Abstract 抽象
The orchid mantises achieve camouflage with morphological modifications in body color and pattern, providing an interesting model for understanding phenotypic innovation. However, a reference genome is lacking for the order Mantodea. To unveil the mechanisms of plant-mimicking body coloration and patterns, we performed de novo assembly of two chromosome-level genomes of the orchid mantis and its close relative, the dead leaf mantis. Comparative genomic analysis revealed that the Scarlet gene plays an important role in the synthesis of xanthommatin, an important pigment for mantis camouflage coloration. Combining developmental transcriptomic analysis and genetic engineering experiments, we found that the cuticle was an essential component of the ‘petal-like’ enlargement, and specific expression in the ventral femur was controlled by Wnt signaling. The prolonged expression of Ultrabithorax (Ubx) accompanied by femoral expansion suggested that Ubx determines leg remodeling in the early developmental stage. We also found evidence of evolution of the Trypsin gene family for insectivory adaptation and ecdysone-dependent sexual dimorphism in body size. Overall, our study presents new genome catalogs and reveals the genetic and evolutionary mechanisms underlying the unique camouflage of the praying mantis, providing evolutionary developmental insights into phenotypic innovation and adaptation.
兰花螳螂通过体色和图案的形态改变来实现伪装,为理解表型创新提供了一个有趣的模型。然而,缺乏 Mantodea 目 的参考基因组。为了揭示模拟植物的身体颜色和图案的机制,我们对兰花螳螂及其近亲枯叶螳螂的两个染色体水平基因组进行了从头组装。比较基因组分析显示,Scarlet 基因在黄素的合成中起重要作用,黄马素是螳螂伪装着色的重要色素。结合发育转录组学分析和基因工程实验,我们发现角质层是“花瓣状”增大的重要组成部分,股骨腹侧的特异性表达受 Wnt 信号传导控制。Ultrabithorax (Ubx) 的长期表达伴有股骨扩张表明 Ubx 决定早期发育阶段的腿部重塑。我们还发现了胰蛋白酶基因家族进化的证据,用于食虫适应和蜕皮激素依赖性体型二态性。总体而言,我们的研究提出了新的基因组目录,并揭示了螳螂独特伪装背后的遗传和进化机制,为表型创新和适应提供了进化发育见解。
Similar content being viewed by others
其他人正在查看类似内容
Introduction 介绍
As predator‒prey interactions are a substantial component of all biological communities, camouflage arising from natural selection is a key adaptive strategy for avoiding predators or attracting prey1,2,3,4. Animal camouflage involves morphological modifications in body colors and patterns that reduce visual detection or recognition5,6. To date, most studies have focused on antipredator camouflage, as a wide range of prey animals benefit from masquerading as inedible and often inanimate objects, such as twigs, leaves, stones or bird droppings7. However, very little attention has been given to predatory camouflage strategies8.
由于捕食者与猎物的互动是所有生物群落的重要组成部分,因此自然选择产生的伪装是避开捕食者或吸引猎物的关键适应性策略 1,2,3,4。动物伪装涉及体色和图案的形态改变,这会降低视觉检测或识别5,6。迄今为止,大多数研究都集中在反捕食者的伪装上,因为许多猎物都受益于伪装成不可食用且通常无生命的物体,例如树枝、树叶、石头或鸟粪7。然而,很少有人关注掠夺性伪装策略8。
Many clades of predatory insects exhibit a remarkable range of morphological camouflage adaptations in response to predation pressure and to attract prey9,10,11. One of the most striking, yet poorly understood, cases occurs in the Mantodea, which are carnivorous insects that exhibit rich diversity, with approximately 2500 species possessing diverse morphological and ecological characteristics12. Despite their popularity and appeal, praying mantises are understudied. Many species in this lineage use strategies involving spectacular morphological modifications in body color and pattern to mimic plant parts, such as flowers, dead leaves, sticks, and barks, and mosses13.
许多捕食性昆虫的分支表现出显着的形态伪装适应能力,以响应捕食压力并吸引猎物 9,10,11。最引人注目但知之甚少的案例之一发生在 Mantodea,这是一种肉食性昆虫,表现出丰富的多样性,大约有 2500 个物种具有不同的形态和生态特征12。尽管它们很受欢迎和有吸引力,但螳螂的研究不足。该谱系中的许多物种使用涉及体色和图案的壮观形态变化的策略来模仿植物部分,例如花朵、枯叶、棍棒和树皮以及苔藓13。
The orchid mantis Hymenopus coronatus (Mantodea: Hymenopodidae), a popular and charismatic species, has developed spectacular flower-mimicking strategies12,14,15. The white and pink coloration in combination with its “petal-shaped” femoral lobes and broad abdomen allow the orchid mantis to mimic generalized flowers. Interestingly, this floral simulation alone attracts more insect pollinators than real flowers16. The trade-off between antipredator camouflage and predatory camouflage of the orchid mantis makes it a master of camouflage. Another popular plant-mimicking mantis is the dead leaf mantis Deroplatys lobata (Mantodea: Deroplatyidae), which shows coloring ranging from brown to gray and possesses a broad prothorax that looks like a ripped and crumpled leaf17. The false leaf vein on the adult’s tucked wings and leaf petiole residue-like legs further its dead-leaf-like appearance. In addition to the uncanny combination of body colors and patterns, these mantises also display features generalizable to any mantis, including insectivory and extreme sexual dimorphism in body size18,19. However, the genetic mechanisms underlying these unique phenotypes remain unexplored. The lack of genomic resources for Mantodea lineages has impeded further in-depth molecular and developmental investigation.
兰花螳螂 Hymenopus coronatus(Mantodea:Hymenopodidae)是一种受欢迎且富有魅力的物种,已经开发出了壮观的模仿花策略12,14,15。白色和粉红色与其“花瓣状”股裂片和宽阔的腹部相结合,使兰花螳螂能够模仿广义的花朵。有趣的是,仅这种花卉模拟就比真正的花朵吸引了更多的昆虫传粉者16。兰花螳螂的反捕食者伪装和捕食性伪装之间的权衡使其成为伪装大师。另一种流行的植物模仿螳螂是枯叶螳螂 Deroplatys lobata(Mantodea:Deroplatyidae),它的颜色从棕色到灰色不等,并拥有宽阔的前胸,看起来像一片撕裂和皱巴巴的叶子17。成虫收起的翅膀和叶柄残留物状腿上的假叶脉进一步加深了其枯叶状的外观。除了体色和图案的不可思议的组合外,这些螳螂还显示出任何螳螂都可以推广的特征,包括食虫和体型18,19 的极端性二态性。然而,这些独特表型背后的遗传机制仍未得到探索。Mantodea 谱系基因组资源的缺乏阻碍了进一步深入的分子和发育研究。
In this work, we preform de novo-assembly of the chromosome-level genomes of the flower-mimicking species H. coronatus with dead leaf-mimicking species D. lobata to investigate and compare the genetics and evolutionary mechanism of phenotypic innovation of these species. Comparative genomic and developmental transcriptomic analyses, and genetic engineering experiments based on RNA interference (RNAi) were performed to dissect the genetic basis of these camouflage phenomena. Here, we present reference genomic resources for two species of mantis and explore the genetic mechanisms of adaptive camouflage in the orchid mantis via the combination of genomic and transcriptomic analysis with functional testing. We believe these results will have far-reaching implications for future in-depth study of Mantodea.
在这项工作中,我们对拟花物种 H. coronatus 和模拟枯叶物种 D. lobata 的染色体水平基因组进行了从头组装,以研究和比较这些物种表型创新的遗传学和进化机制。进行了比较基因组和发育转录组分析,以及基于 RNA 干扰 (RNAi) 的基因工程实验,以剖析这些伪装现象的遗传基础。在这里,我们提供了两种螳螂的参考基因组资源,并通过将基因组和转录组分析与功能测试相结合,探索了兰花螳螂适应性伪装的遗传机制。我们相信这些结果将对未来对 Mantodea 的深入研究产生深远的影响。
Results and Discussion 结果与讨论
Genomic signatures in two highly camouflaged mantises
两只高度伪装的螳螂中的基因组特征
Due to the highly camouflaged appearances of the orchid mantis and dead leaf mantis, they are rarely observed in the wild (Fig. 1A), resulting in little knowledge available for their evolution and biology. To decode the genomic signature of two plant-mimicking mantises, H. coronatus and D. lobata, we performed single-molecule real-time sequencing (Nanopore), Illumina paired-end sequencing, and Hi-C sequencing (Fig. 1B). The high-quality Illumina reads were used to correct the short insertions or deletions (indels) and substitutions in the Nanopore assemblies. More than 98.13% of the Hi-C reads were successfully anchored to 23 chromosomes for H. coronatus (2n = 46) and 14 chromosomes for D. lobata (2n = 28) (Supplementary Fig. 1; Supplementary Table S1). Finally, two genome assemblies were obtained with sizes of 2.88 Gb for H. coronatus and 3.96 Gb for D. lobata, showing contig N50 lengths of 15.76 Mb and 6.11 Mb, respectively (Supplementary Table 2). Subsequently, the integrity of the assembly was demonstrated by 99.32%–99.87% mapping rates for Illumina sequencing reads and Nanopore sequencing reads (Supplementary Table 3) and 96.56%–96.74% Benchmarking Universal Single-Copy Orthologs (BUSCO) completeness (Supplementary Table 4). A series of evaluations indicated that the genome assemblies were of high quality (Supplementary Fig. 2).
由于兰花螳螂和枯叶螳螂的外表高度伪装,它们在野外很少被观察到(图 D)。1A),导致可用于它们的进化和生物学的知识很少。为了解码两种植物模拟螳螂 H. coronatus 和 D. lobata 的基因组特征,我们进行了单分子实时测序 (Nanopore)、Illumina 双端测序和 Hi-C 测序(图 D.高质量的 Illumina 读长用于校正 Nanopore 组装体中的短插入或缺失(插入缺失)和替换。超过 98.13% 的 Hi-C 读数成功锚定到冠状嗜血杆菌的 23 条染色体 (2n = 46) 和 D. lobata(2n = 28) 的 14 条染色体上(补充图 D)。1;补充表 S1)。最后,获得了两个基因组组装体,冠状嗜血杆菌大小分别为 2.88 Gb 和 3.96 Gb,显示重叠群 N50 长度分别为 15.76 Mb 和 6.11 Mb(补充表 2)。随后,Illumina 测序读长和纳米孔测序读长的 99.32%–99.87% 映射率(补充表 3)和 96.56%–96.74% 的通用单拷贝直系同源物 (BUSCO) 完整性基准测试(补充表 4)证明了组装的完整性。一系列评估表明,基因组组装是高质量的(补充图 .2).
