Co-Cultures of Lactobacillus acidophilus and Bacillus subtilis Enhance Mucosal Barrier by Modulating Gut Microbiota-Derived Short-Chain Fatty Acids
嗜酸乳杆菌和枯草芽孢杆菌的共培养通过调节肠道微生物群衍生的短链脂肪酸来增强粘膜屏障
浙江大学海南研究院, 三亚市崖州湾科技城甬佑工业园, 中国 三亚 572000
浙江大学动物科学学院, 中国 杭州 310058
Twins Group Corporation, 中国南昌 330096
通信应收件人的作者。
这些作者对这项工作做出了同样的贡献。
营养素2022, 14(21), 4475;https://doi.org/10.3390/nu14214475
收到意见:2022 年 9 月 17 日 / 修订:2022 年 10 月 13 日 / 接受日期:2022 年 10 月 17 日 / 发布时间:2022 年 10 月 25 日
(本文属于专刊 肠道屏障功能障碍的机制和以益生菌为重点的干预措施)
Abstract 抽象
脱机应激会诱导哺乳动物的肠道屏障功能障碍和免疫失调。已经提出了基于肠道微生物群调节的各种干预措施。本研究旨在从肠道菌群代谢功能的角度探讨嗜酸乳杆菌和枯草芽孢杆菌 (FAM®) 共培养对肠粘膜屏障的影响。共将 180 头仔猪分为 3 组,即对照组(C,基础日粮)、FAM 组(F,补充 0.1% FAM 的基础日粮)和抗生素组(A,补充抗生素混合物的基础日粮)。在这里,我们显示 FAM 补充剂显着增加了体重并降低了腹泻的发生率,并伴有粘膜损伤减轻、紧密连接蛋白、血清二胺氧化酶 (DAO) 和抗菌肽水平增加。此外,16S rRNA 测序和代谢组学分析显示,在 FAM 处理的仔猪中,梭菌门、瘤胃球菌科、厚壁菌门和毛霉菌门的相对丰度增加,总短链脂肪酸 (SCFAs) 和丁酸显著增加。FAM 还增加了肠粘膜中的 CD4 + T 细胞和 SIgA + 细胞以及结肠内容物中 SIgA 的产生。此外,FAM 上调了 IL-22 、短链脂肪酸受体 GPR43 和 GPR41 、芳烃受体 (AhR) 和缺氧诱导因子 1α (HIF-1α) 的表达。FAM在肠道健康中显示出良好的应用前景,为婴儿断奶提供了参考。
1. Introduction 1. 引言
肠道屏障作为一种选择性结构来防止环境抗原侵袭,这对免疫抵抗和宿主存活至关重要 [1]。肠道屏障的发育在出生后迅速发生,其特征是肠道通透性降低 [2]。上皮通透性屏障主要受紧密连接调节,紧密连接由细胞内和顶端细胞间膜蛋白组成,包括闭合小带 (ZO)、密蛋白和闭塞 [3,4,5],它们通过选择性调节肠上皮的离子和孔径来调节上皮渗漏 [5].杯状细胞和潘氏细胞等特殊上皮细胞类型也可以通过提供保护性粘液层和分泌抗菌肽来支持肠道屏障功能[6,7]。脱机应激会诱发肠道屏障功能障碍,包括肠上皮交界处缺陷、粘膜层厚度减少和抗菌肽产生缺陷 [8]。
肠道微生物群的组成和活动从出生起就与宿主共同进化,并受营养和生活方式的影响 [9]。一百万年的共同进化导致了微生物群和宿主之间的共生关系,其中肠道微生物群可能介导宿主的生理和代谢[9,10,11]。具体来说,微生物群诱导的细胞信号传导导致粘膜屏障功能、免疫反应和代谢途径发生变化,从而影响宿主生理学和病理生理学[12,13]。大多数人类疾病,如腹泻、克罗恩病 (CD)、肠易激综合征 (IBS) 和肥胖,都与肠道菌群失调和微生物多样性丧失有关 [14,15]。广泛使用抗生素和其他因素可能会减少细菌捕食者的数量,从而导致肠道微生物多样性减少[16,17]。考虑到肠道微生物群与宿主健康之间复杂的相互作用,基于肠道微生物群调节的干预措施被认为是最具潜力的干预措施之一。近年来,越来越多的研究表明,益生菌微生物对人类和动物的健康有益,包括与常驻微生物群的相互作用[18,19]、调节免疫功能[20]、产生抗菌化合物[21]和有机酸[22,23],以及改善肠道屏障的完整性[24]。
猪在肠道组成和功能上与人类有许多相似之处,使猪成为理想的动物模型[25]。在发酵膳食纤维成分方面,猪和人都使用结肠而不是盲肠作为主要部位[25]。此外,猪和人类在胃肠道微生物群功能途径方面具有 96% 的相似性 [26]。在这里,我们使用仔猪模型从肠道菌群的角度研究 FAM® (嗜酸乳杆菌和枯草芽孢杆菌的共培养) 对肠道屏障功能的体内机制。FAM 对肠道屏障的影响与肠道菌群和丁酸盐代谢过程的改变有关。我们的研究可能为帮助了解益生菌发酵对肠道健康的调节及其在动物或人类中的潜在机制提供新的见解。
2. Materials and Methods 2. 材料和方法
2.1. Animals and Experimental Design
2.1. 动物和实验设计
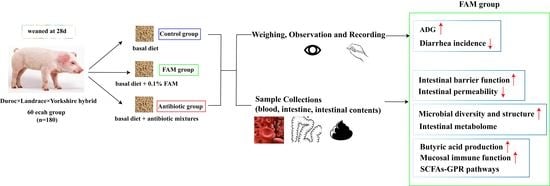
动物实验经浙江大学动物护理与使用专业委员会 (SYXK 2012- 0178) 批准,所有实验程序均符合动物研究的机构指南。将 180 头在 28 日龄断奶的仔猪 (杜洛克 ×长白 × 约克郡杂交种) 以相似的初始体重随机分为三组,即对照组 (C,基础日粮)、FAM 组 (F,补充 0.1% FAM 的基础日粮) 和抗生素组 (A,补充抗生素混合物的基础日粮)。每组有四个重复(即猪栏),每个重复有 15 头仔猪。所有仔猪都被饲养在围栏里,可以免费获得饲料和水。3 个组独立安置在不同的区域,并且 3 个区域的所有环境条件保持一致。基础日粮旨在满足国家研究委员会 (NRC, 2016) 对断奶仔猪的营养需求。FAM®(由浙江康万德川科技有限公司提供,绍兴,中国)由嗜酸乳杆菌 (≥1 × 106 CFU/g) 和枯草芽孢杆菌 (≥1 × 106 CFU/g) 共发酵。抗生素混合物含有 50 mg/kg 喹唑酮、55 mg/kg 基他霉素、75 mg/kg 金霉素和 200 mg/kg 土霉素。经过 7 天的适应期后,仔猪饲喂各自的饮食进行 30 天的实验期。观察并记录每头仔猪的体重、饲料消耗和腹泻发生率。动物和实验设计的示意图如下(方案 1)。
2.2. Sample Collection 2.2. 样本采集
在饲喂试验结束时,随机选择每组 8 头仔猪(每份复制 2 头猪,平均体重)并在禁食 12 小时后人道杀死。收集血样并在 4 °C 下以 3000 × g 离心 15 分钟,得到血清。收集来自回肠和结肠的粘膜样品,并在液氮中快速冷冻,然后储存在 -80 °C 下。 收集来自空肠和回肠的肠道组织 (1 × 1 cm2) 并固定在 4% 多聚甲醛中用于组织形态学和免疫组化分析。收集空肠组织 (0.5 × 0.5 cm2) 并固定在 2.5% 戊二醛固定剂中用于电子显微镜检查。将结肠内容物(中间部分)收集在 1.5 mL 无菌离心管中,并在液氮中快速冷冻并储存在 -80 °C 用于微生物组和代谢物分析。
2.3. Intestinal Histomorphology
2.3. 肠道组织形态学
脱水后,将空肠和回肠样品包埋在石蜡中,并使用切片机切成 5 μm 的切片。进一步用苏木精和伊红 (H&E) 和过碘酸-希夫染色切片进行形态学分析,并使用奥林巴斯 BX 51 显微镜(奥林巴斯公司,日本东京)获取图像。用计算机辅助显微镜 (Micrometrics TM;Nikon ECLIPSE E200,日本东京)。根据之前的研究,进行了透射电子显微镜和扫描电子显微镜可视化 [27]。
2.4. Gene Expression Determined by Real-Time Quantitative PCR (qRT-PCR)
2.4. 实时定量 PCR (qRT-PCR) 测定基因表达
使用设计的引物(如补充表 S1 所示),通过 qRT-PCR 测定回肠粘膜中猪 β 防御素-2 (PBD-2) 、猪 β 防御素-3 (PBD-3) 和再生胰岛衍生的 IIIγ (RegIII. γ) 的相对 mRNA 表达。在 CFX384 实时荧光定量 PCR 系统上使用 Power SYBR Green PCR 预混液 (Applied Biosystems) 进行定量分析。以 GAPDH 为管家基因,基于 2−ΔΔCt 法分析靶基因的相对表达。
2.5. Western Blot (WB) 2.5. 蛋白质印迹 (WB)
将大约 100 mg 组织溶解在 T-PER 组织蛋白提取试剂(包括蛋白酶抑制剂混合物)中进行蛋白质提取。用 BCA 蛋白测定试剂盒测定总蛋白的浓度。根据以前的研究,通过 WB 确定相对蛋白质表达。本研究中使用的一抗如下:MUC2(Novus NB120-11197,美国科罗拉多州利特尔顿)、ZO-1(Thermo Fisher 40-2200,美国马萨诸塞州沃尔瑟姆)、Claudin1(Abcam ab129119,英国剑桥)、Occludin(Abcam ab222691,英国剑桥)、GPR43(Proteintech 19952-1-AP,中国武汉)、GPR41(Abcam ab103718,英国剑桥)、HIF-1α(Thermo Fisher MA1-516,美国马萨诸塞州沃尔瑟姆)、AhR(Novus Biologicals NB100-2289,Littleton、 CO,美国)、IL-22(Affinity DF8343,中国常州)和 GADPH(Abcam ab181602,英国剑桥)。靶蛋白的相对水平针对 GAPDH 进行归一化。
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. 酶联免疫吸附测定 (ELISA)
