1-21.版权所有© 2018 - 2019
CrossRef Full Text | Google Scholar
交叉引用全文|Google Scholar
11. Janssens S, Beyaert R. Role of toll-like receptors in pathogen recognition. Clin Microbiol Rev (2003) 16:637–46. doi: 10.1128/cmr.16.4.637-646.2003
11. Janssens S,Beyaert R. toll样受体在病原体识别中的作用。Clin Microbiol Rev(2003)16:637-46. doi:10.1128/cmr.16.4.637-646.2003
12. Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front Immunol (2014) 300:461. doi: 10.3389/fimmu.2014.00461
13. Bao W, Xia H, Liang Y, Ye Y, Lu Y, Xu X, et al. Toll-like receptor 9 can be activated by endogenous mitochondrial DNA to induce podocyte apoptosis. Sci Rep (2016) 6:22579. doi: 10.1038/srep22579
14. Masum MA, Ichii O, Hosny Ali Elewa Y, Nakamura T, Otani Y, Hosotani M, et al. Overexpression of toll-like receptor 9 correlates with podocyte injury in a murine model of autoimmune membranoproliferative glomerulonephritis. Autoimmunity (2018) 51:386–98. doi: 10.1080/08916934.2018.1549234
15. Burke GW, Mitrofanova A, Fontanella A, Ciancio G, Roth D, Ruiz P, et al. The podocyte: glomerular sentinel at the crossroads of innate and adaptive immunity. Front Immunol (2023) 14:1201619. doi: 10.3389/fimmu.2023.1201619
16. Komada T, Muruve DA. The role of inflammasomes in kidney disease. Nat Rev Nephrol (2019) 15:501–20. doi: 10.1038/s41581-019-0158-z
17. Bai M, Chen Y, Zhao M, Zhang Y, He JC, Huang S, et al. NLRP3 inflammasome activation contributes to aldosterone-induced podocyte injury. Am J Physiol Renal Physiol (2017) 312:F556–F64. doi: 10.1152/ajprenal.00332.2016
18. Zang N, Cui C, Guo X, Song J, Hu H, Yang M, et al. cGAS-STING activation contributes to podocyte injury in diabetic kidney disease. iScience (2022) 25:105145. doi: 10.1016/j.isci.2022.105145
19. Ge Z, Ding S. Regulation of cGAS/STING signaling and corresponding immune escape strategies of viruses. Front Cell Infect Microbiol (2022) 12:954581. doi: 10.3389/fcimb.2022.954581
20. Guey B, Ablasser A. Emerging dimensions of cellular cGAS-STING signaling. Curr Opin Immunol (2022) 74:164–71. doi: 10.1016/j.coi.2022.01.004
21. Mitrofanova A, Fontanella A, Tolerico M, Mallela S, Molina David J, Zuo Y, et al. Activation of stimulator of interferon genes (STING) causes proteinuria and contributes to glomerular diseases. J Am Soc NEPHROL (2022) 33:2153–73. doi: 10.1681/ASN.2021101286
22. Gao Z, Lu L, Chen X, Dobrzyn A. Release of HMGB1 in podocytes exacerbates lipopolysaccharide-induced acute kidney injury. Mediators Inflamm (2021) 2021:1–10. doi: 10.1155/2021/5220226
23. Tesch G, Sourris KC, Summers SA, McCarthy D, Ward MS, Borg DJ, et al. Deletion of bone-marrow-derived receptor for AGEs (RAGE) improves renal function in an experimental mouse model of diabetes. Diabetologia (2014) 57:1977–85. doi: 10.1007/s00125-014-3291-z
24. Jin J, Gong J, Zhao L, Zhang H, He Q, Jiang X. Inhibition of high mobility group box 1 (HMGB1) attenuates podocyte apoptosis and epithelial-mesenchymal transition by regulating autophagy flux. J Diab (2019) 11:826–36. doi: 10.1111/1753-0407.12914
25. Liu T, Li Q, Jin Q, Yang L, Mao H, Qu P, et al. Targeting HMGB1: A potential therapeutic strategy for chronic kidney disease. Int J Biol Sci (2023) 19:5020–35. doi: 10.7150/ijbs.87964
26. Reggiani F, Ponticelli C. Focal segmental glomerular sclerosis: do not overlook the role of immune response. J Nephrol (2016) 29:525–34. doi: 10.1007/s40620-016-0272-y
27. Goldwich A, Burkard M, Ölke M, Daniel C, Amann K, Hugo C, et al. Podocytes are nonhematopoietic professional antigen-presenting cells. J Am Soc Nephrol (2013) 24:906–16. doi: 10.1681/asn.2012020133
28. Li S, Liu Y, He Y, Rong W, Zhang M, Li L, et al. Podocytes present antigen to activate specific T cell immune responses in inflammatory renal disease. J Pathol (2020) 252:165–77. doi: 10.1002/path.5508
29. Luckheeram RV, Zhou R, Verma AD, Xia B. CD4+T cells: differentiation and functions. Clin Dev Immunol (2012) 2012:1–12. doi: 10.1155/2012/925135
30. Ruszkowski J, Lisowska KA, Pindel M, Heleniak Z, Dębska-Ślizień A, Witkowski JM. T cells in IgA nephropathy: role in pathogenesis, clinical significance and potential therapeutic target. Clin Exp Nephrol (2018) 23:291–303. doi: 10.1007/s10157-018-1665-0
31. Novelli R, Benigni A, Remuzzi G. The role of B7-1 in proteinuria of glomerular origin. Nat Rev Nephrol (2018) 14:589–96. doi: 10.1038/s41581-018-0037-z
32. Liu R, Wen X, Peng X, Zhao M, Mi L, Lei J, et al. Immune podocytes in the immune microenvironment of lupus nephritis (Review). Mol Med Rep (2023) 28:204. doi: 10.3892/mmr.2023.13091
33. Nagata M. Podocyte injury and its consequences. Kidney Int (2016) 89:1221–30. doi: 10.1016/j.kint.2016.01.012
34. Gao S, Cui Z, Zhao M-h. Complement C3a and C3a receptor activation mediates podocyte injuries in the mechanism of primary membranous nephropathy. J Am Soc Nephrol (2022) 33:1742–56. doi: 10.1681/asn.2021101384
35. Li X, Ding F, Zhang X, Li B, Ding J. The expression profile of complement components in podocytes. Int J Mol Sci (2016) 17:471. doi: 10.3390/ijms17040471
36. Bruno V, Mühlig AK, Oh J, Licht C. New insights into the immune functions of podocytes: the role of complement. Mol Cell Pedia (2023) 10:3. doi: 10.1186/s40348-023-00157-3
37. Gomes MT, Campos PC, Pereira Gde S, Bartholomeu DC, Splitter G, Oliveira SC. TLR9 is required for MAPK/NF-kappaB activation but does not cooperate with TLR2 or TLR6 to induce host resistance to Brucella abortus. J Leukoc Biol (2016) 99:771–80. doi: 10.1189/jlb.4A0815-346R
38. Fu R, Guo C, Wang S, Huang Y, Jin O, Hu H, et al. Podocyte activation of NLRP3 inflammasomes contributes to the development of proteinuria in lupus nephritis. Arthritis Rheumatol (2017) 69:1636–46. doi: 10.1002/art.40155