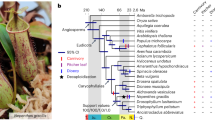
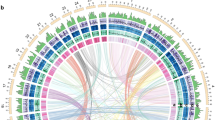
图 1:螳螂的基因组结构和系统发育。
A Photos of the juvenile orchid mantis H. coronatus (upper) and adult dead leaf mantis D. lobata (down) showing their adaptive camouflage. B Circos plot of chromosome-level genome assemblies of H. coronatus and D. lobata. From the inner circle to the outer circle, genome characteristics are indicated successively, including chromosome sizes (a), gene density (b), noncoding RNA density (c), repeat sequence distribution (d), GC content (e), SNP density (f), and homeobox gene clusters (g). C Composition comparison of the repetitive sequences of two mantises and the close relatives Blattella germanica and Zootermopsis nevadensis. The coordinates represent the percentage of the length of each category relative to the corresponding genome size. D Gene structure analysis showing that H. coronatus and D. lobata have larger average intron lengths than B. germanica, Z. nevadensis and Drosophila melanogaster. Abbreviations used throughout: Hcor, H. coronatus. Dlob, D. lobata. Bger, B. germanica. Znev, Z. nevadensis. Dmel, D. melanogaster. E Phylogenomic position of Mantodea. The estimated divergence times and the numbers of families with gene expansions (green) and contractions (red) are displayed on the branches of the phylogenetic tree. The 95% confidence interval of divergence time is indicated in brackets. Three calibration points (red circles) were applied as normal priors. The bootstrap values of most nodes were 100%. O, Ordovician. S, Silurian. D, Devonian. Car, Carboniferous. P, Permian. T, Triassic. J, Jurassic. Cre, Cretaceous. Pa, Paleogene. N, Neogene. Q, Quaternary.
A 幼年兰花螳螂 H. coronatus(上)和成年枯叶螳螂 D. lobata(下)的照片,显示了它们的适应性伪装。B H. coronatus 和 D. lobata 染色体水平基因组组装的 Circos 图。从内圈到外圈依次表示基因组特征,包括染色体大小 (a)、基因密度 (b)、非编码 RNA 密度 (c)、重复序列分布 (d)、GC 含量 (e)、SNP 密度 (f) 和同源框基因簇 (g)。C 两只螳螂和近亲 Blattella germanica 和 Zootermopsis nevadensis 的重复序列的组成比较。坐标表示每个类别的长度相对于相应基因组大小的百分比。D 基因结构分析显示,H. coronatus 和 D. lobata 的平均内含子长度大于 B. germanica、Z. nevadensis 和 Drosophila melanogaster。通篇使用的缩写:Hcor、H. coronatus。Dlob, D. lobata.Bger, B. germanica.Znev, Z. nevadensis.Dmel, D. 黑腹果蝇。E Mantodea 的系统发育位置。估计的分歧时间和基因扩增(绿色)和收缩(红色)的家族数显示在系统发育树的分支上。发散时间的 95% 置信区间用括号表示。三个校准点(红色圆圈)作为正常先验应用。大多数节点的引导值为 100%。O, 奥陶纪。S, 志留纪。D, 泥盆纪。汽车,石炭纪。P, 二叠纪。T, 三叠纪。J, 侏罗纪.Cre,白垩纪。Pa, 古近纪。N, 新近纪。 Q,第四纪。
The genomes of the mantises were larger than most of those sequenced for heterometabolous insects, such as B. germanica (1.79 Gb) and Z. nevadensis (0.48 Gb), containing a high level of repetitive elements amounting to 64.94–66.16% of the genome assemblies (Supplementary Table 5). Among them, 42.36% and 51.92% of the genome sequences were transposable elements (TEs) (1.21 Gb for H. coronatus and 2.06 Gb for D. lobata). The class II retrotransposon DNA transposons (558.37 Mb, 19.39% of genomes) represented the most dominant TE elements, with TcMar-Tc1 being the largest subclass (Fig. 1C, Supplementary Table 5). In addition, class I retrotransposons, including long interspersed nuclear elements (LINEs), short interspersed nuclear elements (SINEs), and long terminal repeats (LTRs), constituted 20.80%–22.39% of the genomes (Supplementary Table 5). Linear regression analysis showed that the length of DNA transposons was positively correlated with the variation in genome size (Supplementary Fig. 3), consistent with the findings of a previous study20, indicating the important role of retrotransposons in mantis genome expansion.
螳螂的基因组比大多数异代谢昆虫测序的基因组大,例如德国芽孢杆菌 (1.79 Gb) 和内华氏芽孢杆菌 (0.48 Gb),含有高水平的重复元件,占基因组组装的 64.94-66.16%(补充表 5)。其中,42.36% 和 51.92% 的基因组序列为转座因子 (TE) (H. coronatus 为 1.21 Gb,D. lobata 为 2.06 Gb)。II 类反转录转座子 DNA 转座子 (558.37 Mb, 19.39% 的基因组) 代表了最显性的 TE 元件,其中 TcMar-Tc1 是最大的亚类(图 .1C,补充表 5)。此外,I 类反转录转座子,包括长穿梭核元件 (LINEs)、短穿散核元件 (SINEs) 和长末端重复序列 (LTR),占基因组的 20.80%–22.39%(补充表 5)。线性回归分析显示,DNA 转座子的长度与基因组大小的变化呈正相关(补充图3),与之前的研究结果一致20,表明反转录转座子在螳螂基因组扩增中的重要作用。
Based on the transcript dataset combining ab initio and homology-based approaches, we identified 16,294 and 17,691 protein-coding genes with average lengths of 77.17–83.96 kb in these two mantis genomes, containing 95.5%–98.2% of complete conserved orthologs within insects (Supplementary Tables 6 and 7). More strikingly, the average length of introns was 12.77–14.97 kb, larger than that in other insect taxa, while the average exon length (218.37–222.83 bp) was similar to that in other insects, resulting in the extension of gene length (Fig. 1D, Supplementary Fig. 4). Finally, ~90% of the genes were functionally annotated (Supplementary Table 8). Analysis of gene family evolution revealed 794 and 1045 expanded gene families in the orchid and dead leaf mantises, respectively (Fig. 1E).
基于结合 ab initio 和基于同源方法的转录数据集,我们在这两个螳螂基因组中鉴定了 16,294 个和 17,691 个蛋白质编码基因,平均长度为 77.17-83.96 kb,包含 95.5%-98.2% 的昆虫内完整保守的直系同源物(补充表 6 和 7)。更引人注目的是,内含子的平均长度为 12.77-14.97 kb,大于其他昆虫类群,而平均外显子长度(218.37-222.83 bp)与其他昆虫相似,导致基因长度延长(图 D)。1D,补充图最后,~90% 的基因进行了功能注释(补充表 8)。基因家族进化分析显示,兰花和枯叶螳螂中分别有 794 和 1045 个扩展的基因家族(图 D)。1E)。
Taken together, these results present two high-quality chromosome-level genome assemblies and gene catalogs for the Mantodea, enabling further insights into the biology and evolution of these extraordinary camouflaged mantises.
综上所述,这些结果为 Mantodea 提供了两个高质量的染色体水平基因组组装和基因目录,从而能够进一步了解这些非凡的伪装螳螂的生物学和进化。
Resolving the Mantodea phylogenetic position and evolutionary history
解析 Mantodea 的系统发育位置和进化历史
Phylogenomic analysis was performed to test the phylogenetic position of Mantodea. 324 single-copy orthologs identified in these two mantises and 16 other insects from 15 key extant insect orders were used to reconstruct a phylogenetic tree (Fig. 1E, Supplementary Fig. 5). The phylogenetic result was congruent with a recent insect phylogeny21, with all the bootstrap values of the concatenated tree above 96% (Fig. 1E). The phylogenetic analysis supported the most recent phylogenetic hypothesis that Mantodea is a sister group to Blattodea, and their divergence was estimated to approximately the Permian–Triassic period (211.89–277.30 million years ago (Ma)) (Fig. 1E), in agreement with the divergence date estimated by morphological and molecular data22.
进行系统发育分析以测试 Mantodea 的系统发育位置。在这两个螳螂中鉴定出的 324 个单拷贝直系同源物和来自 15 个现存关键昆虫目的其他 16 种昆虫用于重建系统发育树(图 D)。1E,补充图5). 系统发育结果与最近的昆虫系统发育21 一致,连接树的所有 bootstrap 值都在 96% 以上(图 D)。系统发育分析支持最新的系统发育假说,即 Mantodea 是 Blattodea 的姊妹群,它们的分化估计大约在二叠纪-三叠纪时期(211.89-2.7730 亿年前 (马))(图 D)。1E),与形态学和分子学数据估计的分歧日期一致22.
The time of divergence of flower- and dead leaf-mimicking camouflage is estimated to be 36.50 Ma ago [95% highest posterior density (HPD) 24.15–55.31 Ma] (Fig. 1E), following the transition to widespread angiosperm-dominated biomes in the Paleocene (66–56 Ma)23. Insect pollination, as the dominant mode of angiosperm pollination, probably drove the diversification and ecological expansion of flower-mimicking mantises24.