按照制造商的建议,用猪 ELISA 试剂盒 (南京建成生物工程研究所) 检测血清中二胺氧化酶 (DAO) 、内毒素 (ET) 和 D-乳酸的蛋白质水平以及回肠内容物中的 SIgA。通过 BCA 蛋白测定试剂盒 (Beyotime Institute of Biotechnology , Shanghai, China) 测量总蛋白的浓度以使目标蛋白浓度正常化。
2.7. Immunohistochemistry
2.7. 免疫组化
根据既往研究,通过免疫组化分析 SIgA+ 细胞和 CD4+ T 细胞的密度。简而言之,石蜡包埋的回肠切片被脱蜡和再水化。揭开抗原后,用 SIgA(猪 IgA 抗体,1:1000;Bethyl Laboratories,美国德克萨斯州蒙哥马利)和 CD4(兔 CD4 抗体,1:500;Servicebio,GB11064) 一抗在 4 °C 下过夜,然后用链霉亲和素-辣根过氧化物酶处理二抗。最后,用二氨基联苯胺检测信号。
2.8. Colonic Content Microbiome Analysis
2.8. 结肠内容物微生物组分析
按照制造商的说明,使用 QIAamp Stool DNA mini kits (Qiagen, New York, NY, USA) 提取结肠内容物的微生物组基因组 DNA,然后通过聚合酶链反应 (PCR) 扩增 16s rRNA 基因的可变区 4 (V4)。将 PCR 产物混合,随后用 Qiagen 凝胶提取试剂盒 (Qiagen) 纯化。根据制造商的建议,使用 TruSeq DNA PCR-free Sample Preparation Kits (Illumina) 生成测序文库,并在 Illumina HiSeq2500 平台上测序以获得 250 bp 的双端读数,进一步与 FLASH (版本 1.2.7, http://ccb.jhu.edu/software/FLASH/) 合并以生成原始标签。在数据过滤和嵌合体删除后,生成了有效的标签以供进一步分析。通过 Uparse 软件 (Version 7.0.1001, http://drive5.com/uparse/) 将相似度> 97% 的序列归类到相同的业务分类单元 (OTU),并使用 GreenGene 数据库对代表性序列的分类信息进行注释。在 MUSCLE 软件 (Version 3.8.31, http://www.drive5.com/muscle/) 上进行多序列比对,以确定不同 OTUs 与不同类群优势种的系统发育关系。使用 Spearman 的相关性分析(IBM SPSS Inc.,芝加哥,伊利诺伊州,美国)进行选定的预测功能。
2.9. Untargeted Metabolomic Analysis
2.9. 非靶向代谢组学分析
通过多种质谱 (MS) 平台对结肠内容物进行代谢组学分析,包括气相色谱质谱/飞行时间 (GC-MS/TOF) 和超高效液相色谱/质谱 (UHPLC/MS)。在Agilent 7890A气相色谱仪系统与Pegasus HT TOF MS (Leco)联用系统上进行GC-MS/TOF分析,在Agilent 1290 UHPLC系统与TripleTOF 6600系统联用(Q-TOF,AB Sciex,Concord,ON,Canada)联用上进行UHPLC/MS分析。在 SIMCA 软件(版本 14.1,MKS Data Analytics Solutions,Concord,ON,Canada)上进行进一步的多变量统计分析。使用潜在结构判别分析的正交投影 (OPLS-DA) 分析组差异和组分离变量。获得预测能力参数 Q2 和拟合优度参数 R2Y,用于估计 7 倍交叉验证后的模型质量。进行代谢物集富集分析和通路分析,以分别在基于网络的工具 MetaboAnalyst 上生成每个差异代谢物和生物标志物代谢通路的相关京都基因和基因组百科全书 (KEGG) 通路(http://www.meta-boanalyst.ca,于 2021 年 3 月 17 日访问)。
2.10. Targeted Metabonomic Analysis
2.10. 靶向代谢组学分析
使用以下程序定量结肠内容物中的短链脂肪酸(乙酸、丙酸、丁酸、异丁酸、戊酸、异戊酸和己酸)。简而言之,使用配备Waters ACQUITY UPLC BEH酰胺柱或Waters ACQUITY UPLC BEH C18色谱柱的Agilent 1290 Infinity系列UHPLC系统对目标代谢物进行MS分析。对标准溶液进行 UPLC-平行反应监测 (PRM)-MS/MS 分析,获得标准曲线。最后,从样品和标准溶液中获得目标分析物的提取离子色谱仪 (EIC),并计算每个样品中结肠内容物的代谢物浓度 (nmol/g)。
2.11. Statistical Analysis
2.11. 统计分析
本文中的数据表示为均值±均值的标准误差 (SEM)。通过 Mann-Whitney U 检验或未配对的学生检验分析两组之间的统计差异,通过单因素方差分析评估三组之间的数据,然后进行 Tukey 多重比较 (SPSS 23.0)。概率值 p < 0.05 被认为具有统计学意义,0.05 < p < 0.10 被认为是一种趋势。
微生物丰富度根据丰富度指数 (Chao1、观察物种、ACE) 测量,并通过多样性指数 (Simpson 和 Shannon) 获取微生物多样性。使用 Bray-Curtis 方法进行 Anosim 分析。β集是根据主坐标分析 (PCoA) 和非度量多维刻度 (NMDS) 确定的。微生物菌群的相对丰度以中位数百分比表示。线性判别分析效应量 (LEfSe) 分析在在线 LEfSe 工具(http://huttenhower.sph.harvard.edu/galaxy,2021 年 9 月 21 日访问)上访问。
3. Results 3. 结果
3.1. FAM Supplementation Promoted Intestinal Barrier Function
3.1. 补充 FAM 促进肠道屏障功能
如表 1 所示,FAM 和抗生素显着增加了断奶仔猪的体重并降低了腹泻发生率。此外,在 FAM 和抗生素组中观察到微绒毛和线粒体数量增加,上皮连接恢复,表明 FAM 或抗生素改善了肠道形态(图 1A)。此外,还测量了断奶仔猪的肠道屏障功能。如图 1B、D 所示,与对照和抗生素处理的仔猪相比,FAM 处理的仔猪具有更高的紧密连接蛋白(ZO-1 和 Occludin)和 MUC2 蛋白表达水平以及更高的杯状细胞数量。通过 qPCR 分析抗菌肽 (AMPs) 的相对 mRNA 表达,涉及猪 β 防御素-2 (PBD-2)、猪 β 防御素-3 (PBD-3) 和再生胰岛衍生的 IIIγ (RegIIIγ) (图 1E)。结果显示,FAM 或抗生素显著上调 PBD-2 、 PBD-3 和 RegIIIγ 的表达,且 FAM 组 PBD-2 表达远高于抗生素。为了进一步阐明补充 FAM 后肠上皮功能得到改善,检测血清内毒素、 DAO 和 D-乳酸水平。如图 1F 所示,FAM 治疗后 DAO 水平显著降低(图 1G),表明 FAM 改善了肠道屏障功能和肠道通透性。
图 1.仔猪的肠道屏障稳态。(A) 仔猪空肠横截面的代表性电子显微镜图像。从上到下:1-3 绒毛形态的扫描电子显微镜图像,比例尺 = 300 μm;微绒毛形态的 4-6 张 SEM 图像,比例尺 = 100 μm;7-9 微绒毛结构的透射电子显微镜图像,包括微绒毛形态:红色,上皮细胞连接:蓝色,线粒体:黄色,比例尺 = 1 μm。(B) 向上,通过 WB 测定测定紧密连接蛋白 ZO-1、Claudin-1 和 Occludin 的相对表达。下,对紧密连接蛋白水平进行定量分析。(C) 左图,回肠和结肠杯状细胞的 PAS 染色图像。右图,杯状细胞的定量分析。比例尺 = 40μm。(D) 左图,回肠中 MUC2 的 WB 测定。右图为 MUC2 蛋白表达的定量分析。(E) qPCR 检测先天免疫因子 PBD-2 、 PBD-3 和 RegIIIγ 的相对 mRNA 表达。(F) 通过 ELISA 测定法测量的血清 DAO、内毒素和 D-乳酸含量 (n = 8)。** p < 0.01, * p < 0.05.
3.2. FAM Regulated Microbial Diversity and Structure in the Colon
为了检测 FAM 对肠道微生物组丰富度和多样性的直接影响,进行了α多样性分析。如图 2A 所示,FAM 组观察到的物种、Chao1、ACE 和 PD 整棵树与抗生素组相比显著增加,表明 FAM 可以增加肠道微生物组的丰富度和多样性。为了检查肠道微生物群的组成改变,进行了β多样性分析。UPGMA 聚类分析的聚类树表明,在门水平上,三组肠道微生物群的组成存在显著差异(补充图 S1A)。PCoA 和 NMDS 分析中分别聚集的肠道微生物群也证实了三组结肠微生物组的差异(图 2B)。
图 2.仔猪肠道菌群的多样性和结构变化。(A) 通过观察到的物种、结肠中的 Chao1、ACE 和 PD 整棵树估计的微生物群落的 α 多样性。(B) 基于加权 Unifrac 距离的 PCoA 散点图。基于 BrayCurtis 距离的细菌群落 NMDS 图将三组分开。(C) 通过 LDA 效应大小 (LEfSe) 分析获得组间差异显著的结肠微生物群的 LDA 评分图。LDA 评分高于 2 的微生物群被视为生物标志物微生物群 (p: 门水平,c: 类水平, o: 目水平, f: 科水平, g: 属水平)。C: 对照组, F: FAM 组, A: 抗生素组。** p < 0.01, * p < 0.05.