39. Xiang H, Zhu F, Xu Z, Xiong J. Role of inflammasomes in kidney diseases via both canonical and non-canonical pathways. Front Cell Dev Biol (2020) 8:106. doi: 10.3389/fcell.2020.00106
40. Wang Z, Zhang S, Xiao Y, Zhang W, Wu S, Qin T, et al. NLRP3 inflammasome and inflammatory diseases. Oxid Med Cell Longev (2020) 2020:4063562. doi: 10.1155/2020/4063562
41. Wu M, Yang Z, Zhang C, Shi Y, Han W, Song S, et al. Inhibition of NLRP3 inflammasome ameliorates podocyte damage by suppressing lipid accumulation in diabetic nephropathy. Metabolism (2021) 118:154748. doi: 10.1016/j.metabol.2021.154748
42. Mitrofanova A, Fontanella A, Tolerico M, Mallela S, Molina David J, Zuo Y, et al. Activation of stimulator of IFN genes (STING) causes proteinuria and contributes to glomerular diseases. J Am Soc NEPHROL (2022) 33:2153–73. doi: 10.1681/ASN.2021101286
43. Yang H, Wang H, Andersson U. Targeting inflammation driven by HMGB1. Front Immunol (2020) 11:484. doi: 10.3389/fimmu.2020.00484
44. Zhong H, Li X, Zhou S, Jiang P, Liu X, Ouyang M, et al. Interplay between RAGE and TLR4 regulates HMGB1-induced inflammation by promoting cell surface expression of RAGE and TLR4. J Immunol (2020) 205:767–75. doi: 10.4049/jimmunol.1900860
45. Zhang H, Deng Z, Wang Y. Molecular insight in intrarenal inflammation affecting four main types of cells in nephrons in IgA nephropathy. Front Med (2023) 10:1128393. doi: 10.3389/fmed.2023.1128393
46. Daehn I, Casalena G, Zhang T, Shi S, Fenninger F, Barasch N, et al. Endothelial mitochondrial oxidative stress determines podocyte depletion in segmental glomerulosclerosis. J Clin Invest (2014) 124:1608–21. doi: 10.1172/jci71195
47. Qi H, Casalena G, Shi S, Yu L, Ebefors K, Sun Y, et al. Glomerular endothelial mitochondrial dysfunction is essential and characteristic of diabetic kidney disease susceptibility. Diabetes (2017) 66:763–78. doi: 10.2337/db16-0695
48. Tufro A, Veron D. VEGF and podocytes in diabetic nephropathy. Semin Nephrol (2012) 32:385–93. doi: 10.1016/j.semnephrol.2012.06.010
49. Gnudi L. Angiopoietins and diabetic nephropathy. Diabetologia (2016) 59:1616–20. doi: 10.1007/s00125-016-3995-3
50. Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, et al. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med (2000) 6:460–3. doi: 10.1038/74725
51. Suyama M, Miyazaki Y, Matsusaka T, Sugano N, Ueda H, Kawamura T, et al. Forced expression of vascular endothelial growth factor-A in podocytes decreases mesangial cell numbers and attenuates endothelial cell differentiation in the mouse glomerulus. Clin Exp Nephrol (2017) 22:266–74. doi: 10.1007/s10157-017-1450-5
52. Karpanen T, Bry M, Ollila HM, Seppänen-Laakso T, Liimatta E, Leskinen H, et al. Overexpression of vascular endothelial growth factor-B in mouse heart alters cardiac lipid metabolism and induces myocardial hypertrophy. Circ Res (2008) 103:1018–26. doi: 10.1161/circresaha.108.178459
53. Zanatta CM, Veronese FV, Loreto M, Sortica DA, Carpio VN, Eldeweiss MIA, et al. Endothelin-1 and endothelin A receptor immunoreactivity is increased in patients with diabetic nephropathy. Renal Fail (2012) 34:308–15. doi: 10.3109/0886022x.2011.647301
54. Ebefors K, Wiener RJ, Yu L, Azeloglu EU, Yi Z, Jia F, et al. Endothelin receptor-A mediates degradation of the glomerular endothelial surface layer via pathologic crosstalk between activated podocytes and glomerular endothelial cells. Kidney Int (2019) 96:957–70. doi: 10.1016/j.kint.2019.05.007
55. Ferland-McCollough D, Slater S, Richard J, Reni C, Mangialardi G. Pericytes, an overlooked player in vascular pathobiology. Pharmacol Ther (2017) 171:30–42. doi: 10.1016/j.pharmthera.2016.11.008
56. Lodyga M, Hinz B. TGF-β1 – A truly transforming growth factor in fibrosis and immunity. Semin Cell Dev Biol (2020) 101:123–39. doi: 10.1016/j.semcdb.2019.12.010
57. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science (2020) 367:eaau6977. doi: 10.1126/science.aau6977
58. Wu X-m, Gao Y-b, Cui F-q, Zhang N. Exosomes from high glucose-treated glomerular endothelial cells activate mesangial cells to promote renal fibrosis. Biol Open (2016) 5:484–91. doi: 10.1242/bio.015990
59. Wu X, Gao Y, Xu L, Dang W, Yan H, Zou D, et al. Exosomes from high glucose-treated glomerular endothelial cells trigger the epithelial-mesenchymal transition and dysfunction of podocytes. Sci Rep (2017) 7:9371. doi: 10.1038/s41598-017-09907-6
60. Ghayur A, Margetts PJ. Transforming growth factor-beta and the glomerular filtration barrier. Kidney Res Clin Pract (2013) 32:3–10. doi: 10.1016/j.krcp.2013.01.003
61. Purohit S, Piani F, Ordoñez FA, de Lucas-Collantes C, Bauer C, Cara-Fuentes G. Molecular mechanisms of proteinuria in minimal change disease. Front Med (2021) 8:761600. doi: 10.3389/fmed.2021.761600
62. Kopp JB, Anders H-J, Susztak K, Podestà MA, Remuzzi G, Hildebrandt F, et al. Podocytopathies. Nat Rev Dis Prime (2020) 31:167–74. doi: 10.1038/s41572-020-0196-7
63. Vivarelli M, Massella L, Ruggiero B, Emma F. Minimal change disease. Clin J Am Soc Nephrol (2017) 12:332–45. doi: 10.2215/cjn.05000516
64. Araya CE, Wasserfall CH, Brusko TM, Mu W, Segal MS, Johnson RJ, et al. A case of unfulfilled expectations. Cytokines in idiopathic minimal lesion nephrotic syndrome. Pediatr Nephrol (2006) 21:603–10. doi: 10.1007/s00467-006-0026-5
65. Lai K-W, Wei C-L, Tan L-K, Tan P-H, Chiang GSC, Lee CGL, et al. Overexpression of interleukin-13 induces minimal-change–like nephropathy in rats. J Am Soc Nephrol (2007) 18:1476–85. doi: 10.1681/asn.2006070710
66. Garin E, West L, Zheng W. Effect of interleukin-8 on glomerular sulfated compounds and albuminuria. Pediatr NEPHROL (1997) 11:274–9. doi: 10.1007/s004670050276