模拟花叶和枯叶伪装的发散时间估计为 36.50 马 [95% 最高后密度 (HPD) 24.15–55.31 马](图1E),在古新世 (66-56 马) 过渡到广泛的被子植物为主的生物群落之后23。昆虫授粉作为被子植物授粉的主要方式,可能推动了拟花螳螂的多样化和生态扩张24。
To better understand the demographic history of plant-mimicking camouflage in Mantodea, pairwise sequential Markovian coalescent (PSMC) analysis was performed to assess the evolutionary history and dynamic effective population size25. H. coronatus and D. lobata populations underwent stepwise expansion in the early Pleistocene (Supplementary Fig. 6). Subsequently, H. coronatus experienced a population reduction at approximately 1 Ma, whereas the D. lobata population experienced an expansion approximately 300 thousand years ago (kya) and subsequently declined, coinciding with dramatic temperature changes, such as intensified amplitudes of glacial cycles during the Penultimate Glaciation (PG, 200–130 kya) and the Last Glaciation (LG, 70–10 kya) (Supplementary Fig. 6). The distribution of the orchid mantis has been reported in the rainforest across Malaysia, Indonesia, India, Thailand, Vietnam and Southern China15, while that of the dead leaf mantis is mainly in the rainforest of Malaysia and Philippines26. Both of them showed intrinsic susceptibility to extreme environmental fluctuations. The distinct demographic histories between the two mantis species demonstrated that differential coloration probably confers host-heterogeneous responses to environmental change; a similar finding has been reported in the oakleaf butterfly27. Overall, these results provide a reliable phylogenetic framework and date estimates for a better understanding the origin and evolution of praying mantis camouflage.
为了更好地了解 Mantodea 中模拟植物伪装的种群历史,进行了成对顺序马尔可夫聚结 (PSMC) 分析以评估进化历史和动态有效种群规模25。H. coronatus 和 D. lobata 种群在更新世早期经历了逐步扩张(补充图随后,H. coronatus 在大约 1 马 时经历了种群减少,而 D. lobata 种群在大约 30 万年前经历了扩张 (kya) 并随后下降,与剧烈的温度变化相吻合,例如倒数第二次冰期 (PG, 200-130 kya) 和最后一次冰期 (LG, 70-10 kya) 期间冰川周期的振幅增强(补充图6). 兰花螳螂的分布已在马来西亚、印度尼西亚、印度、泰国、越南和中国南部的热带雨林中报道15,而枯叶螳螂的分布主要在马来西亚和菲律宾的热带雨林中 26。它们都表现出对极端环境波动的内在敏感性。两种螳螂物种之间不同的种群历史表明,不同的颜色可能赋予宿主对环境变化的异质反应;橡叶蝴蝶27 中也报告了类似的发现。总体而言,这些结果为更好地了解螳螂伪装的起源和进化提供了可靠的系统发育框架和日期估计。
Genetic and biochemical bases of camouflage coloration
伪装着色的遗传和生化基础
Animal colors have been an intriguing scientific issue for centuries28. The orchid mantis displays unique color variation over the course of its development (Fig. 2A). The first-instar larva (L1) depends largely on its aposematic coloration to evade predators, mimicking the larva of a poisonous red and black assassin bug (Hemiptera: Reduviidae), exhibits red and black aposematic colors and subsequently changes to a white and pink color pattern starting in the second-instar larval stage (L2), beginning to mimic a flower blossom (Fig. 2A). This floral resemblance is most apparent in juvenile orchid mantises that have yet to develop wings. The adult orchid mantis resembles withering flowers. These results demonstrate that the orchid mantis changes its body color throughout its lifespan to successively mimic poisonous reduviids, blossoming flowers and withering flowers.
几个世纪以来,动物的颜色一直是一个有趣的科学问题28.兰花螳螂在其发育过程中表现出独特的颜色变化(图 D)。第一龄幼虫 (L1) 在很大程度上依赖于其着色来躲避捕食者,模仿有毒的红色和黑色刺客虫(半翅目:Reduviidae)的幼虫,表现出红色和黑色的斑点颜色,随后从第二龄幼虫阶段 (L2) 开始变为白色和粉红色图案,开始模仿花朵(图 D.这种花的相似性在尚未长出翅膀的幼年兰花螳螂中最为明显。成年兰花螳螂类似于枯萎的花朵。这些结果表明,兰花螳螂在其一生中会改变其体色,以连续模仿有毒的 reduviids、开花和枯萎的花朵。
图 2:伪装着色的遗传和生化基础。
A Body color variation during the lifespan of the female orchid mantis shows aposematism in the first instar and camouflage coloration from the onset of the second instar. B Phylogenetic analysis of the Scarlet gene. The degree of support for each node is shown as a probability percentage. The estimated divergence times of the Scarlet gene are displayed on the branches. C The Scarlet (Hcor-13g0747, marked with an asterisk to represent an orthologous gene among Insecta) and White gene loci. D Schematic of the insect xanthommatin synthesis pathway in pigment granules. E The concentrations of the substrates for xanthommatin synthesis, kynurenine and 3-hydroxy-kynurenine, were determined using high-performance liquid chromatography across developmental stages. Abbreviations used throughout: L1, first-instar larva. L2, second-instar larva. L5, fifth-instar larva. Data represent the mean ± standard deviation of five replicates. *p = 0.04, **p = 0.0006 and 0.0009, respectively, by two-sided Student’s t tests. (F) In vitro redox reactions with the pigment extracted from the L2 orchid mantis. Vc, Ascorbic acid.
A 雌性兰花螳螂一生中的体色变化在第一龄表现出伪装性,从第二龄开始就表现出伪装色。B Scarlet 基因的系统发育分析。每个节点的支持程度显示为概率百分比。Scarlet 基因的估计分化时间显示在分支上。C 猩红 (Hcor-13g0747,用星号标记,代表昆虫中的直系同源基因)和白色基因位点。D 色素颗粒中昆虫黄瘤素合成途径的示意图。E 使用高效液相色谱法测定黄马青素合成底物犬尿氨酸和 3-羟基犬尿氨酸的浓度,贯穿整个发育阶段。通篇使用的缩写:L1,第一龄幼虫。L2,二龄幼虫。L5,五龄幼虫。数据表示 5 次仿行的均值±标准差。*p = 0.04、**p = 0.0006 和 0.0009,分别通过双侧学生 t 检验。(F) 与从 L2 兰花螳螂中提取的色素的体外氧化还原反应。Vc,抗坏血酸。
Histological analysis showed a black color in the exoskeleton, which consisted of three layers (epicuticle, exocuticle, and endocuticle), while a red color was mainly distributed in the epidermis under the exoskeleton (Supplementary Fig. 7A, B). This finding was also supported by the black pigment being retained in the cuticle shell of L1 after molting (Supplementary Fig. 7C, D). In addition, transmission electron microscopy observations confirmed the presence of pigments in the endocuticle of the red-body color area (Supplementary Fig. 8).
组织学分析显示外骨骼呈黑色,由三层(表皮层、外角质层和内皮层)组成,而红色主要分布在外骨骼下的表皮中(补充图 D)。7A, B)。蜕皮后 L1 角质层壳中保留的黑色色素也支持这一发现(补充图 D)。7C, D)。此外,透射电子显微镜观察证实了红体颜色区的内皮层中存在色素(补充图 D)。8).
Gene family analysis showed that the ABCG gene family was significantly expanded in H. coronatus (Supplementary Fig. 9). The ABCG subfamily belongs to the ABC superfamily and plays an important role in compound transport29. Within the ABCG subfamily, the Scarlet gene underwent an expansion in both sequenced mantises, with three and two copies in H. coronatus and D. lobata, respectively. Two duplications of Scarlet occurred at approximately 4.19 Ma and 2.14 Ma (Fig. 2B). Comparative genome analysis showed highly conserved synteny in Scarlet and White genes among B. germanica, H. coronatus and D. lobata (Fig. 2C). Notably, eight exons of the Scarlet gene were lost in D. lobata (Supplementary Fig. 10), which might explain the deficiency of a red or pink color in D. lobata. The Scarlet protein in combination with the White protein, forms a heterodimer responsible for transporting 3-hydroxykynurenine into pigment granules to synthesize xanthommatin (Fig. 2D), which is a widespread invertebrate pigment belonging to the ommochromes30.
基因家族分析显示,ABCG 基因家族在 H. coronatus 中显著扩增(补充图 D)。ABCG 亚家族属于 ABC 超家族,在化合物转运中起重要作用29。在 ABCG 亚家族中,Scarlet 基因在两个测序的螳螂中都经历了扩增,分别在 H. coronatus 和 D. lobata 中具有 3 个和 2 个拷贝。Scarlet 的两次重复发生在大约 4.19 马 和 2.14 马 (图 .比较基因组分析显示,德国双歧杆菌、H. coronatus 和 D. lobata的猩红和白色基因的同线高度保守(图值得注意的是,Scarlet 基因的 8 个外显子在 D. lobata 中丢失(补充图 D.10),这可能解释了 D. lobata 中红色或粉红色的缺陷。猩红蛋白与 White 蛋白结合,形成一个异二聚体,负责将 3-羟基犬尿氨酸转运到色素颗粒中以合成黄瘤素(图 D)。2D),这是一种广泛分布的无脊椎动物色素,属于 ommochromes30。
To further validate the role of xanthommatin in the formation of red and pink colors, the concentrations of the substrates 3-hydroxykynurenine and kynurenine were determined in L1, L2, and fifth-instar (L5) larvae by high-performance liquid chromatography (Supplementary Fig. 11). A gradient decrease in the concentration was observed along with fading of the red body color during instar development (Fig. 2E). Xanthommatin pigments can change color under oxidative/reductive conditions: decarboxylated xanthommatin is yellow under oxidative conditions, and xanthommatin is pale red or crimson red under reductive conditions31. To validate the important role of xanthommatin in the formation of the pink color pattern, we further extracted pigment from L2 H. coronatus individuals and treated it in vitro with redox agents. Similar redox-dependent color changes were observed as the color changed from red to pink with the addition of ascorbic acid (Fig. 2F). These results suggest that xanthommatin is an important pigment for camouflage coloration in mantises that can display a redox-dependent red or pink color change. The ABCG subfamily plays an important role in the synthesis of xanthommatin in H. coronatus and D. lobata.