为了探索肠道微生物群结构的具体变化,在三个不同的分类水平上分析了微生物群落的相对丰度(补充图 S1B)。在门水平上,厚壁菌门 (75.16%) 和拟杆菌门 (22.67%) 是两种最主要的菌群。FAM 组 (0.32%) 和抗生素组 (0.35%) 变形菌门的相对丰度低于对照组 (0.52%),表明 FAM 和抗生素可以减少结肠有害细菌。在科水平上,瘤胃球菌科、Prevotellaceae、Lachnospiraceae 和 unidentified_clostridiales 是主要的分类单元。值得注意的是,FAM 组显示 Muribaculaceae 丰度增加,表明 FAM 可能促进肠道功能降解复合碳水化合物。在属水平上,Terrisporobacter、Bacteroides、Blautia、Faecalibacterium 和 Lactobacillus 是主要分类单元。LEfSe 分析进一步显示,FAM 显着增加了 18 种细菌生物标志物的相对丰度,包括梭菌门、瘤胃球菌科、厚壁菌门和毛霉菌门,并降低了涉及猪链球菌属、拟杆菌门和巨单胞菌属的 22 种微生物生物标志物的相对丰度(图 2C)。
3.3. Functional Metagenomics Prediction and Spearman’s Correlation Analysis of Gut Microbiota
3.3. 肠道微生物群的功能宏基因组学预测和 Spearman 相关性分析
为了探讨 FAM 诱导的微生物变化是否调节肠道微生物群的代谢功能,基于 16S rRNA 基因测序对肠道微生物群进行了功能宏基因组学预测(图 3A-C)。我们的结果显示,补充 FAM 后,KEGG 通路第三级的 18 条通路发生显著改变,包括 “丁酸代谢” 、 “丙酮酸代谢” 和 “丙酸代谢” 的比例显著增加,以及 “肽酶” 、 “鞘脂代谢” 和 “伴侣和折叠催化剂” 的比例显着降低。此外,与抗生素处理的仔猪相比,FAM 处理仔猪的“丁酸代谢”、“丙酮酸代谢”、“ABC 转运蛋白”、“丙酸代谢”和“色氨酸代谢”的比例显著增加,而“脂多糖生物合成”和“脂多糖生物合成蛋白”的比例降低。为了进一步分析显著不同的属与特定预测功能之间的相关性,进行了 spearman 相关性分析。如图 3D 所示,厚壁菌门和梭状芽胞杆菌属与“丁酸代谢”、“丙酮酸代谢”、“色氨酸代谢”和“脂肪酸代谢”呈正相关,与“脂多糖生物合成”呈负相关。同时,拟杆菌门、特内里菌门和拟杆菌门与“脂多糖生物合成”呈负相关,与“丁酸代谢”和“丙酮酸代谢”呈正相关。
图 3.肠道微生物群的功能宏基因组学预测和 Spearman 相关性分析。(A) 基于水平 2 和水平 3 的欧几里得距离的主成分分析 (PCA) 散点图。(B) F 组和 C 组之间 KEGG 通路第三级肠道菌群的预测功能。(C) F 组和 A 组之间 KEGG 通路第三级肠道菌群的预测功能。(D) 通过 Spearman 相关检验对特定预测功能与微生物群进行显著差异的关联分析。红色:正相关;蓝色:负相关。C: 对照组, F: FAM 组, A: 抗生素组。** p < 0.01,* p < 0.05,空白表示无显著差异。
3.4. FAM Induced Functional Changes of Intestinal Metabolome in the Colon Contents
3.4. FAM 诱导的结肠内容物肠道代谢组功能变化
微生物群代谢功能的变化通常会导致代谢物的变化。为了检查仔猪肠道代谢组的功能变化,通过 GC-MS 分析结肠内容物。OPLS-DA 评分图显示肠道代谢组分别聚集在三组之间,火山图进一步揭示了组间代谢物的显着差异(图 4A、B)。如图 4C 所示,FAM 组的棕榈油酸、石胆酸和角鲨烯水平远高于对照组和抗生素组。此外,与 FAM 组相比,我们观察到抗生素组的胺 (尸胺、马来酰亚胺和邻磷酸乙醇胺) 浓度显着升高 (图 4D)。为了进一步鉴定生物标志物 KEGG 通路,进行了代谢物集富集分析。结果显示,FAM 处理后氨基酸代谢、氨酰基-tRNA 生物合成和短链脂肪酸代谢受到显著影响 (图 4E),这与肠道菌群的功能预测 (图 3A、B) 不一致,即 FAM 组丁酸代谢显着增加。因此,我们随后探讨了 FAM 在肠道菌群丁酸酯代谢中的调节作用。
图 4.选择显着变化的代谢物和组间富集分析。(A) 通过 GC/MS 获得代谢组学的 OPLS-DA 评分图,从左到右:F 和 C、A 和 C、F 和 A 的比较,以及 F 和 A 和 C。所有个体都被分成符合实验设计的集群。(B) 火山图显示了 log2 (倍数变化) 和 -log10 (p 值) 之间的关系。(C) F 组几种具有有益作用的代谢物显著增加。(D) F 组和 A 组之间表现出差异的胺的相对浓度。(E) F 组和 C 组、F 和 A 组之间差异的前 25 个 KEGG 代谢通路。底部量表显示每条通路的富集率,每个条形的颜色深度表示差异的程度。C: 对照组, F: FAM 组, A: 抗生素组。** p < 0.01,* p < 0.05,空白表示无显著差异。
3.5. FAM Promoted Butyric Acid Production and Enhanced Mucosal Immune Function
3.5. FAM 促进丁酸生成并增强粘膜免疫功能
为了进一步确认非靶向代谢组学分析检测到的丁酸酯代谢变化,采用基于 LC-MS/MS 的靶向代谢组学方法定量短链脂肪酸 (SCFA) 的精确浓度,包括乙酸、丙酸、异丁酸、丁酸、异戊酸和戊酸。如图 5A 所示,FAM 组的丁酸和总 SCFA 水平远高于其他组。与对照组和 FAM 组相比,抗生素组总 SCFAs 、 乙酸 和 丁酸 含量较低。SCFA 是膳食纤维中微生物群的主要代谢产物,已被公认为调节粘膜免疫和肠道稳态的重要介质 [28]。因此,我们检测到粘膜免疫功能和炎症反应。结果表明,FAM 增加了肠粘膜中 CD4+ T 细胞和 SIgA+ 细胞的积分光密度 (IOD) 以及结肠内容物中 SIgA 的产生,表明 FAM 增强了粘膜免疫力(图 5B-D)。与抗生素组相比,FAM 组促炎基因 NF-κB 和 TNF-α 表达水平较低,抗炎基因 IL-22 表达水平较高(图 5E),这与既往研究一致,即肠道菌群衍生的 SCFA 可以调节 CD4+ T 细胞和肠道免疫 [28]。与对照组相比,抗生素组促炎基因 TNF-α 表达较高,抗炎基因 IL-22 水平较低,表明抗生素对仔猪有不良影响。
图 5.FAM 增强粘膜免疫并抑制炎症反应。(A) 结肠内容物中短链脂肪酸的靶向代谢组学分析:乙酸、丙酸、异丁酸、丁酸、异戊酸和戊酸。(B) 三组 SIgA+ 细胞和 CD4+ T 细胞的免疫组化染色。比例尺 = 40 μm。(C) SIgA+ 细胞和 CD4+ T 细胞的积分光密度 (IOD)。(D) 通过 ELISA 分析确定结肠内容物中的分泌型免疫球蛋白 A (SIgA) 浓度。(E) 通过 qPCR 测定炎症相关基因 NF-κB 、 TNF-α 、 IL-22 和 IL-10 的相对 mRNA 表达。** p < 0.01, * p < 0.05.