67. Kim AHJ, Chung J-J, Akilesh S, Koziell A, Jain S, Hodgin JB, et al. B cell–derived IL-4 acts on podocytes to induce proteinuria and foot process effacement. JCI Insight (2017) 2:e81836. doi: 10.1172/jci.insight.81836
68. Cara-Fuentes G, Wasserfall CH, Wang H, Johnson RJ, Garin EH. Minimal change disease: a dysregulation of the podocyte CD80–CTLA-4 axis? Pediatr Nephrol (2014) 29:2333–40. doi: 10.1007/s00467-014-2874-8
69. Bertelli R, Bonanni A, Caridi G, Canepa A, Ghiggeri GM. Molecular and cellular mechanisms for proteinuria in minimal change disease. Front Med (2018) 5:170. doi: 10.3389/fmed.2018.00170
70. Hashimura Y, Nozu K, Kanegane H, Miyawaki T, Hayakawa A, Yoshikawa N, et al. Minimal change nephrotic syndrome associated with immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome. Pediatr Nephrol (2009) 24:1181–6. doi: 10.1007/s00467-009-1119-8
71. Watts AJB, Keller KH, Lerner G, Rosales I, Collins AB, Sekulic M, et al. Discovery of autoantibodies targeting nephrin in minimal change disease supports a novel autoimmune etiology. J Am Soc Nephrol (2022) 33:238–52. doi: 10.1681/asn.2021060794
72. Ruggenenti P, Ruggiero B, Cravedi P, Vivarelli M, Massella L, Marasà M, et al. Rituximab in steroid-dependent or frequently relapsing idiopathic nephrotic syndrome. J Am Soc Nephrol (2014) 25:850–63. doi: 10.1681/asn.2013030251
73. Zhuang J, Zhao Z, Zhang C, Song X, Lu C, Tian X, et al. Case report: Successful outcome of treatment using rituximab in an adult patient with refractory minimal change disease and β-thalassemia complicating autoimmune hemolytic anemia. Front Med (2022) 9:1059740. doi: 10.3389/fmed.2022.1059740
74. Colucci M, Oniszczuk J, Vivarelli M, Audard V. B-cell dysregulation in idiopathic nephrotic syndrome: what we know and what we need to discover. Front Immunol (2022) 13:823204. doi: 10.3389/fimmu.2022.823204
75. Oniszczuk J, Beldi-Ferchiou A, Audureau E, Azzaoui I, Molinier-Frenkel V, Frontera V, et al. Circulating plasmablasts and high level of BAFF are hallmarks of minimal change nephrotic syndrome in adults. Nephrol Dialysis Transplant (2021) 36:609–17. doi: 10.1093/ndt/gfaa279
76. Colucci M, Carsetti R, Rosado MM, Cascioli S, Bruschi M, Candiano G, et al. Atypical IgM on T cells predict relapse and steroid dependence in idiopathic nephrotic syndrome. Kidney Int (2019) 96:971–82. doi: 10.1016/j.kint.2019.04.006
77. Trachtman H, Laskowski J, Lee C, Renner B, Feemster A, Parikh S, et al. Natural antibody and complement activation characterize patients with idiopathic nephrotic syndrome. Am J Physiology-Renal Physiol (2021) 321:F505–F16. doi: 10.1152/ajprenal.00041.2021
78. Jamin A, Berthelot L, Couderc A, Chemouny JM, Boedec E, Dehoux L, et al. Autoantibodies against podocytic UCHL1 are associated with idiopathic nephrotic syndrome relapses and induce proteinuria in mice. J Autoimmun (2018) 89:149–61. doi: 10.1016/j.jaut.2017.12.014
79. McGrogan A, Franssen CFM, de Vries CS. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dialysis Transplant (2010) 26:414–30. doi: 10.1093/ndt/gfq665
80. Rosenberg AZ, Kopp JB. Focal segmental glomerulosclerosis. Clin J Am Soc Nephrol (2017) 12:502–17. doi: 10.2215/cjn.05960616
81. Zhang H, Wang Z, Dong L, Guo Y, Wu J, Zhai S. New insight into the pathogenesis of minimal change nephrotic syndrome: Role of the persistence of respiratory tract virus in immune disorders. Autoimmun Rev (2016) 15:632–7. doi: 10.1016/j.autrev.2016.02.007
82. Morel A, Meuleman M-S, Moktefi A, Audard V. Renal diseases associated with hematologic Malignancies and thymoma in the absence of renal monoclonal immunoglobulin deposits. Diagnostics (2021) 11:710. doi: 10.3390/diagnostics11040710
83. Bakhriansyah M, Souverein PC, van den Hoogen MWF, de Boer A, Klungel OH. Risk of nephrotic syndrome for non-steroidal anti-inflammatory drug users. Clin J Am Soc Nephrol (2019) 14:1355–62. doi: 10.2215/cjn.14331218
84. Davis J, Desmond M, Berk M. Lithium and nephrotoxicity: Unravelling the complex pathophysiological threads of the lightest metal. Nephrology (2018) 23:897–903. doi: 10.1111/nep.13263
85. De Vriese AS, Sethi S, Nath KA, Glassock RJ, Fervenza FC. Differentiating primary, genetic, and secondary FSGS in adults: A clinicopathologic approach. J Am Soc Nephrol (2018) 29:759–74. doi: 10.1681/asn.2017090958
86. Shalhoub R. Pathogenesis of lipoid nephrosis: a disorder of T-cell function. LANCET (1974) 2:556–60. doi: 10.1016/s0140-6736(74)91880-7
87. Kemper MJ, Zepf K, Klaassen I, Link A, Müller-Wiefel DE. Changes of lymphocyte populations in pediatric steroid-sensitive nephrotic syndrome are more pronounced in remission than in relapse. Am J Nephrol (2005) 25:132–7. doi: 10.1159/000085357
88. Ye Q, Zhou C, Li S, Wang J, Liu F, Liu Z, et al. The immune cell landscape of peripheral blood mononuclear cells from PNS patients. Sci Rep (2021) 11:13083. doi: 10.1038/s41598-021-92573-6
89. Zhai S, Sun B, Zhang Y, Zhao L, Zhang L. IL−17 aggravates renal injury by promoting podocyte injury in children with primary nephrotic syndrome. Exp Ther Med (2020) 20:409–17. doi: 10.3892/etm.2020.8698
90. Dryer SE, Otalora L, Chavez E, Watford D, Tueros L, Correa M, et al. Identification of glomerular and podocyte-specific genes and pathways activated by sera of patients with focal segmental glomerulosclerosis. PloS One (2019) 14:e0222948. doi: 10.1371/journal.pone.0222948
91. Dryer SE, Chung C-F, Kitzler T, Kachurina N, Pessina K, Babayeva S, et al. Intrinsic tumor necrosis factor-α pathway is activated in a subset of patients with focal segmental glomerulosclerosis. PloS One (2019) 14:e0216426. doi: 10.1371/journal.pone.0216426
92. Shimada M, Ishimoto T, Lee PY, Lanaspa MA, Rivard CJ, Roncal-Jimenez CA, et al. Toll-like receptor 3 ligands induce CD80 expression in human podocytes via an NF- B-dependent pathway. Nephrol Dialysis Transplant (2011) 27:81–9. doi: 10.1093/ndt/gfr271