为了进一步验证黄素在红色和粉红色形成中的作用,通过高效液相色谱法测定了 L1、L2 和五龄 (L5) 幼虫中底物 3-羟基犬尿氨酸和犬尿氨酸的浓度(补充图 D)。11). 在龄期发育过程中观察到浓度的梯度降低以及红体颜色的褪色(图 D)。黄马素色素在氧化/还原条件下会变色:脱羧黄马素在氧化条件下是黄色的,黄马素在还原条件下是淡红色或深红色31。为了验证黄瘤素在粉红色图案形成中的重要作用,我们进一步从 L2 H. coronatus 个体中提取色素,并用氧化还原剂在体外处理。当添加抗坏血酸后颜色从红色变为粉红色时,观察到类似的氧化还原依赖性颜色变化(图 D)。这些结果表明,黄马素是螳螂伪装着色的重要色素,可以显示氧化还原依赖性的红色或粉红色变化。ABCG 亚家族在 H. coronatus 和 D. lobata 黄马素的合成中起重要作用。
Molecular mechanism underlying the morphogenesis of plant-mimicking femoral appendages
模拟植物股骨附件形态发生的分子机制
The large, flat expansions of the exoskeleton (petal-like femoral lobes) on the femur of the mid and hind legs contribute to the resemblance of the orchid mantis to flowers (Fig. 3A), while the closely related dead leaf mantis also displays leaf petiole residue-like appendages (Fig. 1A). To investigate the makeup of these traits, we performed histological analysis and revealed that the petal-like structures were mainly composed of exoskeleton, along with connective tissues (Supplementary Fig. 12). The cuticle is the major component of insect exoskeletons, and the encoding genes underwent significant expansion in H. coronatus and D. lobata, especially in the former (Fig. 3B). Whole-genome duplication analysis revealed that Cuticle genes were tandemly duplicated, with 35 copies on chromosome 3 (Supplementary Fig. 13). Compared to those in H. coronatus L1, the femoral lobes in the mid and hind legs in L2 were enlarged to resemble petals (Fig. 2A). Integrating evolutionary biology and developmental biology are crucial for bettering understanding the molecular basis underlying key phenotypic innovations32. We performed transcriptome sequencing across the developmental stages of H. coronatus, including L1, L2, and L5 larval stages as well as the adult stage. Comparative analysis revealed that L2 and L5 possessed significantly higher expression of Cuticle in the leg tissues than L1 (Fig. 3C).
外骨骼(花瓣状股骨叶)在中腿和后腿股骨上的大而扁平的扩张有助于兰花螳螂与花朵的相似性(图 D)。3A),而密切相关的枯叶螳螂也显示出叶柄残渣状附属物(图 D)。为了研究这些特征的组成,我们进行了组织学分析,发现花瓣状结构主要由外骨骼和结缔组织组成(补充图 1)。角质层是昆虫外骨骼的主要成分,编码基因在 H. coronatus 和 D. lobata 中经历了显着的扩增,尤其是在前者中(图 D)。全基因组重复分析显示,角质层基因是串联重复的,在 3 号染色体上有 35 个拷贝(补充图 D)。13). 与 H. coronatus L1 相比,L2 中腿和后腿的股叶扩大到类似于花瓣(图 D)。整合进化生物学和发育生物学对于更好地理解关键表型创新背后的分子基础至关重要32.我们对冠状嗜血杆菌的发育阶段进行了转录组测序,包括 L1 、 L2 和 L5 幼虫阶段以及成虫阶段。比较分析表明,L2 和 L5 在腿部组织中角质层的表达明显高于 L1(图 1)。3C)。
图 3:模拟植物伪装的股骨形态发生的分子机制。
A Photos of T2 and T3 legs across the developmental stage. Scale bar = 1 mm. B Gene family analysis showing that the Cuticle genes were significantly expanded in the genomes of H. coronatus and D. lobata. C Heatmaps of the differentially expressed genes in the leg tissues across the developmental stages. The red and blue colors indicate high and low expression levels, respectively. D Expression level analysis of the Arm gene at day 3 after two injections of Arm double-stranded RNA (dsArm). Ctrl, control group. Data represent the mean ± standard deviation of five replicates. *p = 0.02 by two-sided Student’s t tests. E Comparison of the size of ‘petal-like’ femoral lobes (the red dotted area) between the dsArm and control groups. The numbers indicate the areas of these expansions. F Gene Ontology (GO) enrichment of differentially expressed genes with FDR-corrected p value < 0.05 and |log2-fold-change | > 1 in the legs between the dsArm and control groups. The enriched GO terms with corrected p < 0.05 determined by Fisher’s exact test are presented. The size of the circles represents the number of differentially expressed genes for each GO term. The red and cyan colors represent the molecular function and biological process categories, respectively. G Bubble plot showing the expression levels of cuticle mRNAs between the dsArm and control groups. H Reconstruction of gene regulatory pathways related to femoral remodeling. The red and blue colors represent significantly up- and downregulated genes in the leg tissues of the fifth-instar larvae compared to the first-instar larvae. co, coxa. tr, trochanter. fe, femur. ti, tibia. ts, tarsus.
A 发育阶段 T2 和 T3 腿的照片。比例尺 = 1 毫米。B 基因家族分析表明,角质层基因在 H. coronatus 和 D. lobata 的基因组中显着扩增。C 跨发育阶段腿部组织中差异表达基因的热图。红色和蓝色分别表示高表达水平和低表达水平。D 两次注射 Arm 双链 RNA 后第 3 天 Arm 基因的表达水平分析 (dsArm)。Ctrl 键,控制组。数据表示 5 次仿行的均值±标准差。*p = 0.02(通过双侧学生 t 检验)。E dsArm 和对照组之间“花瓣状”股叶(红色虚线区域)大小的比较。这些数字表示这些扩展的区域。F 基因本体论 (GO) 富集差异表达基因,FDR 校正的 p 值< 0.05 和 |log2 倍变化 |> 1 在 ds组和对照组之间的腿部。给出了由 Fisher 精确检验确定的校正 p < 0.05 的富集 GO 项。圆圈的大小代表每个 GO 项的差异表达基因的数量。红色和青色分别代表分子功能和生物过程类别。G 气泡图显示了 ds组和对照组之间角质层 mRNA 的表达水平。H 重建与股骨重塑相关的基因调控途径。 与第一龄幼虫相比,红色和蓝色代表 5 龄幼虫腿部组织中显著上调和下调的基因。co, coxa.tr, 转子.fe, 股骨。ti, 胫骨。TS, 跗骨。
We also found a higher expression level of the homeodomain transcription factor Ultrabithorax (Ubx) in L1 and L2 leg tissues than in L5 leg tissues (Fig. 3C). Ubx has been proven to specify segment identity along the animal body axis and controls the expression of genes that regulate tissue growth and patterning during early development. We also compared the size of mesothoracic (T2) and metathoracic (T3), and detected the expression level of Ubx in femur, tibia and tarsus, respectively. We found that the T3 tibia and tarsus of H. coronatus were enlarged both in length and area with higher expression level of Ubx mRNA compared to their T2 counterparts (Supplementary Fig. 14A, B), consistent with the trend observed in Tenodera aridifolia33. We detected a higher expression level of Ubx in T3 femur than that in T2, consistent with the observation of more enlarged T3 femur (Supplementary Fig. 14C). Compared to T. aridifolia, H. coronatus exhibits “petal-like” femoral expansion from onset of L2 stage. It was remarkably that we found that a high mRNA expression lasted in the L2 stage in T2 and T3, which might result in the occurrence of “petal-like” cuticular enlargement (Supplementary Fig. 14D). These data suggest that the prolonged expression of Ubx in the femur in the early developmental stage plays a key role in the modulation of the petal-like femoral enlargement.
我们还发现,同源域转录因子 Ultrabithorax (Ubx) 在 L1 和 L2 腿部组织中的表达水平高于 L5 腿部组织(图 D)。Ubx 已被证明可以指定沿动物身体轴的片段身份,并控制在早期发育过程中调节组织生长和模式化的基因的表达。我们还比较了中胸 (T2) 和后胸 (T3) 的大小,并分别检测了 Ubx 在股骨、胫骨和跗骨中的表达水平。我们发现,与 T2 对应物相比,H. coronatus 的 T3 胫骨和跗骨在长度和面积上都扩大了,Ubx mRNA 的表达水平更高(补充图 D)。14A, B),与在 Tenodera aridifolia33 中观察到的趋势一致。我们检测到 T3 股骨中 Ubx 的表达水平高于 T2,这与观察到的 T3 股骨更增大一致(补充图 1)。与 T. aridifolia 相比,H. coronatus 从 L2 阶段开始就表现出“花瓣状”股骨扩张。值得注意的是,我们发现 T2 和 T3 的 L2 阶段持续高 mRNA 表达,这可能导致“花瓣状”表皮增大的发生(补充图 D.这些数据表明,Ubx 在股骨早期发育阶段的长期表达在调节花瓣状股骨增大中起关键作用。
We also observed significant upregulation of the extracellular matrix (ECM)-related gene Mmp2, the ECM protease-encoding gene Stubble, and the downstream RhoA signaling-related genes in the leg tissue of L2 and L5 (Fig. 3C), which are known to be important for epithelial morphogenesis of leg imaginal discs34.
我们还观察到 L2 和 L5 腿部组织中细胞外基质 (ECM) 相关基因 Mmp2、ECM 蛋白酶编码基因 Stubble 和下游 RhoA 信号转导相关基因的显著上调(图 2)。3C),已知对腿部想象盘的上皮形态发生很重要34。
To further investigate why flat expansion occurred along the ventral axis of the femur, we then focused on the Wnt signaling pathway, which is known to play important roles in axis elongation during leg development35. Correspondingly, we found that genes related to the Wnt signaling pathway were significantly differentially expressed between L1 and L5. Compared to those in L1, Wnt/Wg and the receptors Wls were upregulated in L5, subsequently inhibiting the expression of intracellular Apc and Axin (Fig. 3C). Consequently, the key transcription factor Arm was upregulated in the leg tissue of L5 (Fig. 3C). To verify the indispensable role of the Wnt signaling pathway in ventral enlargement, we conducted RNAi of Arm, the key effector in this pathway, from the onset of L2 by two injections of Arm double-stranded RNA (dsArm). As expected, the mRNA expression level of Arm was dramatically decreased in the dsArm group, as detected by real-time quantitative PCR at day 3 postinjection (Fig. 3D), suggesting successful silencing of Arm. Notably, a specific reduction in petal-like femoral lobes was observed in the mid- and hind legs after the next molt (Fig. 3E). Further transcriptomic analysis showed that significantly differentially expressed genes (DEGs) were enriched in structural constituents of the cuticle and the Wnt signaling pathway (Fig. 3F), with Cuticle genes significantly downregulated in the dsArm group (Fig. 3G), suggesting that the Wnt signaling pathway drives the ventral flat expansion by enhancing Cuticle expression (Fig. 3H).