3.6. FAM Upregulated IL-22 Production and Enhanced GPR41 and GPR43 Activation
3.6. FAM 上调 IL-22 产生并增强 GPR41 和 GPR43 激活
从上述结果,我们得出结论,FAM 增强了肠道屏障功能并上调了肠道微生物群衍生的 SCFA 的产生。为了进一步证明 FAM 如何调节肠道屏障,我们评估了包括 GPR43 、 GPR41 和 GPR109A 在内的短链脂肪酸受体的表达。结果表明,FAM 组中 GPR43 和 GPR41 的相对表达远高于对照组和抗生素组(图 6A)。基于 GPR43 、 GPR41 和 IL-22 的变化,我们进一步确定了它们的相对蛋白表达。一致地,FAM 显着增加了 GPR43 、 GPR41 和 IL-22 的蛋白表达 (图 6B)。碳氢化合物受体 (AhR) 和缺氧诱导因子 1α (HIF-1α) 被认为是 CD4+ T 细胞中 SCFAs-GPR41-IL-22 通路的重要因子。因此,我们确定了肠粘膜 AhR 和 HIF-1α 的相对蛋白表达。与对照组和抗生素组相比,FAM 组 AhR 和 HIF-1α 蛋白表达较高 (图 6B),表明 FAM 可能在 SCFAs-GPR41-AhR/HIF1α-IL-22 通路和 SCFAs-GPR43-IL-22 通路两个途径上调肠道菌群来源的 SCFA 并调节 CD4+ T 细胞中 IL-22 的产生。此外,抗生素组 GPR43 、 GPR41 、 AhR 、 HIF-1α 和 IL-22 蛋白水平显著低于对照组和 FAM 组。
4. Discussion
脱机应激会诱导哺乳动物的肠道屏障功能障碍和免疫失调。在这里,我们使用仔猪模型从肠道菌群代谢功能的角度探讨 FAM (一种由嗜酸乳杆菌和枯草芽孢杆菌共培养的益生菌) 对肠道粘膜屏障的影响。乳酸菌和芽孢杆菌孢子因其对肠道免疫系统的免疫刺激特性而已被用作人类和动物食用的益生菌[29]。据报道,使用单个嗜酸乳杆菌,CRL 1014 会影响乳酸杆菌群落组成和微生物代谢,从而改善人类的肠道健康 [30]。单一枯草芽孢杆菌通过调节肠道菌群、免疫反应和血液代谢组学来降低断奶仔猪的大肠杆菌感染和腹泻发生率[31]。然而,在肠道微生物群环境中相互补充并占据不同生态位的细菌菌株的组合可能会导致对宿主免疫反应和健康的影响增强[32]。乳酸菌与蜡样芽孢杆菌共培养可以刺激乳酸菌株的生物合成能力 [33]。凝结芽孢杆菌 BC198 和副干酪乳杆菌 S38 的组合似乎在减少体内脂肪堆积和调节肠道微生物群方面显示出比单一菌株更强的功效,即使在相对较低的剂量下也是如此 [34]。 另一项研究报道,口服 Lactobacillus delbrüeckii 和 Bacillus subtilis 联合给药对金头鲷鱼细胞先天免疫反应的影响优于单独给药 [32]。我们的结果表明,嗜酸乳杆菌和枯草芽孢杆菌 (FAM) 的共培养增加了仔猪的体重并降低了腹泻的发生率,表明 FAM 在断奶应激中发挥了保护作用。
肠道屏障作为一种选择性结构来防止环境抗原侵袭,这对免疫抵抗和宿主存活至关重要 [1]。肠道屏障的发育在出生后迅速发生,其特征是肠道通透性降低 [2]。脱机应激会诱发肠道屏障功能障碍,包括肠上皮连接处缺陷、粘膜层厚度减少和抗菌肽产生缺陷 [8]。在本研究中,在断奶仔猪中观察到绒毛损伤和上皮连接受损。FAM 治疗改善了肠道形态结构以及紧密连接蛋白 (Occludin 和 ZO-1) 和 AMPs 的表达。Occludin 和 ZO-1 是顶端连接复合体 (AJC) 中的主要跨膜蛋白,它们调节离子和小分子跨上皮屏障的细胞旁扩散 [35,36]。据报道,AJC 疾病会导致上皮屏障功能受损,是许多炎症性疾病的常见特征 [37]。本研究中 Occludin 和 ZO-1 表达的增加表明 FAM 治疗后上皮通透性屏障得到改善。杯状细胞及其分泌产物粘液是粘膜屏障中防御环境抗原的重要组成部分 [38]。据报道,地衣芽孢杆菌—枯草芽孢杆菌混合物可以增加回肠中杯状细胞的数量。我们的研究表明,FAM 增加了杯状细胞和 MUC2 水平,证明了 FAM 仔猪的粘膜屏障增强。 PBD-2、PBD-3 和 RegIII. γ属于抗菌肽家族,可刺激先天免疫 [39]。PBD-2 可以通过与 Toll 样受体 4 相互作用并抑制下游 NF-κB 信号通路来缓解炎症 [7]。RegIIIγ 具有经典的 C 型凝集素结构域,可与细菌细胞壁的肽聚糖结合,对革兰氏阳性菌具有直接的抗菌活性,从而保护肠粘膜的上皮屏障功能 [28]。FAM 组 PBD-2 、 PBD-3 和 RegIII. γ 水平高于对照组,表明 FAM 对黏膜屏障有保护作用。此外,FAM 降低了血清内毒素、 DAO 和 D-乳酸水平,表明 FAM 可以促进粘膜屏障功能的发育,降低肠道通透性。
研究表明,益生菌可以调节宿主肠道菌群的结构和多样性 [40]。我们目前的研究表明,在 FAM 处理的仔猪中观察到的肠道微生物群物种 Chao1 、 ACE 和 PD 全树显著增加,表明 FAM 可以改善肠道菌群的α多样性。关于微生物群落的结构改变,一项研究表明,益生菌可以增加厚壁菌门:拟杆菌门的比率并减少仔猪模型中的腹泻 [41]。在本实验中,FAM 处理后厚壁菌门增加,而拟杆菌门减少,与之前的研究一致。此外,LEfSe 分析进一步揭示 FAM 显着增加了 18 种细菌生物标志物的相对丰度,包括梭菌门、瘤胃球菌科、厚壁菌门和毛霉菌门,降低了涉及猪链球菌属、拟杆菌门和巨单胞菌属的 22 种微生物生物标志物的相对丰度。瘤胃球菌科是将初级胆汁酸转化为次级胆汁酸并进一步促进肠道吸收和先天防御的主要细菌[42,43]。基因组分析显示,Muribaculaceae (S24-7) 在降解复合碳水化合物方面具有功能多样性 [44]。结果表明,FAM 调节微生物多样性并促进有益微生物群的相对丰度。
为探讨 FAM 诱导的微生物改变与肠道屏障功能调节之间的联系,基于 16S rRNA 基因测序对肠道菌群进行了功能性宏基因组学预测。我们发现 FAM 改变了 KEGG 通路第三级的 18 条通路,包括 'butanoate metabolism' 、 'pyruvate metabolism' 和 'propanoate metabolism'。Spearman 的相关性分析进一步显示,与“丁酸代谢”、“丙酮酸代谢”、“丙酸代谢”和“脂肪酸代谢”相关的细菌 (厚壁菌门和梭菌属) 比例较高,表明 FAM 可能通过调节肠道菌群的代谢功能重建了肠道屏障稳态。为了进一步确认肠道菌群代谢功能的变化,我们进行了多个基于 MS 平台的非靶向代谢组学分析。FAM 组结肠内容物代谢组与棕榈油酸、石胆酸和角鲨烯的显著增加有关,对宿主免疫和炎症有有益作用 [45,46,47]。此外,代谢物集富集分析表明,FAM 后氨基酸代谢、氨酰基-tRNA 生物合成和短链脂肪酸 (SCFA) 代谢受到显着影响。SCFA 是膳食纤维中肠道微生物群的主要代谢产物,已被公认为调节粘膜屏障功能的重要介质 [40].非靶向代谢组学分析中 SCFA 代谢的改变与肠道菌群的功能预测一致,表明 FAM 调节断奶仔猪肠道菌群的 SCFA 代谢。
为了进一步确认非靶向代谢组学分析检测到的 SCFA 代谢变化,我们进行了靶向代谢组学分析以量化 SCFA 代谢。先前的研究表明,益生菌通过调节细菌产生SCFAs,对人类和动物的肠道健康产生保护作用[48,49,50]。在本研究中,FAM 后结肠内容物中丁酸和总 SCFA 的产生显着增加。SCFA 被认为是肠道微生物组和免疫系统之间通讯的重要介质 [51]。微生物群衍生的 SCFA 已被证明可以通过调节不同的免疫细胞来预防多种疾病 [52,53,54]。因此,我们检测到结肠组织中的粘膜免疫功能和炎症反应。肠道相关淋巴组织和抗体是黏膜免疫的重要组成部分。B 细胞分化为 SIgA+ 细胞需要 CD4+ T 细胞分泌的细胞因子,而 SIgA+ 细胞将 SIgA 分泌到粘液中。在之前的研究中,接受益生菌补充剂的配方奶粉喂养婴儿在治疗期间保持了较高的粪便 SIgA 水平 [55,56]。在本研究中,我们观察到 FAM 治疗后肠粘膜中 CD4 + T 细胞和 SIgA + 细胞以及结肠内容物中 SIgA 水平较高,这与之前的研究一致。最近的一项研究报道,微生物群衍生的 SCFA 可以调节 CD4+ T 细胞 IL-22 的产生和肠道免疫 [54]。 在我们的研究中,FAM 降低了促炎基因的表达并增加了抗炎基因 Il22 的表达,表明 FAM 通过调节 SCFAs 代谢和 IL-22 的产生来增强粘膜免疫力。SCFA 已被证明可以通过调节宿主免疫来减少肠道炎症,其中 SCFAs-GPR 通路被认为是最重要的机制之一。游离脂肪酸受体 (FFARs) 属于 G 蛋白偶联受体 (GPCRs),包括 GPR41、GPR43 和 GPR109A,主要在肠上皮细胞和免疫细胞中表达,并被 SCFA 激活 [57]。最近的一项研究发现,丁酸盐可以通过 GPR41 和 HDAC 抑制促进 CD4+ T 细胞和 ILC 中 IL-22 的产生,其中 AhR 和 HIF-1α 参与 IL-22 的产生 [58]。在我们的研究中,我们评估了 IL-22 和短链脂肪酸受体(包括 GPR43、GPR41 和 GPR109A)的表达。结果显示,FAM 处理仔猪中 GPR41 和 GPR43 表达以及 AHR 和 HIF1α 表达显著增加,提示 SCFAs-GPR41 通路可能参与 IL-22 上调。粘膜 IL-22 和肠道菌群的相互作用在维持肠道稳态方面起着关键作用。一方面,IL-22 调整肠道菌群组成以改善肠道屏障。另一方面,肠道微生物群的代谢物促进了 IL-22 的产生 [58,59]。
5. Conclusions 5. 结论
总之,我们的结果表明,FAM 通过调节肠道菌群和 SCFA 调节肠道屏障稳态和免疫功能。GPR41 、 GPR43 、 AhR 和 HIF1α 可能介导丁酸诱导 IL-22。因此,我们的研究将 FAM 作为调节肠道稳态的新型益生菌干预措施,并可能为帮助了解它们在动物或人类中的潜在机制提供新的见解。
Supplementary Materials 补充材料
以下内容可在 https://www.mdpi.com/article/10.3390/nu14214475/s1 在线获得,图 S1:门水平肠道微生物群的相对丰度,表 S1:qRT-PCR 实验中使用的引物序列。
Author Contributions 作者贡献
概念化,Z.X. 和 M.L.;方法学,M.Q.;软件,ML;验证、Z.X.、M.L. 和 M.Q.;形式分析,Z.X.;调查,Z.Y.;资源,ML;数据管理,M.L. 和 Z.X.;写作——原始草稿准备、M.L. 和 M.Q.;写作——审查和编辑,X.H.;可视化,M.Q.;监督,X.H.;项目管理,X.H.;资金获取,X.H.所有作者均已阅读并同意手稿的已发表版本。
Funding 资金
这项研究由浙江省重点研发计划(2022C02043)、海南省三亚亚州湾科技城特别博士科学研究基金(资助号.HSPHDSRF-2022-04-008)和三亚崖湾科技城 2020 年研究计划(Grant No.SKJC- 511 2020-02-007)。
Institutional Review Board Statement
机构审查委员会声明
动物研究方案经浙江大学动物护理与使用委员会 (SYXK 2012-0178) 批准,所有实验程序均符合动物研究的机构指南。
Informed Consent Statement
知情同意书
Data Availability Statement
数据可用性声明
Conflicts of Interest 利益冲突
作者声明没有利益冲突。
References 引用
- Blikslager, A.T.; Moeser, A.J.; Gookin, J.L.; Jones, S.L.; Odle, J. Restoration of barrier function in injured intestinal mucosa. Physiol. Rev. 2007, 87, 545–564. [Google Scholar] [CrossRef]