93. Ishimoto T, Shimada M, Gabriela G, Kosugi T, Sato W, Lee PY, et al. Toll-like receptor 3 ligand, polyIC, induces proteinuria and glomerular CD80, and increases urinary CD80 in mice. Nephrol Dialysis Transplant (2012) 28:1439–46. doi: 10.1093/ndt/gfs543
94. Reiser J, von Gersdorff G, Loos M, Oh J, Asanuma K, Giardino L, et al. Induction of B7-1 in podocytes is associated with nephrotic syndrome. J Clin Invest (2004) 113:1390–7. doi: 10.1172/jci20402
95. Hoffman W, Lakkis FG, Chalasani G. B cells, antibodies, and more. Clin J Am Soc Nephrol (2016) 11:137–54. doi: 10.2215/cjn.09430915
96. Ye Q, Zhang Y, Zhuang J, Bi Y, Xu H, Shen Q, et al. The important roles and molecular mechanisms of annexin A2 autoantibody in children with nephrotic syndrome. Ann Trans Med (2021) 9:1452–. doi: 10.21037/atm-21-3988
97. Hattori M, Shirai Y, Kanda S, Ishizuka K, Kaneko N, Ando T, et al. Circulating nephrin autoantibodies and posttransplant recurrence of primary focal segmental glomerulosclerosis. Am J Transplant (2022) 22:2478–80. doi: 10.1111/ajt.17077
98. Batal I, Khairallah P, Weins A, Andeen NK, Stokes MB. The role of HLA antigens in recurrent primary focal segmental glomerulosclerosis. Front Immunol (2023) 14:1124249. doi: 10.3389/fimmu.2023.1124249
99. Kawahara K, Mukai T, Iseki M, Nagasu A, Nagasu H, Akagi T, et al. SH3BP2 deficiency ameliorates murine systemic lupus erythematosus. Int J Mol Sci (2021) 22:4169. doi: 10.3390/ijms22084169
100. Srivastava T, Garola RE, Zhou J, Boinpelly VC, Rezaiekhaligh MH, Joshi T, et al. Scaffold protein SH3BP2 signalosome is pivotal for immune activation in nephrotic syndrome. JCI Insight (2023) e170055. doi: 10.1172/jci.insight.170055
101. Cattran DC, Brenchley PE. Membranous nephropathy: integrating basic science into improved clinical management. Kidney Int (2017) 91:566–74. doi: 10.1016/j.kint.2016.09.048
102. Sethi S, Beck LH, Glassock RJ, Haas M, De Vriese AS, Caza TN, et al. Mayo Clinic consensus report on membranous nephropathy: proposal for a novel classification. Kidney Int (2023) 104:1092–102. doi: 10.1016/j.kint.2023.06.032
103. Bally S, Debiec H, Ponard D, Dijoud F, Rendu J, Fauré J, et al. Phospholipase A2 receptor–related membranous nephropathy and mannan-binding lectin deficiency. J Am Soc Nephrol (2016) 27:3539–44. doi: 10.1681/asn.2015101155
104. Couser WG. Primary membranous nephropathy. Clin J Am Soc Nephrol (2017) 12:983–97. doi: 10.2215/cjn.11761116
105. Hoxha E, Reinhard L, Stahl RAK. Membranous nephropathy: new pathogenic mechanisms and their clinical implications. Nat Rev Nephrol (2022) 18:466–78. doi: 10.1038/s41581-022-00564-1
106. Ronco P, Debiec H. Membranous nephropathy: current understanding of various causes in light of new target antigens. Curr Opin Nephrol Hypertension (2021) 30:287–93. doi: 10.1097/mnh.0000000000000697
107. Beck L, Bonegio R, Lambeau G, Beck D, Powell D, Cummins T, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med (2009) 361:11–21. doi: 10.1056/NEJMoa0810457
108. Tomas NM, Beck LH, Meyer-Schwesinger C, Seitz-Polski B, Ma H, Zahner G, et al. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. New Engl J Med (2014) 371:2277–87. doi: 10.1056/NEJMoa1409354
109. Tomas NM, Dehde S, Meyer-Schwesinger C, Huang M, Hermans-Borgmeyer I, Maybaum J, et al. Podocyte expression of human phospholipase A2 receptor 1 causes immune-mediated membranous nephropathy in mice. Kidney Int (2023) 103:297–303. doi: 10.1016/j.kint.2022.09.008
110. Al-Rabadi LF, Caza T, Trivin-Avillach C, Rodan AR, Andeen N, Hayashi N, et al. Serine protease HTRA1 as a novel target antigen in primary membranous nephropathy. J Am Soc Nephrol (2021) 32:1666–81. doi: 10.1681/asn.2020101395
111. Reinhard L, Machalitza M, Wiech T, Gröne H, Lassé M, Rinschen M, et al. Netrin G1 is a novel target antigen in primary membranous nephropathy. J Am Soc NEPHROL (2022) 33:1823–31. doi: 10.1681/ASN.2022050608
112. Le Quintrec M, Teisseyre M, Bec N, Delmont E, Szwarc I, Perrochia H, et al. Contactin-1 is a novel target antigen in membranous nephropathy associated with chronic inflammatory demyelinating polyneuropathy. Kidney Int (2021) 100:1240–9. doi: 10.1016/j.kint.2021.08.014
113. Sethi S, Debiec H, Madden B, Vivarelli M, Charlesworth MC, Ravindran A, et al. Semaphorin 3B–associated membranous nephropathy is a distinct type of disease predominantly present in pediatric patients. Kidney Int (2020) 98:1253–64. doi: 10.1016/j.kint.2020.05.030
114. Sethi S, Madden BJ, Debiec H, Charlesworth MC, Gross L, Ravindran A, et al. Exostosin 1/exostosin 2–associated membranous nephropathy. J Am Soc Nephrol (2019) 30:1123–36. doi: 10.1681/asn.2018080852
115. Sethi S, Debiec H, Madden B, Charlesworth MC, Morelle J, Gross L, et al. Neural epidermal growth factor-like 1 protein (NELL-1) associated membranous nephropathy. Kidney Int (2020) 97:163–74. doi: 10.1016/j.kint.2019.09.014
116. Sethi S, Madden B, Debiec H, Morelle J, Charlesworth MC, Gross L, et al. Protocadherin 7–associated membranous nephropathy. J Am Soc Nephrol (2021) 32:1249–61. doi: 10.1681/asn.2020081165
117. Caza TN, Hassen SI, Kuperman M, Sharma SG, Dvanajscak Z, Arthur J, et al. Neural cell adhesion molecule 1 is a novel autoantigen in membranous lupus nephritis. Kidney Int (2021) 100:171–81. doi: 10.1016/j.kint.2020.09.016
118. Fanouriakis A, Kostopoulou M, Cheema K, Anders H-J, Aringer M, Bajema I, et al. 2019 Update of the Joint European League Against Rheumatism and European Renal Association–European Dialysis and Transplant Association (EULAR/ERA–EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis (2020) 79:713–23. doi: 10.1136/annrheumdis-2020-216924