为了进一步研究为什么沿股骨腹轴发生平坦扩张,我们随后关注了 Wnt 信号通路,已知该通路在腿部发育过程中的轴伸长中起重要作用35。相应地,我们发现与 Wnt 信号通路相关的基因在 L1 和 L5 之间显著差异表达。与 L1 相比,Wnt/Wg 和受体 Wls 在 L5 中上调,随后抑制细胞内 Apc 和 Axin 的表达(图 1)。因此,关键转录因子 Arm 在 L5 的腿部组织中上调(图 D)。为了验证 Wnt 信号通路在腹侧扩大中不可或缺的作用,我们从 L2 开始通过两次注射 Arm 双链 RNA (dsArm) 对该通路中的关键效应子 Arm 进行了 RNAi。正如预期的那样,dsArm 组的 mRNA 表达水平显着降低,正如在注射后第 3 天通过实时定量 PCR 检测到的那样(图 D)。3D),表明 Arm 成功沉默。值得注意的是,在下一次蜕皮后,在中腿和后腿中观察到花瓣状股叶的特异性减少(图 D)。进一步的转录组学分析表明,显著差异表达基因 (DEG) 在角质层和 Wnt 信号通路的结构成分中富集(图 D)。3F),其中 dsArm 组的角质层基因显著下调(图 D)。3G),表明 Wnt 信号通路通过增强角质层表达来驱动腹侧平坦扩张(图 D)。3H)。
Overall, these results suggested that the cuticle was the major structural constituent of the petal-like femoral lobes, and the enlargement in the ventral femur exoskeleton was regulated by Wnt signaling and the prolonged expression of Ubx in the early development stage.
总体而言,这些结果表明角质层是花瓣状股叶的主要结构成分,腹侧股骨外骨骼的增大受 Wnt 信号传导和发育早期 Ubx 表达延长的调节。
Ecdysone-dependent sexual dimorphism in body size
蜕皮激素依赖性体型二态性
Sexually dimorphic traits are important for sexual selection and species survival36,37. The body size of the female orchid mantis is much larger than that of the male, representing a spectacular case of sexual dimorphism (Fig. 4A). Female orchid mantises and dead leaf mantises usually undergo 2–3 more molts, and as adults, are 60-65 mm long, i.e., 2–3 times larger than males (25-30 mm) (Fig. 4B). Compared to that in a close relative of mantises, B. germanica, which usually requires 5 instars for both sexes38, the dimorphism in mantises was unusually extreme (Fig. 4B). The predominant explanation is that increased female size can increase fecundity and facilitate attracting more prey, whereas the small and mobile male facilitates mate-finding and reproductive success18. To investigate this regulatory mechanism, comparative transcriptomic analysis was performed between female and male adults of H. coronatus (Supplementary Fig. 15). We observed 1,892 downregulated and 1,858 upregulated transcripts in female mantises (Benjamini‒Hochberg-corrected p < 0.05), most of which were enriched in the Hippo signaling pathway and insect hormone biosynthesis (Fig. 4C). As expected, sex steroid hormone biosynthesis was also significantly enriched between the two sexes.
性二态性状对性选择和物种生存很重要36,37。雌性兰花螳螂的体型比雄性大得多,代表了性二态性的壮观案例(图 D)。雌性兰花螳螂和枯叶螳螂通常会多蜕皮 2-3 次,成年后长 60-65 毫米,即比雄性大 2-3 倍(25-30 毫米)(图 D)。与螳螂的近亲 B. germanica 相比,通常两性都需要 5 龄38,螳螂的二态性异常极端(图 D)。主要的解释是,增加雌性体型可以提高繁殖力并促进吸引更多的猎物,而体型小且移动的雄性则有助于寻找配偶和繁殖成功18。为了研究这种调节机制,对 H. coronatus 的雌性和雄性成虫进行了比较转录组学分析(补充图 .15). 我们在雌性螳螂中观察到 1,892 个下调和 1,858 个上调转录本 (Benjamini\u2012Hochberg 校正的 p < 0.05),其中大多数富含 Hippo 信号通路和昆虫激素生物合成 (图 4C)。正如预期的那样,性类固醇激素的生物合成在两性之间也显著丰富。
A Comparison of body size between male and female adults. B Lifetime average number of molts in both sexes showing sexual distinction in molting development of the praying mantises. The red and cyan colors represent females and males, respectively. The data graphed for Bger (B. germanica), Choo (Clitarchus hookeri), Lmig (Locusta migratoria), and Bmor (Bombyx mori) are from the literature68,69,70,71. C KEGG pathway enrichment of differentially expressed genes with FDR-corrected p value < 0.05 and |log2-fold-change | > 1 between male and female orchid mantises. The size of the circle indicates the number of genes. The red and blue colors indicate low and high Benjamini-Hochberg corrected p values, respectively. D Gene set enrichment analysis (GSEA) showing the correlations of the genes involved in the Hippo signaling pathway and insect hormone biosynthesis with sex. E Crosstalk between ecdysone synthesis and the Hippo signaling pathway induces sexual size dimorphism in mantis. The red font represents the upregulated genes in females.
The Hippo pathway is a key regulator of tissue size and an evolutionarily conserved signal regulating numerous biological processes, including cell growth, organ size control, and regeneration39. The insect hormone biosynthesis pathway controls the growth, development, metamorphosis, and reproduction of insects40. Gene set enrichment analysis (GSEA) revealed that genes representing the Hippo signaling pathway, including the major effector Yki, were significantly upregulated in females (Fig. 4D). In addition, genes related to the synthesis of the ecdysteroid 20-hydroxyecdysone (20E), such as Nvd, Spo, Phm, Dib, Sad and Shd, were highly expressed in females (Fig. 4D, E). A previous study proved that the Tai gene is controlled by 20E, which can interact with the Hippo transcriptional coactivator Yki to promote the expression of canonical Yki-targeting genes41. Consistently, the responsive genes of Yki, such as Diap1, Dally, Dmyc, Fj, Ex, and Crb, were highly expressed in females (Fig. 4E), suggesting a potential role of ecdysis development in the induction of sexual dimorphism in body size of mantises through crosstalk between 20E and Hippo signaling.
Expansion of the Trypsin, CYP450, and UGT gene families for adaptation to insectivory
Praying mantises are charismatic predators exclusively feeding on insects that are rich in protein, whereas close relatives are omnivorous, such as cockroaches, or herbivorous, such as termites, stick insects and crickets (Fig. 5A). To uncover the key genes involved in insectivory adaptation, comparative genomic analysis was performed, which revealed significant expansion of Trypsin in H. coronatus and D. lobata, with 17 copies tandemly arranged on chromosome 18 of H. coronatus and chromosome 6 of D. lobata (Fig. 5B, C). Trypsin is a serine protease that breaks polypeptide chains into smaller peptides, facilitating dietary protein degradation42. We found that all the Trypsin genes were specifically expressed in the abdomen rather than in the head, thorax, or leg tissues (Fig. 5D). To further prove the correlation between Trypsin and insectivory, the enzyme activity of intestinal Trypsin of H. coronatus was determined by chemiluminescence with an herbivorous cricket as a control. Significantly higher enzyme activity of Trypsin was found in H. coronatus than in the cricket (Fig. 5E), further indicating the important role of Trypsin in insectivory adaptation in mantises.
A Comparison of dietary protein content between praying mantises and other insects. B Phylogenetic analysis showing that the Trypsin gene family was significantly expanded in H. coronatus and D. lobata. C Schematic representation of the tandem replication of the Trypsin gene cluster in praying mantises. Each arrow indicates a complete gene oriented in the 5′ to 3′ direction. D A color diagram showing the expression level of Trypsin in the head, thorax, leg, and abdomen tissues. E Detection of trypsin enzyme activity in the intestines of a carnivorous mantis and an herbivorous cricket. Data represent the mean ± standard deviation of four replicates. * p = 0.04 by two-sided Student’s t tests.
As insectivores, mantises may frequently encounter poisonous prey, resulting in the accumulation of biotoxins and other xenobiotic matter. As a consequence, the cytochrome P450 (CYP450) and UDP-glucuronosyltransferase (UGT) gene families, especially Cyp9e2 and Ugt2b, which are known to be involved in xenobiotic metabolism, underwent significant expansion in the genomes of H. coronatus and D. lobata (Supplementary Fig. 16). These results suggest that expansion of the Trypsin, CYP450, and UGT gene families plays an important role in adaptation to an insectivorous diet.
Due to their exceptional plant-mimicking phenotypes and suite of evolutionary adaptations, mantises can serve as an outstanding model for studying complex phenotypic innovation and adaptive evolution. Reference genomes and accurate annotations are critical for evolutionary, developmental, and functional analyses43. Our study presents two reference genomes and gene catalogs for the Mantodea and reveals the genetic and biochemical bases of plant-mimicking coloration and femoral appendages in the praying mantis by comparative genomic and transcriptomic analyses in combination with functional validation. Moreover, we found evidence to support the molecular basis of ecdysone-dependent sexually dimorphic body size and adaptation to an insectivorous diet. In conclusion, our study provides valuable genomic resources, as well as evolutionary developmental insights into camouflage phenotypic innovation and adaptation in arthropods.
Methods
Sample collection
Captive breeding individuals of H. coronatus (Mantodea, Hymenopodidae) hatched from the same ootheca that was collected from the Xishuangbanna rainforest, Yunnan Province, China in 2018. Individuals of D. lobata (Mantodea, Deroplatyidae) were collected from a captive breeding center in Beijing, China in 2018. All individuals were housed in semitransparent cages (7 cm × 7 cm × 9 cm) in an incubator with a temperature range of 26–27 °C, 80% relative humidity, and a 16 h/8 h light/dark photoperiod. Larvae from the first to third instars were fed fruit flies and switched to crickets from the fourth instar onward. To ensure sufficient output of nucleic acids for Nanopore sequencing and Illumina paired-end sequencing, adult female H. coronatus and D. lobata were used for nucleic acid preps. DNA was extracted using the DNeasy Blood and Tissue Kit following the manufacturer’s protocol (Qiagen, Valencia, CA, USA). The quantity and quality of DNA were checked by a Qubit 2.0 (Thermo Fisher Scientific) and agarose gel electrophoresis, respectively. RNA was extracted using TRIzol reagent (Thermo Fisher Scientific).