Blikslager, A.T.;莫泽,AJ;古金,JL;琼斯,S.L.;Odle, J. 受损肠粘膜屏障功能的恢复。生理学修订版2007, 87, 545–564。[谷歌学术搜索][交叉引用] - Catassi, C.; Bonucci, A.; Coppa, G.V.; Carlucci, A.; Giorgi, P.L. Intestinal permeability changes during the first month: Effect of natural versus artificial feeding. J. Pediatr. Gastroenterol. Nutr. 1995, 21, 383–386. [Google Scholar] [CrossRef]
卡塔西,C.;博努奇,A.;科帕,GV;卡鲁奇,A.;Giorgi, PL 第一个月肠道通透性变化:自然喂养与人工喂养的影响。J. 儿科杂志。胃肠醇。营养。1995, 21, 383–386。[谷歌学术搜索][交叉引用] - Edelblum, K.L.; Turner, J.R. The tight junction in inflammatory disease: Communication breakdown. Curr. Opin. Pharm. 2009, 9, 715–720. [Google Scholar] [CrossRef] [Green Version]
埃德尔布鲁姆,KL;特纳 J.R.炎症性疾病的紧密连接:沟通中断。电流。意见。药学。2009, 9, 715–720.[谷歌学术搜索][交叉引用][绿色版] - Marchiando, A.; Graham, W.V.; Turner, J. Epithelial Barriers in Homeostasis and Disease. Annu. Rev. Pathol. 2010, 5, 119–144. [Google Scholar] [CrossRef]
马尔奇安多,A.;格雷厄姆,西弗吉尼亚州;Turner, J. 体内平衡和疾病中的上皮屏障。Annu. Rev. Pathol.2010, 5, 119–144.[谷歌学术搜索][交叉引用] - Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef]
Turner, J.R. 肠道粘膜屏障功能在健康和疾病中的作用。Nat. Rev. 免疫学。2009, 9, 799–809.[谷歌学术搜索][交叉引用] - Gustafsson, J.K.; Johansson, M.E.V. The role of goblet cells and mucus in intestinal homeostasis. Nat. Rev. Gastro. Hepat. 2022, 1–19. [Google Scholar] [CrossRef]
古斯塔夫森,JK;约翰逊,M.E.V.杯状细胞和粘液在肠道稳态中的作用。Nat. Rev. Gastro.肝。2022 年,1-19。[谷歌学术搜索][交叉引用] - Bevins, C.L.; Salzman, N.H. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat. Rev. Microbiol. 2011, 9, 356–368. [Google Scholar] [CrossRef]
贝文斯,CL;Salzman, NH 潘氏细胞、抗菌肽和维持肠道稳态。Nat. Rev. 微生物学。2011, 9, 356–368.[谷歌学术搜索][交叉引用] - Bonaz, B.L.; Bernstein, C.N. Brain-gut interactions in inflammatory bowel disease. Gastroenterology 2013, 144, 36–49. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [Green Version]
- Maruvada, P.; Leone, V.; Kaplan, L.M.; Chang, E.B. The Human Microbiome and Obesity: Moving beyond Associations. Cell Host Microbe 2017, 22, 589–599. [Google Scholar] [CrossRef]
马鲁瓦达,P.;莱昂内,V.;卡普兰,LM;张 E.B.人类微生物组和肥胖:超越关联。细胞宿主微生物2017, 22, 589–599。[谷歌学术搜索][交叉引用] - Chen, L.; Wang, D.; Garmaeva, S.; Kurilshikov, A.; Vich Vila, A.; Gacesa, R.; Sinha, T.; Segal, E.; Weersma, R.K.; Wijmenga, C.; et al. The long-term genetic stability and individual specificity of the human gut microbiome. Cell 2021, 184, 2302–2315. [Google Scholar] [CrossRef]
陈,L.;王 D.;加尔马耶娃,S.;Kurilshikov, A.;维赫·维拉,A.;加塞萨,R.;辛哈,T.;西格尔,E.;Weersma, R.K.;维伊门加,C.;等。人类肠道微生物组的长期遗传稳定性和个体特异性。细胞2021, 184, 2302–2315。[谷歌学术搜索][交叉引用] - Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.; Ismagilov, R.; Mazmanian, S.; Hsiao, E.; et al. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef] [Green Version]
矢野,JM;俞 K.;唐纳森,GP;沙斯特里,G.G.;安,P.;马,L.;纳格勒,C.;伊斯马吉洛夫,R.;马兹曼语,S.;萧 E.;来自肠道微生物群的原生细菌调节宿主血清素的生物合成。细胞2015, 161, 264–276。[谷歌学术搜索][交叉引用][绿色版] - Schirmer, M.; Garner, A.; Vlamakis, H.; Xavier, R.J. Microbial genes and pathways in inflammatory bowel disease. Nat. Rev. Microbiol. 2019, 17, 497–511. [Google Scholar] [CrossRef]
席尔默,M.;加纳,A.;弗拉马基斯,H.;Xavier, RJ 炎症性肠病中的微生物基因和途径。Nat. Rev. 微生物学。2019, 17, 497–511.[谷歌学术搜索][交叉引用] - Willing, B.P.; Dicksved, J.; Halfvarson, J.; Andersson, A.F.; Lucio, M.; Zheng, Z.; Järnerot, G.; Tysk, C.; Jansson, J.K.; Engstrand, L. A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology 2010, 139, 1844–1854. [Google Scholar] [CrossRef]
Willing, BP;迪克斯维德,J.;哈夫瓦森,J.;安德森,AF;卢西奥,M.;郑 Z.;Järnerot, G.;蒂斯克,C.;詹森,JK;恩斯特兰德,L.一项针对双胞胎的焦磷酸测序研究表明,胃肠道微生物特征因炎症性肠病表型而异。胃肠病学2010, 139, 1844–1854。[谷歌学术搜索][交叉引用] - Turnbaugh, P.J.; Bäckhed, F.; Fulton, L.; Gordon, J.I. Diet-Induced Obesity Is Linked to Marked but Reversible Alterations in the Mouse Distal Gut Microbiome. Cell Host Microbe 2008, 3, 213–223. [Google Scholar] [CrossRef] [Green Version]
Turnbaugh, PJ;Bäckhed, F.;富尔顿,L.;Gordon, J.I. 饮食诱导的肥胖与小鼠远端肠道微生物组的显着但可逆的改变有关。细胞宿主微生物2008, 3, 213–223。[谷歌学术搜索][交叉引用][绿色版] - Carroll, I.M.; Ringel-Kulka, T.; Siddle, J.P.; Ringel, Y. Alterations in composition and diversity of the intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol. Motil. 2012, 24, 248–521. [Google Scholar] [CrossRef] [Green Version]
卡罗尔,IM;林格尔-库尔卡,T.;西德尔,JP;Ringel, Y. 腹泻为主的肠易激综合征患者肠道微生物群组成和多样性的改变。神经胃肠醇。莫蒂尔。2012, 24, 248–521.[谷歌学术搜索][交叉引用][绿色版] - Mosca, A.; Leclerc, M.; Hugot, J.P. Gut Microbiota Diversity and Human Diseases: Should We Reintroduce Key Predators in Our Ecosystem? Front. Microbiol. 2016, 7, 455. [Google Scholar] [CrossRef] [Green Version]
莫斯卡,A.;勒克莱尔,M.;Hugot, JP 肠道微生物群多样性和人类疾病:我们应该在我们的生态系统中重新引入关键捕食者吗?前面。微生物学。2016, 7, 455.[谷歌学术搜索][交叉引用][绿色版] - Hegarty, J.W.; Guinane, C.M.; Ross, R.P.; Hill, C.; Cotter, P.D. Bacteriocin production: A relatively unharnessed probiotic trait? F1000Res 2016, 5, 2587. [Google Scholar] [CrossRef]
赫加蒂,JW;吉南,CM;罗斯,R.P.;希尔,C.;Cotter, P.D. 细菌素生产:一种相对未被利用的益生菌特性?F1000Res2016, 5, 2587.[谷歌学术搜索][交叉引用] - van Baarlen, P.; Wells, J.M.; Kleerebezem, M. Regulation of intestinal homeostasis and immunity with probiotic lactobacilli. Trends Immunol. 2013, 34, 208–215. [Google Scholar] [CrossRef]
范巴伦,P.;威尔斯,JM;Kleerebezem, M. 益生菌乳酸杆菌对肠道稳态和免疫的调节。趋势免疫学。2013, 34, 208–215.[谷歌学术搜索][交叉引用] - Klaenhammer, T.R.; Kleerebezem, M.; Kopp, M.V.; Rescigno, M. The impact of probiotics and prebiotics on the immune system. Nat. Rev. Immunol. 2012, 12, 728–734. [Google Scholar] [CrossRef]
Klaenhammer, T.R.;Kleerebezem, M.;科普,MV;雷西尼奥,M.益生菌和益生元对免疫系统的影响。Nat. Rev. 免疫学。2012, 12, 728–734.[谷歌学术搜索][交叉引用] - Bali, V.; Panesar, P.S.; Bera, M.B.; Kennedy, J.F. Bacteriocins: Recent Trends and Potential Applications. Crit. Rev. Food Sci. Nutr. 2016, 56, 817–834. [Google Scholar] [CrossRef]