119. Sakhi H, Moktefi A, Bouachi K, Audard V, Hénique C, Remy P, et al. Podocyte injury in lupus nephritis. J Clin Med (2019) 8:1340. doi: 10.3390/jcm8091340
120. Oliva-Damaso N, Payan J, Oliva-Damaso E, Pereda T, Bomback AS. Lupus podocytopathy: an overview. Adv Chronic Kidney Disease (2019) 26:369–75. doi: 10.1053/j.ackd.2019.08.011
121. Tsai C-Y, Li K-J, Shen C-Y, Lu C-H, Lee H-T, Wu T-H, et al. Decipher the immunopathological mechanisms and set up potential therapeutic strategies for patients with lupus nephritis. Int J Mol Sci (2023) 24:10066. doi: 10.3390/ijms241210066
122. Zou X, Cheng H, Zhang Y, Fang C, Xia Y. The antigen-binding fragment of anti-double-stranded DNA IgG enhances F-actin formation in mesangial cells by binding to alpha-actinin-4. Exp Biol Med (2012) 237:1023–31. doi: 10.1258/ebm.2012.012033
123. Yung S, Cheung KF, Zhang Q, Chan TM. Anti-dsDNA antibodies bind to mesangial annexin II in lupus nephritis. J Am Soc Nephrol (2010) 21:1912–27. doi: 10.1681/asn.2009080805
124. Deocharan B, Qing X, Lichauco J, Putterman C. α-actinin is a cross-reactive renal target for pathogenic anti-DNA antibodies. J Immunol (2002) 168:3072–8. doi: 10.4049/jimmunol.168.6.3072
125. Crispin J, Liou L-b, Huang C-c. Sialyltransferase and neuraminidase levels/ratios and sialic acid levels in peripheral blood B cells correlate with measures of disease activity in patients with systemic lupus erythematosus and rheumatoid arthritis: A pilot study. PloS One (2016) 11:e0151669. doi: 10.1371/journal.pone.0151669
126. Yung S, Chan TM. Mechanisms of kidney injury in lupus nephritis – the role of anti-dsDNA antibodies. Front Immunol (2015) 6:475. doi: 10.3389/fimmu.2015.00475
127. Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol (2010) 11:373–84. doi: 10.1038/ni.1863
128. Sato Y, Tamura M, Yanagita M. Tertiary lymphoid tissues: a regional hub for kidney inflammation. Nephrol Dialysis Transplant (2023) 38:26–33. doi: 10.1093/ndt/gfab212
129. Sato Y, Silina K, van den Broek M, Hirahara K, Yanagita M. The roles of tertiary lymphoid structures in chronic diseases. Nat Rev Nephrol (2023) 19:525–37. doi: 10.1038/s41581-023-00706-z
130. Murata H, Matsumura R, Koyama A, Sugiyama T, Sueishi M, Shibuya K, et al. T cell receptor repertoire of T cells in the kidneys of patients with lupus nephritis. Arthritis Rheum (2002) 46:2141–7. doi: 10.1002/art.10432
131. Kitching AR, Alikhan MA. CD8+ cells and glomerular crescent formation: outside-in as well as inside-out. J Clin Invest (2018) 128:3231–3. doi: 10.1172/jci122045
132. Iwata Y, Boström EA, Menke J, Rabacal WA, Morel L, Wada T, et al. Aberrant macrophages mediate defective kidney repair that triggers nephritis in lupus-susceptible mice. J Immunol (2012) 188:4568–80. doi: 10.4049/jimmunol.1102154
133. Klarquist J, Zhou Z, Shen N, Janssen EM. Dendritic cells in systemic lupus erythematosus: from pathogenic players to therapeutic tools. Mediators Inflamm (2016) 2016:1–12. doi: 10.1155/2016/5045248
134. Zhou X-J, Klionsky DJ, Zhang H. Podocytes and autophagy: a potential therapeutic target in lupus nephritis. Autophagy (2019) 15:908–12. doi: 10.1080/15548627.2019.1580512
135. Jin J, Zhao L, Zou W, Shen W, Zhang H, He Q. Activation of cyclooxygenase-2 by ATF4 during endoplasmic reticulum stress regulates kidney podocyte autophagy induced by lupus nephritis. Cell Physiol Biochem (2018) 48:753–64. doi: 10.1159/000491904
136. Papadimitraki ED, Tzardi MB G, Sotsiou E, Boumpas DT. Glomerular expression of toll-like receptor-9 in lupus nephritis but not in normal kidneys: implications for the amplification of the inflammatory response. LUPUS (2009) 18:831–5. doi: 10.1177/0961203309103054
137. Kwok S-K, Tsokos GC. New insights into the role of renal resident cells in the pathogenesis of lupus nephritis. Korean J Internal Med (2018) 33:284–9. doi: 10.3904/kjim.2017.383
138. Lv F, He Y, Xu H, Li Y, Han L, Yan L, et al. CD36 aggravates podocyte injury by activating NLRP3 inflammasome and inhibiting autophagy in lupus nephritis. Cell Death Disease (2022) 13:729. doi: 10.1038/s41419-022-05179-9
139. Teisseyre M, Cremoni M, Boyer-Suavet S, Ruetsch C, Graça D, Esnault VLM, et al. Advances in the management of primary membranous nephropathy and rituximab-refractory membranous nephropathy. Front Immunol (2022) 13:859419. doi: 10.3389/fimmu.2022.859419
140. Xu C, Ha X, Yang S, Tian X, Jiang H. Advances in understanding and treating diabetic kidney disease: focus on tubulointerstitial inflammation mechanisms. Front Endocrinol (2023) 14:1232790. doi: 10.3389/fendo.2023.1232790
141. Müller-Deile J, Schenk H, Schiffer M. Minimal-change-Glomerulonephritis und fokal-segmentale Glomerulosklerose. Der Internist (2019) 60:450–7. doi: 10.1007/s00108-019-0590-y
142. Cruz-Topete D, Cidlowski JA. One hormone, two actions: anti- and pro-inflammatory effects of glucocorticoids. Neuroimmunomodulation (2015) 22:20–32. doi: 10.1159/000362724
143. Ponticelli C, Locatelli F. Glucocorticoids in the treatment of glomerular diseases. Clin J Am Soc Nephrol (2018) 13:815–22. doi: 10.2215/cjn.12991117
144. Broek M, Smeets B, Schreuder MF, Jansen J. The podocyte as a direct target of glucocorticoids in nephrotic syndrome. Nephrol Dialysis Transplant (2022) 37:1808–15. doi: 10.1093/ndt/gfab016
145. Li X, Chuang PY, D’Agati VD, Dai Y, Yacoub R, Fu J, et al. Nephrin preserves podocyte viability and glomerular structure and function in adult kidneys. J Am Soc Nephrol (2015) 26:2361–77. doi: 10.1681/asn.2014040405
146. Agrawal S, Chanley MA, Westbrook D, Nie X, Kitao T, Guess AJ, et al. Pioglitazone enhances the beneficial effects of glucocorticoids in experimental nephrotic syndrome. Sci Rep (2016) 6:24392. doi: 10.1038/srep24392