All sample collections and animal experimental procedures were approved by the Institutional Animal Care and Use Committee of the Institute of Zoology, Chinese Academy of Sciences. All efforts were made to minimize the suffering of the animals.
Genome sequencing, Hi-C sequencing, and genome assembly
A combination of Nanopore sequencing, Illumina sequencing and Hi-C sequencing was used to generate mantis genome assemblies. Paired-end libraries with insert sizes of 400 bp were constructed and sequenced using the Illumina HiSeq X-Ten platform following the standard manufacturer’s protocol (San Diego, USA), yielding 155.6 Gb of clean reads for H. coronatus and 286.3 Gb for D. lobata, followed by quality filtering (Supplementary Table 9). The high-quality reads were used for genome size estimation by the K-mer method. 1D libraries were constructed according to the manufacturer’s instructions (SQK-LSK109, Oxford Nanopore Technologies, UK) and sequenced on the Nanopore PromethION platform (Oxford Nanopore Technologies, UK). In total, 297.60 Gb of subreads for H. coronatus and 258.05 Gb for D. lobata were generated with a high sequencing depth (approximately, 66–103×) (Supplementary Table 9). To further improve the continuity of the assembled genomes and anchor the assemblies into chromosomes, Hi-C sequencing was performed to order and orient the contigs, as well as to correct misjoined sections and merge overlaps. To construct Hi-C libraries, tissue samples were fixed immediately in formaldehyde solution once isolated. DNA was digested with the restriction enzyme DpnII overnight. Hi-C libraries with a mean size of 350 bp were constructed using NEBNext Ultra enzymes and Illumina-compatible adaptors and sequenced using the Illumina HiSeq 2500 platform. All libraries were quantified by a Qubit 2.0 (Thermo Fisher Scientific), and insert size was checked using an Agilent 2100.
Contigs were assembled by NextDenovo software (v2.0-beta.1, https://github.com/Nextomics/NextDenovo), and the assembled contig-level genomes were polished by NextPolish (v1.0.5)44 and Pilon (v1.22)45. Then, the contigs were anchored into chromosomes by Hi-C sequencing reads through the Juicer (v1.5)46 and 3D-DNA (v180922)47 software workflows. To further improve the chromosome-scale assembly, it was subjected to manual review and refinement using Juicebox Assembly Tools (https://github.com/theaidenlab/juicebox). Finally, genome quality was estimated with BUSCO (insecta_odb9, v3.0.2)48 and k-mer analysis and by mapping the initial reads back to the assembly.
Transcriptome sequencing and analysis
RNA from whole individuals of H. coronatus (first-, second-, and fifth-instar larvae and adults) was used for Nanopore full-length cDNA sequencing. Meanwhile, samples of the brain, thorax, abdomen, and mid and hind legs of each individual were sequenced using Illumina HiSeq 2500 sequencing. Fifth-instar larvae of D. lobata were used for Nanopore full-length cDNA sequencing and Illumina HiSeq 2500 sequencing. RNA from female and male subadults was extracted and subjected to Illumina HiSeq 2500 sequencing to investigate sex-related DEGs. At least three replicates were used for each group.
For H. coronatus, 222.31 Gb of Nanopore full-length cDNA sequencing data and 573.28 Gb of Illumina read cDNA sequencing data were obtained across different developmental stages and assembled into transcript datasets, yielding 13,817 transcripts (Supplementary Table 9). For D. lobata, 123.60 Gb of Nanopore full-length cDNA sequencing data and 68.21 Gb of Illumina read cDNA sequencing data were obtained from adult individuals and yielded 15,494 transcripts (Supplementary Table 9).
Relative gene expression was measured as transcripts per million reads, and DEGs were identified using DESeq2 (v1.0)49 with false discovery rate (FDR) corrected p value (q-value) set to less than 0.05 and |log2-fold-change| set to greater than 1. Gene ontology functional enrichment and KEGG pathway analysis of the DEGs were performed with Goatools and KOBAS50, respectively, with a Benjamini‒Hochberg-corrected p value less than 0.05 indicating statistical significance.
Genome annotation
Transposable elements were identified using RepeatMasker (open-4.0.7), RepeatModeler (v1.0.8), and MITE-Hunter (v1.0.8)51. Gene structures were determined by combining ab initio and homology methods. For ab initio annotation, we used Augustus (v3.2.1)52 and GENSCAN (v1.0)53 to analyze the repeat-masked genome. For homolog-based annotation, protein sequences of the fruit fly (Drosophila melanogaster), cockroach (Blattella germanica), honeybee (Apis mellifera), mosquito (Aedes aegypti), and small brown planthopper (Laodelphax striatella) genomes were aligned to mantis genome sequences using BLAST software (v2.3.0)54. Together with transcriptomic data, gene sets from these three methods were then integrated by EVidenceModeler software (v1.1.1)55. For gene functional annotation, the integrated gene set was aligned against public databases, including KEGG, Swiss-Prot, TrEMBL, COG, and NR, with BLAST (v2.3.0)54 and merged with annotations by InterProScan (v4.8) software56. Annotation integrity was estimated by comparison with reference genome annotations and BUSCO (v3.0.2)48, resulting in 95.5–98.2% completeness according to BUSCO analysis, suggesting the high quality of the annotation.
Identification of orthologous groups and phylogenomic analysis
To cluster families of protein-coding genes, we extracted protein sequences from the genomes of H. coronatus, D. lobata, and 16 other species of Insecta: Zootermopsis nevadensis (Blattodea: Termopsidae), B. germanica (Blattodea: Ectobiidae), D. melanogaster (Diptera: Drosophilidae), Locusta migratoria (Orthoptera: Acrididae), Clitarchus hookeri (Phasmatodea: Phasmatidae), Bombyx mori (Lepidoptera: Bombycidae), A. mellifera (Hymenoptera: Apidae), Ctenocephalides felis (Siphonaptera: Pulicidae), Stenopsyche tienmushanensis (Trichoptera: Stenopsychidae), Folsomia candida (Entomobryomorpha: Isotomidae), Ladona fulva (Odonata: Libellulidae), Pediculus humanus (Phthiraptera: Pediculidae), Photinus pyralis (Coleoptera: Lampyridae), Campodea augens (Diplura: Campodeidae), Rhopalosiphum maidis (Hemiptera: Aphididae), and Ephemera danica (Ephemeroptera: Ephemeridae). Protein sequences showing redundancy caused by alternative splicing variations or premature codons were removed from the protein-coding genes. The protein sequences were aligned reciprocally (i.e., all-vs.-all) using BLASTP programs with an E-value ≤ 1e − 5 and then clustered using OrthoMCL (v2.0.9)57. Finally, 22,455 orthogroups were identified among the 18 species.
To reveal the phylogenetic relationships among Mantodea and other insects, the protein sequences of 324 1:1 orthologs from all 18 species were aligned with MUSCLE (v3.8.31)58 and then concatenated using in-house Perl scripts. RAxML (v8.2.10)59 was used to construct a phylogenetic tree for the superalignment using the GTRGAMMA model with Campodea augens and Folsomia candida as outgroups. We used the best-fitting substitution model as deduced by ProteinModelSelection.pl and 1000 replicates for bootstrap support. The MCMCTree program of the PAML (v4.8)60 package was used to determine divergence times with the approximate likelihood calculation method and three dated fossil records (Baissatermes lapideus (145–99.6 Ma), Raphogla rubra (260–251 Ma), and Rhyniella praecursor (412.3–391.9 Ma)) from TimeTree (http://www.timetree).
Expansion and contraction of gene families
Family expansion/contraction analysis was performed by CAFÉ (v3.1)61 calculations with the parameters lambda -s and p < 0.01 based on the phylogenetic tree constructed above. Gene expansion and contraction results for each branch of the phylogenetic tree were obtained.
Demographic history reconstruction
To trace the demographic history of H. coronatus and D. lobata, we employed PSMC to estimate changes in effective population size using heterozygous sites25, with the following set of parameters: −N 30 –t 15 –r 5 −p 4 + 25 × 2 + 4 + 6. H. coronatus and D. lobata take approximately 8 months to reach adulthood and then mature sexually after 25–35 days, so the generation time (g) was set to 0.75 years. The nucleotide mutation rate (μ) of H. coronatus was estimated to be 1.85 × 10–9 mutations per site per generation with D. lobata as the reference species for comparison using the following formula: μ = D × g/2T62, where D is the observed frequency of pairwise differences between the two species, T is the estimated divergence time, and g is the estimated generation time for H. coronatus and D. lobata. Global mean surface temperature was estimated from benthic d18O63.
Genome duplication
To investigate the genome evolution of H. coronatus and D. lobata, we searched for genome-wide duplications with B. germanica as an outgroup. We identified different modes of gene duplication as whole-genome duplications (WGDs), tandem duplicates (TDs), proximal duplicates (PDs, gene distance on the same chromosome less than 10), transposed duplicates (TRDs, transposed gene duplications), or dispersed duplicates (DSDs) using DupGen_finder64 with default parameters. Five duplication categories were identified in the H. coronatus and D. lobata genomes, including DSD (53.59%), TD (23.12%), TRD (14.12%), PD (8.42%), and WGD (0.76%). Then, the overlap between the various types of duplicated genes and expanded genes was identified, yielding 796 tandem replication genes belonging to expanded gene families, including the Cuticle gene family.
To verify whether the identified tandem gene clusters existed, we mapped the Illumina and Nanopore reads back to these two genome assemblies and examined whether the sequencing depth of each tandem replicated gene was consistent with the sequencing depth of the whole genome. Finally, a uniform sequencing depth of all the duplicated genes consistent with the sequence depth of the whole genome was observed, confirming that these tandem gene clusters were real.