巴厘岛,V.;帕内萨,PS;贝拉,MB;Kennedy, JF 细菌素:最近的趋势和潜在应用。Crit. Rev. 食品科学 Nutr.2016, 56, 817–834.[谷歌学术搜索][交叉引用] - Rios-Covian, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de Los, R.C.; Salazar, N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
里奥斯-科维安,D.;Ruas-Madiedo, P.;玛戈尔斯,A.;盖蒙德,M.;德洛斯,RC;Salazar, N. 肠道短链脂肪酸及其与饮食和人类健康的联系。前面。微生物学。2016, 7, 185.[谷歌学术搜索][交叉引用][公共医学][绿色版] - Sanna, S.; van Zuydam, N.R.; Mahajan, A.; Kurilshikov, A.; Vich, V.A.; Vosa, U.; Mujagic, Z.; Masclee, A.A.M.; Jonkers, D.M.A.E.; Oosting, M.; et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat. Genet. 2019, 51, 600–605. [Google Scholar] [CrossRef] [PubMed]
桑娜,S.;van Zuydam, N.R.;马哈詹,A.;Kurilshikov, A.;弗吉尼亚州维奇;美国沃萨;穆贾吉奇,Z.;马斯克利,A.A.M.;琼克斯,D.M.A.E.;奥斯廷,M.;等。肠道微生物组、短链脂肪酸和代谢疾病之间的因果关系。Nat. Genet.2019, 51, 600–605.[谷歌学术搜索][交叉引用][公共医学] - Han, X.; Lee, A.; Huang, S.; Gao, J.; Spence, J.R.; Owyang, C. Lactobacillus rhamnosus GG prevents epithelial barrier dysfunction induced by interferon-gamma and fecal supernatants from irritable bowel syndrome patients in human intestinal enteroids and colonoids. Gut. Microbes 2019, 10, 59–76. [Google Scholar] [CrossRef] [Green Version]
韩 X.;李,A.;黄 S.;高 J.;小斯宾塞;Owyang, C. 鼠李糖乳杆菌 GG 可预防人肠类肠杆菌和结肠样结肠综合征患者肠易激综合征患者的干扰素-γ 和粪便上清液诱导的上皮屏障功能障碍。肠。微生物2019, 10, 59-76。[谷歌学术搜索][交叉引用][绿色版] - Roura, E.; Koopmans, S.; Lallès, J.; Le Huerou-Luron, I.; de Jager, N.; Schuurman, T.; Val-Laillet, D. Critical review evaluating the pig as a model for human nutritional physiology. Nutr. Res. Rev. 2016, 29, 60–90. [Google Scholar] [CrossRef]
罗拉,E.;考夫曼斯,S.;拉莱斯,J.;Le Huerou-Luron, I.;de Jager, N.;舒尔曼,T.;Val-Laillet, D. 批判性评论评估猪作为人类营养生理学模型。营养研究修订版2016, 29, 60–90。[谷歌学术搜索][交叉引用] - Xiao, L.; Estellé, J.; Kiilerich, P.; Ramayo-Caldas, Y.; Xia, Z.; Feng, Q.; Liang, S.; Pedersen, A.O.; Kjeldsen, N.J.; Liu, C.; et al. A reference gene catalogue of the pig gut microbiome. Nat. Microbiol. 2016, 1, 16161. [Google Scholar] [CrossRef] [Green Version]
肖 L.;埃斯特莱,J.;基勒里奇,P.;拉马约-卡尔达斯,Y.;夏 Z.;冯 Q.;梁 S.;佩德森,AO;新泽西州凯尔德森;刘 C.;等。猪肠道微生物组的参考基因目录。自然微生物学。2016, 1, 16161.[谷歌学术搜索][交叉引用][绿色版] - Geng, S.; Cheng, S.; Li, Y.; Wen, Z.; Ma, X.; Jiang, X. Faecal Microbiota Transplantation Reduces Susceptibility to Epithelial Injury and Modulates Tryptophan Metabolism of the Microbial Community in a Piglet Model. J. Crohn’s Colitis 2018, 12, 1359–1374. [Google Scholar] [CrossRef]
耿, S.;程 S.;李英;温,Z.;马,X.;江, X. 粪便微生物群移植降低了仔猪模型中上皮损伤的易感性并调节微生物群落的色氨酸代谢。J. 克罗恩结肠炎2018, 12, 1359–1374。[谷歌学术搜索][交叉引用] - Huang, J.; Qi, Y.; Wang, A.; Huang, C.; Liu, X.; Yang, X.; Li, L.; Zhou, R. Porcine β-defensin 2 inhibits proliferation of pseudorabies virus in vitro and in transgenic mice. Virol. J. 2020, 17, 18. [Google Scholar] [CrossRef]
黄 J.;齐,Y.;王 A.;黄 C.;刘晓波;杨 X.;李,L.;周,R. Porcine β-defensin 2 在体外和转基因小鼠中抑制伪狂犬病病毒的增殖。Virol. J.2020, 17, 18.[谷歌学术搜索][交叉引用] - Casula, G.; Cutting, S.M. Bacillus Probiotics: Spore Germination in the Gastrointestinal Tract. Appl. Environ. Microb. 2002, 68, 2344–2352. [Google Scholar] [CrossRef] [Green Version]
卡苏拉,G.;Cutting, SM Bacillus Probiotics:胃肠道中的孢子萌发。Appl. Environ.微。2002, 68, 2344–2352.[谷歌学术搜索][交叉引用][绿色版] - Sivieri, K.; Morales, M.L.V.; Adorno, M.A.T.; Sakamoto, I.K.; Saad, S.M.I.; Rossi, E.A. Lactobacillus acidophilus CRL 1014 improved “gut health” in the SHIME® reactor. BMC Gastroenterol. 2013, 13, 100. [Google Scholar] [CrossRef]
西维耶里,K.;莫拉莱斯,M.L.V.;阿多诺,M.A.T.;坂本,I.K.;萨德,S.M.I.;Rossi, E.A. 嗜酸乳杆菌 CRL 1014 改善了 SHIME® 反应器中的“肠道健康”。BMC 胃肠醇。2013, 13, 100.[谷歌学术搜索][交叉引用] - Luise, D.; Bertocchi, M.; Motta, V.; Salvarani, C.; Bosi, P.; Luppi, A.; Fanelli, F.; Mazzoni, M.; Archetti, I.; Maiorano, G.; et al. Bacillus sp. probiotic supplementation diminish the Escherichia coli F4ac infection in susceptible weaned pigs by influencing the intestinal immune response, intestinal microbiota and blood metabolomics. J. Anim. Sci. Biotechnol. 2019, 10, 74. [Google Scholar] [CrossRef] [PubMed]
露易丝,D.;贝尔托奇,M.;莫塔,V.;萨尔瓦拉尼,C.;博斯,P.;卢皮,A.;法内利,F.;马佐尼,M.;阿切蒂,I.;马奥拉诺,G.;等人 芽孢杆菌益生菌补充剂通过影响肠道免疫反应、肠道微生物群和血液代谢组学来减少易感断奶仔猪的大肠杆菌 F4ac 感染。J. Anim. Sci. 生物技术。2019, 10, 74.[谷歌学术搜索][交叉引用][公共医学] - Salinas, I.; Cuesta, A.; Esteban, M.Á.; Meseguer, J. Dietary administration of Lactobacillus delbrüeckii and Bacillus subtilis, single or combined, on gilthead seabream cellular innate immune responses. Fish Shellfish Immun. 2005, 19, 67–77. [Google Scholar] [CrossRef]
萨利纳斯,I.;库斯塔,A.;埃斯特班,M.Á.;Meseguer, J. Lactobacillus delbrüeckii 和 Bacillus subtilis 的饮食给药,单独或联合用于金头鲷细胞先天免疫反应。鱼 贝类免疫。2005, 19, 67–77.[谷歌学术搜索][交叉引用] - Røssland, E.; Langsrud, T.; Granum, P.E.; Sørhaug, T. Production of antimicrobial metabolites by strains of Lactobacillus or Lactococcus co-cultured with Bacillus cereus in milk. Int. J. Food Microbiol. 2005, 98, 193–200. [Google Scholar] [CrossRef] [PubMed]
Røssland, E.;朗斯鲁德,T.;Granum,PE;Sørhaug, T. 与蜡样芽孢杆菌在牛奶中共培养的乳酸菌或乳球菌菌株产生抗菌代谢物。国际食品微生物学杂志。2005, 98, 193–200.[谷歌学术搜索][交叉引用][公共医学] - Hsieh, C.; You, F.; Dai, F.; Tung, Y.; Chen, W.; Chu, H.; Wu, S.-H.; Vhau, C.-F. Bacillus coagulans BC198 and Lactobacillus paracasei S38 in combination reduce body fat accumulation and modulate gut microbiota. CyTA-J. Food 2020, 18, 764–775. [Google Scholar] [CrossRef]
谢 C.;你,F.;戴,F.;董 Y.;陈 W.;朱 H.;吴 S.-H.;Vhau, C.-F.凝结芽孢杆菌BC198 和副干酪乳杆菌 S38 联合使用可减少体内脂肪堆积并调节肠道微生物群。CyTA-J 的。食品2020, 18, 764–775。[谷歌学术搜索][交叉引用] - Severson, E.A.; Kwon, M.; Hilgarth, R.S.; Parkos, C.A.; Nusrat, A. Glycogen Synthase Kinase 3 (GSK-3) influences epithelial barrier function by regulating Occludin, Claudin-1 and E-cadherin expression. Biochem. Biophys. Res. Commun. 2010, 397, 592–597. [Google Scholar] [CrossRef] [Green Version]