147. Zhao X, Khurana S, Charkraborty S, Tian Y, Sedor JR, Bruggman LA, et al. α Actinin 4 (ACTN4) regulates glucocorticoid receptor-mediated transactivation and transrepression in podocytes. J Biol Chem (2017) 292:1637–47. doi: 10.1074/jbc.M116.755546
148. Lombel RM, Gipson DS, Hodson EM. Treatment of steroid-sensitive nephrotic syndrome: new guidelines from KDIGO. Pediatr Nephrol (2012) 28:415–26. doi: 10.1007/s00467-012-2310-x
149. Liu Y, Yang R, Yang C, Dong S, Zhu Y, Zhao M, et al. Cyclophosphamide versus cyclosporine A therapy in steroid-resistant nephrotic syndrome: a retrospective study with a mean 5-year follow-up. J Int Med Res (2018) 46:4506–17. doi: 10.1177/0300060518782017
150. Grenda R, Obrycki Ł. Second and third generational advances in therapies of the immune-mediated kidney diseases in children and adolescents. Children (2022) 9:536. doi: 10.3390/children9040536
151. Hackl A, Zed SEDA, Diefenhardt P, Binz-Lotter J, Ehren R, Weber LT. The role of the immune system in idiopathic nephrotic syndrome. Mol Cell Pedia (2021) 8:18. doi: 10.1186/s40348-021-00128-6
152. Zhang M, Lv Y, Liu H. The efficacy of cyclophosphamide combined with prednisone in membranous nephropathy patients with different cytochrome P450 2B6 gene polymorphisms and analysis of factors influencing the efficacy. Drug Design Dev Ther (2022) 16:3655–62. doi: 10.2147/dddt.S373487
153. Bao L, Hao C, Wang J, Wang D, Zhao Y, Li Y, et al. High-dose cyclophosphamide administration orchestrates phenotypic and functional alterations of immature dendritic cells and regulates th cell polarization. Front Pharmacol (2020) 11:775. doi: 10.3389/fphar.2020.00775
154. Stangou M, Marinaki S, Papachristou E, Kolovou K, Sambani E, Zerbala S, et al. Immunosuppressive regimens based on Cyclophospamide or Calcineurin inhibitors: Comparison of their effect in the long term outcome of Primary Membranous Nephropathy. PloS One (2019) 14:e0217116. doi: 10.1371/journal.pone.0217116
155. Gallon L, Traitanon O, Yu Y, Shi B, Leventhal JR, Miller J, et al. Differential effects of calcineurin and mammalian target of rapamycin inhibitors on alloreactive th1, th17, and regulatory T cells. Transplantation (2015) 99:1774–84. doi: 10.1097/tp.0000000000000717
156. Li Y, Guptill JT, Russo MA, Massey JM, Juel VC, Hobson-Webb LD, et al. Tacrolimus inhibits Th1 and Th17 responses in MuSK-antibody positive myasthenia gravis patients. Exp Neurol (2019) 312:43–50. doi: 10.1016/j.expneurol.2018.11.006
157. Niazi M, Shirpoor A, Taghizadeh Afshari A, Naderi R, Bagheri M, Pourjabali M, et al. Cyclosporine A induces kidney dysfunction by the alteration of molecular mediators involved in slit diaphragm regulation and matrix metalloproteins: the mitigating effect of curcumin. Expert Opin Drug Metab Toxicol (2020) 16:1223–31. doi: 10.1080/17425255.2020.1822323
158. Li X, Ding F, Wang S, Li B, Ding J. Cyclosporine A protects podocytes by regulating WAVE1 phosphorylation. Sci Rep (2015) 5:17694. doi: 10.1038/srep17694
159. Li X, Zhang X, Li X, Wang X, Wang S, Ding J. Cyclosporine A protects podocytes via stabilization of cofilin-1 expression in the unphosphorylated state. Exp Biol Med (2014) 239:922–36. doi: 10.1177/1535370214530365
160. Chiba Y, Inoue CN. Once-daily low-dose cyclosporine A treatment with angiotensin blockade for long-term remission of nephropathy in frasier syndrome. Tohoku J Exp Med (2019) 247:35–40. doi: 10.1620/tjem.247.35
161. Bobé P, Liao R, Liu Q, Zheng Z, Fan J, Peng W, et al. Tacrolimus protects podocytes from injury in lupus nephritis partly by stabilizing the cytoskeleton and inhibiting podocyte apoptosis. PloS One (2015) 10:e0132724. doi: 10.1371/journal.pone.0132724
162. Peng T, Chang X, Wang J, Zhen J, Yang X, Hu Z. Protective effects of tacrolimus on podocytes in early diabetic nephropathy in rats. Mol Med Rep (2017) 15:3172–8. doi: 10.3892/mmr.2017.6354
163. Wen Y, Liu L, Zhou P, Li H, Wang Z, Zhang Y, et al. Tacrolimus restores podocyte injury and stabilizes the expression of Cabin1 in 5/6 nephrectomized rats. Renal Fail (2016) 38:564–70. doi: 10.3109/0886022x.2016.1148936
164. Ma R, Wang Y, Xu Y, Wang R, Wang X, Yu N, et al. Tacrolimus protects podocytes from apoptosis via downregulation of TRPC6 in diabetic nephropathy. J Diabetes Res (2021) 2021:1–11. doi: 10.1155/2021/8832114
165. Yasuda H, Fukusumi Y, Ivanov V, Zhang Y, Kawachi H. Tacrolimus ameliorates podocyte injury by restoring FK506 binding protein 12 (FKBP12) at actin cytoskeleton. FASEB J (2021) 35:e21983. doi: 10.1096/fj.202101052R
166. Fervenza FC, Appel GB, Barbour SJ, Rovin BH, Lafayette RA, Aslam N, et al. Rituximab or cyclosporine in the treatment of membranous nephropathy. New Engl J Med (2019) 381:36–46. doi: 10.1056/NEJMoa1814427
167. Rovin BH, Teng YKO, Ginzler EM, Arriens C, Caster DJ, Romero-Diaz J, et al. Efficacy and safety of voclosporin versus placebo for lupus nephritis (AURORA 1): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet (2021) 397:2070–80. doi: 10.1016/s0140-6736(21)00578-x
168. Abdel-Kahaar E, Keller F. Clinical pharmacokinetics and pharmacodynamics of voclosporin. Clin Pharmacokinetics (2023) 62:693–703. doi: 10.1007/s40262-023-01246-2
169. Abo Zed SED, Hackl A, Bohl K, Ebert L, Kieckhöfer E, Müller C, et al. Mycophenolic acid directly protects podocytes by preserving the actin cytoskeleton and increasing cell survival. Sci Rep (2023) 13:4281. doi: 10.1038/s41598-023-31326-z
170. Hackl A, Nüsken E, Voggel J, Abo Zed SED, Binz-Lotter J, Unnersjö-Jess D, et al. The effect of mycophenolate mofetil on podocytes in nephrotoxic serum nephritis. Sci Rep (2023) 13:14167. doi: 10.1038/s41598-023-41222-1