Expansion of the ABC, Cuticle, Trypsin, CYP450, and UGT gene families
Genome-wide protein sequences of H. coronatus, D. lobata, Z. nevadensis, B. germanica, and D. melanogaster were extracted, and the conserved nucleotide binding domain (PF00005.24) and transmembrane domain (PF00664.20) were scanned genome-wide for candidate ABC transporter genes using the Hidden Markov Model (HMM) in R (v3.2.1)52. To assign the candidate ABC genes to different subfamilies, multiple alignments of the ABC transporter protein sequences were performed using MUSCLE (v3.8.31)58, and the poorly aligned regions and partial gaps were removed with trimAI (gt = 0.5). Then, the alignments were subjected to phylogenetic analysis using RAxML based on the maximum likelihood method with the following parameters: -f a -x 12345 -N 1000 -p 12345 -m PROTGAMMAJTTX. The resulting trees were displayed and edited using FigTree (v1.4.4, https://github.com/rambaut/figtree/releases). The subfamily assignment of ABC proteins in each species was further confirmed using BLASTP analyses on the NCBI webserver (https://www.ncbi.nlm.nih.gov/blast). In addition, the same analyses were performed to identify Cuticle (pfam:PF00379), Trypsin (pfam:PF00089), UGT (pfam:PF00201), and CYP450 (pfam:PF00067). We also performed manual annotation of these genes to avoid omissions in their general feature format (GFF) files.
Extraction of insect pigments and color reaction in vitro
To extract the pigment of the orchid mantises, second-instar individuals were crushed in liquid nitrogen and dissolved in acidified methanol (hydrochloric acid at 0.5%)65, followed by incubation in a thermostatic shaker for 48 h at 220 rpm and 25 °C. After centrifugation, the supernatant was collected and passed through a 0.22-μm filter. The redox-dependent color changes of the pigments were observed by adding 10 μL of oxidant (NaNO2 with a concentration of 1%) and reductant (ascorbic acid with a concentration of 1%). In acidified methanol, the redox states of the ommochrome pigments were stable, and they were stored at −20 °C. Five individuals were assigned to each group.
Transmission electron microscopy
The ultrastructure of the mid and hind legs was investigated by transmission electron microscopy. The legs were dissected from fifth- instar H. coronatus and fixed with 3% glutaraldehyde in 0.2 mol/L phosphate buffer (pH 7.2) for 48 h at 4 °C. The legs were then rinsed 3 times with phosphate buffer followed by postfixation in 1% osmium tetroxide for 3 h at 4 °C. The legs were washed several times, for 10 min each time, and placed into a series of ascending concentrations of acetone (50, 70, 80, 90, and 100%) for dehydration. The samples were embedded in Epon 812 for 2 h at room temperature. Then, the samples were trimmed to prepare ultrathin sections. The ultrastructure was captured by a JEM-1200EX transmission electron microscope (TEM, JEOL, Japan).
High-performance liquid chromatography analysis
Individuals of the first, second, and fifth instars were used to determine the concentrations of kynurenine and 3-hydroxykynurenine. The same amount of leg tissue was homogenized directly, added to a methanol solution containing the internal standard, homogenized again, and then centrifuged at 4 °C and 18,000 × g for 20 mins (Microfuge 20 R, Beckman Coulter, Inc., Indianapolis, IN, USA). The supernatant was used to detect the concentrations of kynurenine and 3-hydroxykynurenine according to the protocol of a previous study66. The standards of kynurenine (CAS No. 343-65-7) and 3-hydroxykynurenine (CAS No. 484-78-6) were purchased from Sigma‒Aldrich. Five individuals were assigned to each group.
Histological analysis of the petal-like femoral lobes and size measurement of leg tissue
Hematoxylin-eosin staining was performed for structural observation of the leg tissue of H. coronatus. Briefly, the mid and hind legs of H. coronatus were fixed in 4% paraformaldehyde overnight at 4 °C. Then, paraffin-embedded, 4 μm tissue slices were cut and placed on slides for hematoxylin-eosin staining, with hematoxylin staining the nucleus and eosin staining the cytoplasm.
ImageJ2 (http://imagej.net/) was used to measure the length and area of the femur, tibia and tarsus of T2 and T3 legs of H. coronatus. Briefly, each segment of leg was dissected and taken photos. The scale bar of image was set followed by marking the target region by color threshold to automatically measure the area. The length of the target was measured by straight line.
RNA interference
To validate the function of Arm in the morphological development of legs, double-stranded RNAs (dsRNAs) were synthesized for each gene. The dsRNA sequences are shown in Supplementary Table 10. dsRNA targeting each gene was injected into the right midleg of first-instar individuals at 0.1 μL (660 ng/μL) using a microinjection system, and the second injection was conducted as described above at day 5 after the first injection. Control group individuals were injected with the same amount of PBS. Four individuals were assigned to each group. The mRNA expression levels of these genes were detected at day 3 after the second injection. One individual per group was cultured to molting for observation of the phenotypic outcome.
Real-time quantitative polymerase chain reaction (PCR)
Total RNA was extracted from the femur, tibia and tarsus of T2 and T3 legs using RNAprep Pure Micro Kit (DP420, TIANGEN Biotech (Beijing) Co.,Ltd). RNA quality was determined using a NanoDrop 2000 (Thermo Fisher Scientific Inc.), and RNA of suitable quantity was reverse transcribed using a PrimeScript RT Reagent Kit (RR037A, TaKaRa Biotechnology Co., Ltd.). Quantitative real-time PCR was performed using TB Green Premix Ex Taq II (Tli RNaseH Plus) (RR820A, TaKaRa Biotechnology Co., Ltd.) on an Mx3000P Real-Time PCR System (Agilent Technologies, Inc.). The relative expression level of the Ubx and Arm genes was quantified using GAPDH as the internal reference. Relative fold-change in gene expression was calculated using the delta-delta cycle threshold method67. Three biological repeats were assigned to each group. Genes with a |fold-change| of greater than 2 and an adjusted p value less than 0.05 were identified as DEGs.
Enzyme activity of trypsin
Insect guts were isolated for Trypsin detection. Samples were immediately frozen in liquid nitrogen and stored at −80 °C before use. The enzyme activity of trypsin was detected using a commercial kit from Solarbio Technology Company (BC2315; Beijing, China). In this experiment, N-benzoyl-L-arginine-ethylester (BAEE) was used as the substrate. Under the catalysis of Trypsin, the ester bond of BAEE was hydrolyzed to produce a molecule of N-benzoyl-L-arginine (BA) and ethanol. The UV absorption of BA at 253 nm was much higher than that of BAEE. The amount of enzyme that increased by 0.001 per minute was calculated as one unit of activity. Four individuals were assigned to each group.
Statistical analysis
Measurements of continuous variables are expressed as the means ± SDs. Unless otherwise stated, differences between two groups were assessed using Student’s t tests, and differences among three or more groups were assessed using one-way analyses of variance in SPSS v18 (SPSS). Dunnett’s post hoc tests were used to compare treatment groups to controls. Adjusted p < 0.05 was considered statistically significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The sequence data and the genome assemblies have been deposited to the National Genomics Data Center, China (https://ngdc.cncb.ac.cn/), with accession number CRA010804 (https://bigd.big.ac.cn/gsa/browse/CRA010804). Source data are provided as a Source Data file. Source data are provided with this paper.
References
Wallace, A. R. Darwinism—An Exposition of the Theory of Natural Section with Some of its Applications., (MacMillan, 1889).
Anderson, B. & de Jager, M. L. Natural selection in mimicry. Biol. Rev. Camb. Philos. Soc. 95, 291–304 (2020).
Vereecken, N. J. & Schiestl, F. P. The evolution of imperfect floral mimicry. Proc. Natl Acad. Sci. USA 105, 7484–7488 (2008).
Qu, M. et al. The genetic basis of the leafy seadragon’s unique camouflage morphology and avenues for its efficient conservation derived from habitat modeling. Sci. China Life Sci. 66, 1213–1230 (2023).
Cuthill, I. C. et al. The biology of color. Science 357, https://doi.org/10.1126/science.aan0221 (2017).
Caro, T. Wallace on Coloration: Contemporary Perspective and Unresolved Insights. Trends Ecol. Evol. 32, 23–30 (2017).
Skelhorn, J., Rowland, H. M., Speed, M. P. & Ruxton, G. D. Masquerade: camouflage without crypsis. Science 327, 51 (2010).
Pembury Smith, M. Q. R. & Ruxton, G. D. Camouflage in predators. Biol. Rev. Camb. Philos. Soc. 95, 1325–1340 (2020).
Nishikawa, H. et al. A genetic mechanism for female-limited Batesian mimicry in Papilio butterfly. Nat. Genet 47, 405–409 (2015).
Garrouste, R. et al. Insect mimicry of plants dates back to the Permian. Nat. Commun. 7, 13735 (2016).
Zhang, W., Westerman, E., Nitzany, E., Palmer, S. & Kronforst, M. R. Tracing the origin and evolution of supergene mimicry in butterflies. Nat. Commun. 8, 1269 (2017).
Svenson, G. J., Hardy, N. B., Cahill Wightman, H. M. & Wieland, F. Of flowers and twigs: phylogenetic revision of the plant-mimicking praying mantises (Mantodea: Empusidae and Hymenopodidae) with a new suprageneric classification. Syst. Entomol. 40, 789–834 (2015).
Rivera, J. & Svenson, G. J. The Neotropical ‘polymorphic earless praying mantises’ – Part I: molecular phylogeny and revised higher‐level systematics (Insecta: Mantodea, Acanthopoidea). Syst. Entomol. 41, 607–649 (2016).
O’Hanlon, J. C., Holwell, G. I. & Herberstein, M. E. Pollinator deception in the orchid mantis. Am. Nat. 183, 126–132 (2014).
O’Hanlon, J. C. Orchid mantis. Curr. Biol. 26, R145–R146 (2016).
Herberstein, M. E., Holwell, G. I. & O’Hanlon, J. C. Predatory pollinator deception: Does the orchid mantis resemble a model species. Curr. Zool. 60, 90–103 (2014).
Agudelo, R. A., Maldaner, C. & Rafael, J. A. Dry leaf or twig mantis? A new genus and species of Acanthopidae with sexually dimorphic cryptic strategies (Insecta: Mantodea). Zootaxa 4560, zootaxa 4560 4562 4566, https://doi.org/10.11646/zootaxa.4560.2.6 (2019).