塞弗森,EA;权,M.;希尔加斯,R.S.;加利福尼亚州帕科斯;Nusrat, A. 糖原合酶激酶 3 (GSK-3) 通过调节 Occludin、Claudin-1 和 E-cadherin 表达来影响上皮屏障功能。生化。生物物理学。Res. Commun.2010, 397, 592–597.[谷歌学术搜索][交叉引用][绿色版] - Gumbiner, B. Structure, biochemistry, and assembly of epithelial tight junctions. Am. J. Physiol.-Cell Physiol. 1987, 253, C749–C758. [Google Scholar] [CrossRef] [PubMed]
Gumbiner, B. 上皮紧密连接的结构、生物化学和组装。美国生理学杂志-细胞生理学。1987, 253, C749–C758.[谷歌学术搜索][交叉引用][公共医学] - Schmitz, H.; Barmeyer, C.; Fromm, M.; Runkel, N.; Foss, H.; Bentzel, C.J.; Riechen, E.-O.; Schulzke, J.-D. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology 1999, 116, 301–309. [Google Scholar] [CrossRef]
施密茨,H.;巴梅尔,C.;弗洛姆,M.;朗克尔,N.;福斯,H.;本茨,CJ;Riechen, E.-O.;舒尔茨克,法学博士紧密连接结构的改变导致溃疡性结肠炎的上皮屏障功能受损。胃肠病学1999, 116, 301–309。[谷歌学术搜索][交叉引用] - McDole, J.R.; Wheeler, L.W.; McDonald, K.G.; Wang, B.; Konjufca, V.; Knoop, K.A. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature 2012, 483, 345–349. [Google Scholar] [CrossRef] [Green Version]
麦克多尔,J.R.;惠勒,L.W.;麦克唐纳,K.G.;王 B.;孔朱夫卡,V.;Knoop, K.A. Goblet 细胞将管腔抗原递送至小肠中的 CD103+ 树突状细胞。自然2012, 483, 345–349。[谷歌学术搜索][交叉引用][绿色版] - Cash, H.L.; Whitham, C.V.; Behrendt, C.L.; Hooper, L.V. Symbiotic Bacteria Direct Expression of an Intestinal Bactericidal Lectin. Science 2006, 313, 1126–1130. [Google Scholar] [CrossRef] [Green Version]
卡什,HL;惠特姆,CV;贝伦特,CL;Hooper, L.V. 共生细菌直接表达肠道杀菌凝集素。科学2006, 313, 1126–1130。[谷歌学术搜索][交叉引用][绿色版] - Fan, L.; Qi, Y.; Qu, S.; Chen, X.; Li, A.; Hendi, M.; Xu, C.; Wang, L.; Hou, T.; Si, J.; et al. B. adolescentis ameliorates chronic colitis by regulating Treg/Th2 response and gut microbiota remodeling. Gut Microbes 2021, 13, 1–17. [Google Scholar] [CrossRef]
范,L.;齐,Y.;曲 S.;陈 X.;李 A.;亨迪,M.;徐 C.;王 L.;侯 T.;Si, J.;et al. B. adolescentis 通过调节 Treg/Th2 反应和肠道微生物群重塑来改善慢性结肠炎。肠道微生物2021, 13, 1-17。[谷歌学术搜索][交叉引用] - Molist, F.; Manzanilla, E.G.; Pérez, J.F.; Nyachoti, C.M. Coarse, but not finely ground, dietary fibre increases intestinal Firmicutes: Bacteroidetes ratio and reduces diarrhoea induced by experimental infection in piglets. Br. J. Nutr. 2012, 108, 9–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
莫利斯特,F.;Manzanilla, E.G.;佩雷斯,JF;Nyachoti, C.M. 粗但不细磨的膳食纤维增加肠道厚壁菌门:拟杆菌门比例并减少仔猪实验性感染引起的腹泻。Br. J. Nutr.2012, 108, 9-15.[谷歌学术搜索][交叉引用][公共医学][绿色版] - Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Nunez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef] [PubMed]
皮卡德,JM;曾, M.Y.;卡鲁索,R.;Nunez, G. 肠道微生物群:在病原体定植、免疫反应和炎症性疾病中的作用。免疫学修订版2017, 279, 70–89。[谷歌学术搜索][交叉引用][公共医学] - Shapiro, H.; Kolodziejczyk, A.A.; Halstuch, D.; Elinav, E. Bile acids in glucose metabolism in health and disease. J. Exp. Med. 2018, 215, 383–396. [Google Scholar] [CrossRef] [PubMed]
夏皮罗,H.;Kolodziejczyk,AA;哈尔斯图赫,D.;Elinav, E. 健康和疾病中葡萄糖代谢中的胆汁酸。J. Exp. 医学。2018, 215, 383–396.[谷歌学术搜索][交叉引用][公共医学] - Lagkouvardos, I.; Lesker, T.R.; Hitch, T.; Galvez, E.; Smit, N.; Neuhaus, K.; Wang, J.; Baines, J.F.; Abt, B.; Stecher, B.; et al. Sequence and cultivation study of Muribaculaceae reveals novel species, host preference, and functional potential of this yet undescribed family. Microbiome 2019, 7, 28. [Google Scholar] [CrossRef] [Green Version]
Lagkouvardos, I.;莱斯克,TR;希奇,T.;加尔维兹,E.;斯密特,N.;诺伊豪斯,K.;王 J.;贝恩斯,JF;阿布特,B.;施特赫尔,B.;等。Muribaculaceae 的序列和培养研究揭示了这个尚未描述的科的新物种、宿主偏好和功能潜力。微生物组2019, 7, 28。[谷歌学术搜索][交叉引用][绿色版] - Andersen, V.; Olsen, A.; Carbonnel, F.; Tjønneland, A.; Vogel, U. Diet and risk of inflammatory bowel disease. Dig. Liver Dis. 2012, 44, 185–194. [Google Scholar] [CrossRef]
安徒生,V.;奥尔森,A.;卡博内尔,F.;Tjønneland, A.;Vogel, U. 饮食和炎症性肠病的风险。挖。肝脏疾病2012, 44, 185–194.[谷歌学术搜索][交叉引用] - Ye, J.; Lv, L.; Wu, W.; Li, Y.; Shi, D.; Fang, D.; Guo, F.; Jiang, H.; Yan, R.; Ye, W.; et al. Butyrate protects mice against methionine–choline-deficient diet-induced non-alcoholic steatohepatitis by improving gut barrier function, attenuating inflammation and reducing endotoxin levels. Front. Microbiol. 2018, 9, 1967. [Google Scholar] [CrossRef] [Green Version]
叶,J.;吕,L.;吴,W.;李英;石 D.;方 D.;郭 F.;江 H.;严,R.;叶,W.;等。丁酸盐通过改善肠道屏障功能、减轻炎症和降低内毒素水平,保护小鼠免受蛋氨酸-胆碱缺乏症饮食诱导的非酒精性脂肪性肝炎的侵害。前面。微生物学。2018, 9, 1967.[谷歌学术搜索][交叉引用][绿色版] - Zhang, J.; Zhang, L.; Ye, X.; Chen, L.; Zhang, L.; Gao, Y.; Kang, J.X.; Cai, C. Characteristics of fatty acid distribution is associated with colorectal cancer prognosis. Prostaglandins Leukot. Essent. Fatty Acid. 2013, 88, 355–360. [Google Scholar] [CrossRef]
张 J.;张 L.;叶 X.;陈,L.;张 L.;高 Y.;康,JX;Cai, C. 脂肪酸分布特征与结直肠癌预后相关。前列腺素 Leukot。埃森特。脂肪酸。2013, 88, 355–360.[谷歌学术搜索][交叉引用] - Kim, H.K.; Rutten, N.; Besseling-van Der Vaart, I.; Niers, L.; Choi, Y.H.; Rijkers, G.T.; van Hemert, S. Probiotic supplementation influences faecal short chain fatty acids in infants at high risk for eczema. Benef. Microbes 2015, 6, 783–790. [Google Scholar] [CrossRef]
Kim, H.K.;鲁滕,N.;Besseling-van Der Vaart, I.;尼尔斯,L.;崔,YH;Rijkers, GT;van Hemert, S. 益生菌补充剂会影响湿疹高危婴儿的粪便短链脂肪酸。贝内夫。微生物2015, 6, 783–790。[谷歌学术搜索][交叉引用] - Markowiak-Kopeć, P.; Śliżewska, K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef] [Green Version]
Markowiak-Kopeć, P.;Śliżewska, K.益生菌对人类肠道微生物组产生短链脂肪酸的影响。营养素2020, 12, 1107。[谷歌学术搜索][交叉引用][绿色版] - Zhuang, M.; Shang, W.; Ma, Q.; Strappe, P.; Zhou, Z. Abundance of probiotics and butyrate-production microbiome manages constipation via short-chain fatty acids production and hormones secretion. Mol. Nutr. Food Res. 2019, 63, 1801187. [Google Scholar] [CrossRef]