171. Seo J-W, Kim YG, Lee SH, Lee A, Kim D-J, Jeong K-H, et al. Mycophenolate mofetil ameliorates diabetic nephropathy in db/db mice. BioMed Res Int (2015) 2015:1–11. doi: 10.1155/2015/301627
172. Lin D-W, Chang C-C, Hsu Y-C, Lin C-L. New insights into the treatment of glomerular diseases: when mechanisms become vivid. Int J Mol Sci (2022) 23:3525. doi: 10.3390/ijms23073525
173. Sun K, Verhoog N, Allie-Reid F, Vanden Berghe W, Smith C, Haegeman G, et al. Inhibition of corticosteroid-binding globulin gene expression by glucocorticoids involves C/EBPβ. PloS One (2014) 9:e110702. doi: 10.1371/journal.pone.0110702
174. Achuthan A, Aslam ASM, Nguyen Q, Lam P-Y, Fleetwood AJ, Frye AT, et al. Glucocorticoids promote apoptosis of proinflammatory monocytes by inhibiting ERK activity. Cell Death Disease (2018) 9:267. doi: 10.1038/s41419-018-0332-4
175. Faul C, Donnelly M, Merscher-Gomez S, Chang YH, Franz S, Delfgaauw J, et al. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med (2008) 14:931–8. doi: 10.1038/nm.1857
176. Schlöndorff J, del Camino D, Carrasquillo R, Lacey V, Pollak MR. TRPC6 mutations associated with focal segmental glomerulosclerosis cause constitutive activation of NFAT-dependent transcription. Am J Physiology-Cell Physiol (2009) 296:C558–C69. doi: 10.1152/ajpcell.00077.2008
177. Léger C, Pitard I, Sadi M, Carvalho N, Brier S, Mechaly A, et al. Dynamics and structural changes of calmodulin upon interaction with the antagonist calmidazolium. BMC Biol (2022) 20:176. doi: 10.1186/s12915-022-01381-5
178. Wei C, Möller CC, Altintas MM, Li J, Schwarz K, Zacchigna S, et al. Modification of kidney barrier function by the urokinase receptor. Nat Med (2007) 14:55–63. doi: 10.1038/nm1696
179. Lee HW, Khan SQ, Faridi MH, Wei C, Tardi NJ, Altintas MM, et al. A podocyte-based automated screening assay identifies protective small molecules. J Am Soc Nephrol (2015) 26:2741–52. doi: 10.1681/asn.2014090859
180. Gong R. The renaissance of corticotropin therapy in proteinuric nephropathies. Nat Rev Nephrol (2011) 8:122–8. doi: 10.1038/nrneph.2011.190
181. Fornoni A, Sageshima J, Wei C, Merscher-Gomez S, Aguillon-Prada R, Jauregui AN, et al. Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci Trans Med (2011) 3:85ra46. doi: 10.1126/scitranslmed.3002231
182. Lattermann C, Gomoll AH, Cole BJ. Arthroscopic partial meniscectomy for degenerative meniscal tear. New Engl J Med (2014) 370:1259–61. doi: 10.1056/NEJMc1401128
183. Walsh PR, Johnson S. Eculizumab in the treatment of Shiga toxin haemolytic uraemic syndrome. Pediatr Nephrol (2018) 34:1485–92. doi: 10.1007/s00467-018-4025-0
184. Bruchfeld A, Magin H, Nachman P, Parikh S, Lafayette R, Potarca A, et al. C5a receptor inhibitor avacopan in immunoglobulin A nephropathy—an open-label pilot study. Clin Kidney J (2022) 15:922–8. doi: 10.1093/ckj/sfab294
185. Lafayette RA, Rovin BH, Reich HN, Tumlin JA, Floege J, Barratt J. Safety, tolerability and efficacy of narsoplimab, a novel MASP-2 inhibitor for the treatment of igA nephropathy. Kidney Int Rep (2020) 5:2032–41. doi: 10.1016/j.ekir.2020.08.003
186. Kaegi C, Wuest B, Schreiner J, Steiner UC, Vultaggio A, Matucci A, et al. Systematic review of safety and efficacy of rituximab in treating immune-mediated disorders. Front Immunol (2019) 10:1990. doi: 10.3389/fimmu.2019.01990
187. Seitz-Polski B, Dahan K, Debiec H, Rousseau A, Andreani M, Zaghrini C, et al. High-dose rituximab and early remission in PLA2R1-related membranous nephropathy. Clin J Am Soc Nephrol (2019) 14:1173–82. doi: 10.2215/cjn.11791018
189. Zonozi R, Laliberte K, Huizenga NR, Rosenthal JK, Jeyabalan A, Collins AB, et al. Combination of rituximab, low-dose cyclophosphamide, and prednisone for primary membranous nephropathy: A case series with extended follow up. Am J Kidney Diseases (2021) 78:793–803. doi: 10.1053/j.ajkd.2021.04.014
190. Masoud S, McAdoo SP, Bedi R, Cairns TD, Lightstone L. Ofatumumab for B cell depletion in patients with systemic lupus erythematosus who are allergic to rituximab. Rheumatology (2018) 57:1156–61. doi: 10.1093/rheumatology/key042
191. Bonanni A, Rossi R, Murtas C, Ghiggeri GM. Low-dose ofatumumab for rituximab-resistant nephrotic syndrome. BMJ Case Rep (2015) 2015:bcr2015210208. doi: 10.1136/bcr-2015-210208
192. Ramachandran R, Kumar V, Bharati J, Rovin B, Nada R, Kumar V, et al. Long-term follow-up of cyclical cyclophosphamide and steroids versus tacrolimus and steroids in primary membranous nephropathy. Kidney Int Rep (2021) 6:2653–60. doi: 10.1016/j.ekir.2021.07.028
193. Ishimoto T, Shimada M, Araya CE, Huskey J, Garin EH, Johnson RJ. Minimal change disease: A CD80 podocytopathy? Semin Nephrol (2011) 31:320–5. doi: 10.1016/j.semnephrol.2011.06.002
194. Teh YM, Lim SK, Jusoh N, Osman K, Mualif SA, Abu Shahin N. CD80 insights as therapeutic target in the current and future treatment options of frequent-relapse minimal change disease. BioMed Res Int (2021) 2021:1–17. doi: 10.1155/2021/6671552
195. Novelli R, Gagliardini E, Ruggiero B, Benigni A, Remuzzi G. Any value of podocyte B7-1 as a biomarker in human MCD and FSGS? Am J Physiology-Renal Physiol (2016) 310:F335–F41. doi: 10.1152/ajprenal.00510.2015
196. Gagliardini E, Novelli R, Corna D, Zoja C, Ruggiero B, Benigni A, et al. B7–1 is not induced in podocytes of human and experimental diabetic nephropathy. J Am Soc Nephrol (2016) 27:999–1005. doi: 10.1681/asn.2015030266
197. Burke GW, Chandar J, Sageshima J, Ortigosa-Goggins M, Amarapurkar P, Mitrofanova A, et al. Benefit of B7-1 staining and abatacept for treatment-resistant post-transplant focal segmental glomerulosclerosis in a predominantly pediatric cohort: time for a reappraisal. Pediatr Nephrol (2022) 38:145–59. doi: 10.1007/s00467-022-05549-7