Svenson, G. J., Brannoch, S. K., Rodrigues, H. M., O’Hanlon, J. C. & Wieland, F. Selection for predation, not female fecundity, explains sexual size dimorphism in the orchid mantises. Sci. Rep. 6, 37753 (2016).
Prete, F. R., Wells, H., Wells, P. H. & Hurd, L. E. The Praying Mantids. Q. Rev. Biol. 19, 319–336 (1999).
Cong, Y., Ye, X., Mei, Y., He, K. & Li, F. Transposons and non-coding regions drive the intrafamily differences of genome size in insects. iScience 25, 104873 (2022).
Misof, B. et al. Phylogenomics resolves the timing and pattern of insect evolution. Science 346, 763–767 (2014).
Trautwein, M. D., Wiegmann, B. M., Beutel, R., Kjer, K. M. & Yeates, D. K. Advances in insect phylogeny at the dawn of the postgenomic era. Annu Rev. Entomol. 57, 449–468 (2012).
Ramirez-Barahona, S., Sauquet, H. & Magallon, S. The delayed and geographically heterogeneous diversification of flowering plant families. Nat. Ecol. Evol. 4, 1232–1238 (2020).
Hu, S., Dilcher, D. L., Jarzen, D. M. & Winship Taylor, D. Early steps of angiosperm pollinator coevolution. Proc. Natl Acad. Sci. USA 105, 240–245 (2008).
Li, H. & Durbin, R. Inference of human population history from individual whole-genome sequences. Nature 475, 493–496 (2011).
Shi, Y. et al. Complete mitochondrial genomes of four species of praying mantises (Dictyoptera, Mantidae) with ribosomal second structure, evolutionary and phylogenetic analyses. PLoS One 16, e0254914 (2021).
Wang, S. et al. The evolution and diversification of oakleaf butterflies. Cell, https://doi.org/10.1016/j.cell.2022.06.042 (2022).
Caro, T., Stoddard, M. C. & Stuart-Fox, D. Animal coloration research: why it matters. Philos. Trans. R Soc. Lond. B Biol. Sci. 372, https://doi.org/10.1098/rstb.2016.0333 (2017).
Dermauw, W. & Van Leeuwen, T. The ABC gene family in arthropods: comparative genomics and role in insecticide transport and resistance. Insect Biochem. Mol. Biol. 45, 89–110 (2014).
Jiang, Y. & Lin, X. Role of ABC transporters White, Scarlet and Brown in brown planthopper eye pigmentation. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 221–222, 1–10 (2018).
Zhang, H. et al. Pigmentary analysis of eggs of the silkworm Bombyx mori. J. Insect Physiol. 101, 142–150 (2017).
Wei, F. A new era for evolutionary developmental biology in non-model organisms. Sci. China Life Sci. 63, 1251–1253 (2020).
Mahfooz, N. S., Li, H. & Popadic, A. Differential expression patterns of the hox gene are associated with differential growth of insect hind legs. Proc. Natl Acad. Sci. USA 101, 4877–4882 (2004).
Chen, G. C., Gajowniczek, P. & Settleman, J. Rho-LIM kinase signaling regulates ecdysone-induced gene expression and morphogenesis during Drosophila metamorphosis. Curr. Biol. 14, 309–313 (2004).
Beermann, A., Pruhs, R., Lutz, R. & Schroder, R. A context-dependent combination of Wnt receptors controls axis elongation and leg development in a short germ insect. Development 138, 2793–2805 (2011).
Hollis, B., Keller, L. & Kawecki, T. J. Sexual selection shapes development and maturation rates in Drosophila. Evolution 71, 304–314 (2017).
Stewart, A. D. & Rice, W. R. Arrest of sex-specific adaptation during the evolution of sexual dimorphism in Drosophila. Nat. Ecol. Evol. 2, 1507–1513 (2018).
Tanaka, A. & Hasegawa, A. Nymphal Development of the German Cockroach, Blattella germanica LINNE (Blattaria: Blattellidae), with Special Reference to Instar Determination and Intra-Instar Staging. Konchu Kontyu 47, 225–238 (1979).
Ma, S., Meng, Z., Chen, R. & Guan, K. L. The Hippo Pathway: Biology and Pathophysiology. Annu Rev. Biochem. 88, 577–604 (2019).
Hun, L. V. et al. Essential functions of mosquito ecdysone importers in development and reproduction. Proc. Natl Acad. Sci. USA 119, e2202932119 (2022).
Zhang, C. et al. The ecdysone receptor coactivator Taiman links Yorkie to transcriptional control of germline stem cell factors in somatic tissue. Dev. Cell 34, 168–180 (2015).
Qiu, W. Q. et al. Degradation of amyloid beta-protein by a serine protease-alpha2-macroglobulin complex. J. Biol. Chem. 271, 8443–8451 (1996).
Hu, Y. et al. Molecular mechanisms of adaptive evolution in wild animals and plants. Sci. China Life Sci. 66, 453–495 (2023).
Hu, J., Fan, J., Sun, Z. & Liu, S. NextPolish: a fast and efficient genome polishing tool for long-read assembly. Bioinformatics 36, 2253–2255 (2020).
Walker, B. J. et al. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One 9, e112963 (2014).
Durand, N. C. et al. Juicer Provides a One-Click System for Analyzing Loop-Resolution Hi-C Experiments. Cell Syst. 3, 95–98 (2016).
Dudchenko, O. et al. De novo assembly of the Aedes aegypti genome using Hi-C yields chromosome-length scaffolds. Science 356, 92–95 (2017).
Simao, F. A., Waterhouse, R. M., Ioannidis, P., Kriventseva, E. V. & Zdobnov, E. M. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 31, 3210–3212 (2015).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Xie, C. et al. KOBAS 2.0: a web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res 39, W316–W322 (2011).
Bedell, J. A., Korf, I. & Gish, W. MaskerAid: a performance enhancement to RepeatMasker. Bioinformatics 16, 1040–1041 (2000).
Stanke, M. & Waack, S. Gene prediction with a hidden Markov model and a new intron submodel. Bioinformatics 19, ii215–ii225 (2003).
Burge, C. & Karlin, S. Prediction of complete gene structures in human genomic DNA. J. Mol. Biol. 268, 78–94 (1997).
Altschul, S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990).
Haas, B. J. et al. Automated eukaryotic gene structure annotation using EVidenceModeler and the Program to Assemble Spliced Alignments. Genome Biol. 9, R7 (2008).
Zdobnov, E. M. & Apweiler, R. InterProScan–an integration platform for the signature-recognition methods in InterPro. Bioinformatics 17, 847–848 (2001).
Li, L., Stoeckert, C. J. Jr & Roos, D. S. OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res 13, 2178–2189 (2003).
Edgar, R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32, 1792–1797 (2004).
Stamatakis, A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313 (2014).
Yang, Z. PAML: a program package for phylogenetic analysis by maximum likelihood. Comput. Appl. Biosci. 13, 555–556 (1997).
De Bie, T., Cristianini, N., Demuth, J. P. & Hahn, M. W. CAFE: a computational tool for the study of gene family evolution. Bioinformatics 22, 1269–1271 (2006).
Kondrashov, A. S. & Crow, J. F. A molecular approach to estimating the human deleterious mutation rate. Hum. Mutat. 2, 229–234 (1993).
Tierney, J. E. et al. Past climates inform our future. Science 370, https://doi.org/10.1126/science.aay3701 (2020).
Qiao, X. et al. Gene duplication and evolution in recurring polyploidization-diploidization cycles in plants. Genome Biol. 20, 38 (2019).
Mun, S. et al. A Major Facilitator Superfamily protein encoded by TcMucK gene is not required for cuticle pigmentation, growth and development in Tribolium castaneum. Insect Biochem. Mol. Biol. 49, 43–48 (2014).
Riou, M. & Christides, J. P. Cryptic color change in a crab spider (Misumena vatia): identification and quantification of precursors and ommochrome pigments by HPLC. J. Chem. Ecol. 36, 412–423 (2010).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408 (2001).
Kamsoi, O. & Belles, X. Myoglianin triggers the premetamorphosis stage in hemimetabolan insects. FASEB J. 33, 3659–3669 (2019).
Snelling, E. P., Seymour, R. S., Matthews, P. G., Runciman, S. & White, C. R. Scaling of resting and maximum hopping metabolic rate throughout the life cycle of the locust Locusta migratoria. J. Exp. Biol. 214, 3218–3224 (2011).
Meng, X., Zhu, F. & Chen, K. Silkworm: A Promising Model Organism in Life Science. J. Insect. Sci. 17, https://doi.org/10.1093/jisesa/iex064 (2017).
Djordjevic, J., Dumas, Z., Robinson-Rechavi, M., Schwander, T. & Parker, D. J. Dynamics of sex-biased gene expression over development in the stick insect Timema californicum. bioRxiv, https://doi.org/10.1101/2021.01.23.427895 (2021).
Acknowledgements
This study is supported by the Strategic Priority Research Program of Chinese Academy of Sciences (XDB31000000, Fuwen Wei), the National Natural Science Foundation of China (31821001, Fuwen Wei), and the Youth Innovation Promotion Association, CAS (2023090, Guangping Huang). We thank Hao Wang and Chaotai Wei for providing the ecological photos of mantises, thank Zongyi Sun for help with genome annotation amending, and thank Dr Yang Li and Chuang Gao for help with RNA interference.
Author information
Authors and Affiliations
Contributions
F.W. conceived and supervised this study. G.H. performed the sample collection. G.H. and L.S. conducted data analysis and interpretation. L.S. and G.H. performed the gene family analysis with input from X.D. and X.H. L.S. and G.H. conducted the functional experiments. G.H., L.S. and F.W. discussed the results and wrote the paper. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Sven Bradler, Aleksandar Popadic and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, G., Song, L., Du, X. et al. Evolutionary genomics of camouflage innovation in the orchid mantis. Nat Commun 14, 4821 (2023). https://doi.org/10.1038/s41467-023-40355-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-40355-1
This article is cited by
-
Uncovering the functional basis of mantids that resemble plants
Science China Life Sciences (2024)