庄 M.;尚 W.;马,Q.;斯特拉普,P.;周,Z. 丰富的益生菌和丁酸盐生产微生物组通过短链脂肪酸产生和激素分泌管理便秘。Mol. Nutr.食品研究2019, 63, 1801187.[谷歌学术搜索][交叉引用] - Ratajczak, W.; Rył, A.; Mizerski, A.; Walczakiewicz, K.; Sipak, O.; Laszczyńska, M. Immunomodulatory potential of gut microbiome-derived short-chain fatty acids (SCFAs). Acta Biochim. Polon. 2019, 66, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Ratajczak, W.;Rył, A.;米泽斯基,A.;Walczakiewicz, K.;俄亥俄州西帕克;Laszczyńska, M. 肠道微生物组衍生的短链脂肪酸 (SCFA) 的免疫调节潜力。比奥希姆学报。波隆。2019, 66, 1-12.[谷歌学术搜索][交叉引用][公共医学][绿色版] - Chen, L.; Sun, M.; Wu, W.; Yang, W.; Huang, X.; Xiao, Y.; Ma, C.; Xu, L.; Yao, S.; Liu, Z.; et al. Microbiota metabolite butyrate differentially regulates Th1 and Th17 cells’ differentiation and function in induction of colitis. Inflamm. Bowel Dis. 2019, 25, 1450–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
陈,L.;孙,M.;吴,W.;杨 W.;黄 X.;肖 Y.;马,C.;徐 L.;姚 S.;刘 Z.;等。微生物群代谢物丁酸盐差异调节 Th1 和 Th17 细胞的分化和在结肠炎诱导中的功能。炎症。肠病2019, 25, 1450–1461.[谷歌学术搜索][交叉引用][公共医学][绿色版] - Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glikman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [Green Version]
史密斯,PM;霍伊特,M.R.;帕尼科夫,N.;米肖,M.;加里尼,CA;Bohlooly-Y,M.;格里克曼,JN;加勒特,W.S.微生物代谢物短链脂肪酸调节结肠 Treg 细胞稳态。科学2013, 341, 569–573。[谷歌学术搜索][交叉引用][绿色版] - Sun, M.; Wu, W.; Chen, L.; Yang, W.; Huang, X.; Ma, C.; Chen, F.; Xiao, Y.; Zhao, Y.; Ma, C.; et al. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat. Commun. 2018, 9, 3555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
孙,M.;吴,W.;陈,L.;杨 W.;黄 X.;马,C.;陈,F.;肖 Y.;赵 Y.;马,C.;等。微生物群衍生的短链脂肪酸促进 Th1 细胞 IL-10 的产生以维持肠道稳态。Nat. Commun.2018, 9, 3555.[谷歌学术搜索][交叉引用][公共医学][绿色版] - Min, Y.N.; Yang, H.L.; Xu, Y.X.; Gao, Y.P. Effects of dietary supplementation of synbiotics on growth performance, intestinal morphology, sIgA content and antioxidant capacities of broilers. J. Anim. Physiol. Anim. Nutr. 2016, 100, 1073–1080. [Google Scholar] [CrossRef]
闵,YN;杨,HL;徐,YX;Gao, Y.P. 膳食中添加合生元对肉鸡生长性能、肠道形态、sIgA 含量和抗氧化能力的影响。J. Anim. Physiol. Anim. 营养学2016, 100, 1073–1080.[谷歌学术搜索][交叉引用] - Xiao, L.; Gong, C.; Ding, Y.; Ding, G.; Xu, X.; Deng, C.; Ze, X.; Malard, P.; Ben, X. Probiotics maintain intestinal secretory immunoglobulin A levels in healthy formula-fed infants: A randomised, double-blind, placebo-controlled study. Benef. Microbes 2019, 10, 729–739. [Google Scholar] [CrossRef]
肖 L.;龚 C.;丁 Y.;丁 G.;徐旭;邓,C.;泽,X.;马拉德,P.;益生菌维持健康配方奶粉喂养婴儿的肠道分泌免疫球蛋白 A 水平:一项随机、双盲、安慰剂对照研究。贝内夫。微生物2019, 10, 729–739。[谷歌学术搜索][交叉引用] - Sun, M.; Wu, W.; Liu, Z.; Cong, Y. Microbiota metabolite short chain fatty acids, GPCR., and inflammatory bowel diseases. J. Gastroenterol. 2017, 52, 1–8. [Google Scholar] [CrossRef]
孙,M.;吴,W.;刘 Z.;Cong, Y. 微生物群代谢物短链脂肪酸、GPCR. 和炎症性肠病。J. 胃肠醇。2017, 52, 1-8.[谷歌学术搜索][交叉引用] - Zhao, Y.; Chen, F.; Wu, W.; Sun, M.; Bilotta, A.J.; Yao, S.; Xiao, Y.; Huang, X.; Eaves-Pyles, T.D.; Golovko, G.; et al. GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3. Mucosal Immunol. 2018, 11, 752–762. [Google Scholar] [CrossRef] [Green Version]
赵 Y.;陈,F.;吴,W.;孙,M.;比洛塔,AJ;姚 S.;肖 Y.;黄 X.;Eaves-Pyles, T.D.;戈洛夫科,G.;GPR43 通过激活 mTOR 和 STAT3 介导微生物群代谢物 SCFA 对肠上皮细胞中抗菌肽表达的调节。粘膜免疫学。2018, 11, 752–762.[谷歌学术搜索][交叉引用][绿色版] - Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
鲁克斯,M.G.;Garrett, WS 肠道微生物群、代谢物和宿主免疫。Nat. Rev. 免疫学。2016, 16, 341–352.[谷歌学术搜索][交叉引用]
| Item | Control | FAM | Antibiotic |
|---|---|---|---|
| Initial BW, kg | 10.34 ± 0.45 | 10.02 ± 0.46 | 9.96 ± 0.51 |
| Final BW, kg | 23.75 ± 2.99 | 25.98 ± 3.31 | 24.71 ± 2.85 |
| ADG, g/d | 447 ± 22.3 b | 532 ± 28.5 a | 492 ± 19.7 a |
| ADFI, g/d | 688 ± 45.7 ab | 736 ± 47.8 a | 686 ± 38.6 ab |
| F/G | 1.53 ± 0.05 a | 1.36 ± 0.02 b | 1.39 ± 0.03 b |
| Diarrhea incidence | 8.00 ± 0.50 a | 4.14 ± 0.25 b | 4.47 ± 0.32 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. 出版商注:MDPI 对已发布的地图和机构隶属关系中的管辖权主张保持中立。 |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
© 2022 年由作者。被许可人 MDPI,瑞士巴塞尔。本文是根据知识共享署名 (CC BY) 许可 (https://creativecommons.org/licenses/by/4.0/) 的条款和条件分发的开放获取文章。
Share and Cite 分享和引用
Xie, Z.; Li, M.; Qian, M.; Yang, Z.; Han, X.
Co-Cultures of Lactobacillus acidophilus and Bacillus subtilis Enhance Mucosal Barrier by Modulating Gut Microbiota-Derived Short-Chain Fatty Acids. Nutrients 2022, 14, 4475.
https://doi.org/10.3390/nu14214475
谢 Z.;李 M.;钱 M.;杨 Z.;Han, X. 嗜酸乳杆菌和枯草芽孢杆菌的共培养物通过调节肠道微生物群衍生的短链脂肪酸来增强粘膜屏障。营养素2022, 14, 4475。https://doi.org/10.3390/nu14214475
Xie Z, Li M, Qian M, Yang Z, Han X.
Co-Cultures of Lactobacillus acidophilus and Bacillus subtilis Enhance Mucosal Barrier by Modulating Gut Microbiota-Derived Short-Chain Fatty Acids. Nutrients. 2022; 14(21):4475.
https://doi.org/10.3390/nu14214475
Xie Z, Li M, Qian M, Yang Z, Han X. 嗜酸乳杆菌和枯草芽孢杆菌的共培养通过调节肠道微生物群衍生的短链脂肪酸来增强粘膜屏障。营养素。2022;14(21):4475.https://doi.org/10.3390/nu14214475
Xie, Zhengjun, Meng Li, Mengqi Qian, Zhiren Yang, and Xinyan Han.
2022. "Co-Cultures of Lactobacillus acidophilus and Bacillus subtilis Enhance Mucosal Barrier by Modulating Gut Microbiota-Derived Short-Chain Fatty Acids" Nutrients 14, no. 21: 4475.
https://doi.org/10.3390/nu14214475
Xie, Zhengjun, Meng Li, Mengqi Qian, Zhiren Yang, 和 Xinyan Han.2022. “嗜酸乳杆菌和枯草芽孢杆菌的共培养通过调节肠道微生物群衍生的短链脂肪酸增强粘膜屏障”营养物质 14,第 21 期:4475。https://doi.org/10.3390/nu14214475
请注意,从 2016 年第一期开始,本期刊使用文章编号而不是页码。在此处查看更多详细信息。