198. Herrera M, Söderberg M, Sabirsh A, Valastro B, Mölne J, Santamaria B, et al. Inhibition of T-cell activation by the CTLA4-Fc Abatacept is sufficient to ameliorate proteinuric kidney disease. Am J Physiology-Renal Physiol (2017) 312:F748–F59. doi: 10.1152/ajprenal.00179.2016
199. Mühlig AK, Keir LS, Abt JC, Heidelbach HS, Horton R, Welsh GI, et al. Podocytes produce and secrete functional complement C3 and complement factor H. Front Immunol (2020) 11:1833. doi: 10.3389/fimmu.2020.01833
200. Nangaku M, Shankland SJ, Couser WG. Cellular response to injury in membranous nephropathy. J Am Soc Nephrol (2005) 16:1195–204. doi: 10.1681/asn.2004121098
201. Harigai M, Takada H. Avacopan, a selective C5a receptor antagonist, for anti-neutrophil cytoplasmic antibody-associated vasculitis. MOD Rheumatol (2022) 32:475–83. doi: 10.1093/mr/roab104/6497529
202. Khaled SK, Claes K, Goh YT, Kwong YL, Leung N, Mendrek W, et al. Narsoplimab, a mannan-binding lectin-associated serine protease-2 inhibitor, for the treatment of adult hematopoietic stem-cell transplantation–associated thrombotic microangiopathy. J Clin Oncol (2022) 40:2447–57. doi: 10.1200/jco.21.02389
203. Freeman J, Cummings J, Chuidian M, Dudler T. Development of pharmacodynamic assays to assess ex vivo masp-2 inhibition and their use to characterize the pharmacodynamics of narsoplimab (OMS721) in humans and monkeys. Blood (2020) 136:26–7. doi: 10.1182/blood-2020-142208
204. Elhadad S, Redmond D, Huang J, Tan A, Laurence J. MASP2 inhibition by narsoplimab suppresses endotheliopathies characteristic of transplant-associated thrombotic microangiopathy:in vitroandex vivoevidence. Clin Exp Immunol (2023) 213:252–64. doi: 10.1093/cei/uxad055
205. Clement LC, Avila-Casado C, Macé C, Soria E, Bakker WW, Kersten S, et al. Podocyte-secreted angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome. Nat Med (2010) 17:117–22. doi: 10.1038/nm.2261
206. Macé C, Chugh SS. Nephrotic syndrome. J Am Soc Nephrol (2014) 25:2393–8. doi: 10.1681/asn.2014030267
207. Chung H, Lee S-W, Hyun M, Kim SY, Cho HG, Lee ES, et al. Curcumin blocks high glucose-induced podocyte injury via RIPK3-dependent pathway. Front Cell Dev Biol (2022) 10:800574. doi: 10.3389/fcell.2022.800574
208. Yu Q, Zhang M, Qian L, Wen D, Wu G. Luteolin attenuates high glucose-induced podocyte injury via suppressing NLRP3 inflammasome pathway. Life Sci (2019) 225:1–7. doi: 10.1016/j.lfs.2019.03.073
209. Zhang J, Wu C, Gao L, Du G, Qin X. Astragaloside IV derived from Astragalus membranaceus: A research review on the pharmacological effects. Adv Pharmacol (2020) 87:89–112. doi: 10.1016/bs.apha.2019.08.002
210. Shen Q, Fang J, Guo H, Su X, Zhu B, Yao X, et al. Astragaloside IV attenuates podocyte apoptosis through ameliorating mitochondrial dysfunction by up-regulated Nrf2-ARE/TFAM signaling in diabetic kidney disease. Free Radic Biol Med (2023) 203:45–57. doi: 10.1016/j.freeradbiomed.2023.03.022
211. Guo H, Wang Y, Zhang X, Zang Y, Zhang Y, Wang L, et al. Astragaloside IV protects against podocyte injury via SERCA2-dependent ER stress reduction and AMPKalpha-regulated autophagy induction in streptozotocin-induced diabetic nephropathy. Sci Rep (2017) 7:6852. doi: 10.1038/s41598-017-07061-7
212. Chen Z, An X, Liu X, Qi J, Ding D, Zhao M, et al. Hyperoside alleviates adriamycin-induced podocyte injury via inhibiting mitochondrial fission. Oncotarget (2017) 8:88792–803. doi: 10.18632/oncotarget.21287
213. Matoba K, Kawanami D, Nagai Y, Takeda Y, Akamine T, Ishizawa S, et al. Rho-kinase blockade attenuates podocyte apoptosis by inhibiting the notch signaling pathway in diabetic nephropathy. Int J Mol Sci (2017) 18:1795. doi: 10.3390/ijms18081795
214. Matoba K, Takeda Y, Nagai Y, Sekiguchi K, Ukichi R, Takahashi H, et al. ROCK2-induced metabolic rewiring in diabetic podocytopathy. Commun Biol (2022) 5:341. doi: 10.1038/s42003-022-03300-4
215. Linke M, Fritsch SD, Sukhbaatar N, Hengstschlager M, Weichhart T. mTORC1 and mTORC2 as regulators of cell metabolism in immunity. FEBS Lett (2017) 591:3089–103. doi: 10.1002/1873-3468.12711
216. Li Q, Zeng Y, Jiang Q, Wu C, Zhou J. Role of mTOR signaling in the regulation of high glucose-induced podocyte injury. Exp Ther Med (2019) 17:2495–502. doi: 10.3892/etm.2019.7236
217. Yang HC, Fogo AB. Mechanisms of disease reversal in focal and segmental glomerulosclerosis. Adv Chronic Kidney Dis (2014) 21:442–7. doi: 10.1053/j.ackd.2014.04.001
218. Matsusaka T, Asano T, Niimura F, Kinomura M, Shimizu A, Shintani A, et al. Angiotensin receptor blocker protection against podocyte-induced sclerosis is podocyte angiotensin II type 1 receptor-independent. Hypertension (2010) 55:967–73. doi: 10.1161/HYPERTENSIONAHA.109.141994
219. Yu N, Yang L, Ling L, Liu Y, Yu Y, Wu Q, et al. Curcumin attenuates angiotensin II-induced podocyte injury and apoptosis by inhibiting endoplasmic reticulum stress. FEBS Open Bio (2020) 10:1957–66. doi: 10.1002/2211-5463.12946
Keywords: podocyte, immunity response, pathogenesis, proteinuria, treatment
Citation: Jiang H, Shen Z, Zhuang J, Lu C, Qu Y, Xu C, Yang S and Tian X (2024) Understanding the podocyte immune responses in proteinuric kidney diseases: from pathogenesis to therapy. Front. Immunol. 14:1335936. doi: 10.3389/fimmu.2023.1335936
Received: 09 November 2023; Accepted: 29 December 2023;
Published: 15 January 2024.
Edited by:
Xu-jie Zhou, Peking University, ChinaReviewed by:
Giuseppe Remuzzi, Istituto di Ricerche Farmacologiche Mario Negri IRCCS, ItalyTongtong Liu, China Academy of Chinese Medical Sciences, China
Copyright © 2024 Jiang, Shen, Zhuang, Lu, Qu, Xu, Yang and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Jiang, jangh-yt@163.com; Xuefei Tian, xuefei.tian@yale.edu
†These authors have contributed equally to this work and share first authorship