Comparison of different human papillomavirus (HPV) vaccine types and dose schedules for prevention of HPV-related disease in females and males
不同人瘤病毒 (HPV) 疫苗类型和剂量方案预防女性和男性 HPV 相关疾病的比较
- PMID: 31755549
- PMCID: PMC6873216
- DOI: 10.1002/14651858.CD013479
Comparison of different human papillomavirus (HPV) vaccine types and dose schedules for prevention of HPV-related disease in females and males
Abstract
Background:Uptake of human papillomavirus (HPV) vaccine remains low in many countries, although the bivalent and quadrivalent HPV vaccines given as a three-dose schedule are effective in the prevention of precancerous lesions of the cervix in women. Simpler immunisation schedules, such as those with fewer doses, might reduce barriers to vaccination, as may programmes that include males.
背景: 人瘤病毒 (HPV) 疫苗的接种率在许多国家仍然很低,尽管二价和四价 HPV 疫苗作为三剂方案给药可有效预防女性宫颈癌前病变。更简单的免疫接种计划,例如剂量较少的免疫接种计划,可能会减少疫苗接种的障碍,包括男性的计划也可能减少。
Objectives:To evaluate the efficacy, immunogenicity, and harms of different dose schedules and different types of HPV vaccines in females and males.
目的: 评价不同剂量方案和不同类型 HPV 疫苗对女性和男性的疗效、免疫原性和危害。
Search methods:We conducted electronic searches on 27 September 2018 in Ovid MEDLINE, the Cochrane Central Register of Controlled Trials (CENTRAL) (in the Cochrane Library), and Ovid Embase. We also searched the WHO International Clinical Trials Registry Platform, and ClinicalTrials.gov (both 27 September 2018), vaccine manufacturer websites, and checked reference lists from an index of HPV studies and other relevant systematic reviews.
检索方法: 我们于 2018年9月27日在 Ovid MEDLINE 、 Cochrane 对照试验中心注册库 (CENTRAL) (在 Cochrane 图书馆) 和 Ovid Embase 中进行了电子检索。我们还检索了WHO国际临床试验注册平台和 ClinicalTrials.gov(均于2018年9月27日)、疫苗制造商网站,并检查了HPV研究索引和其他相关系统综述的参考文献列表。
Selection criteria:We included randomised controlled trials (RCTs) with no language restriction. We considered studies if they enrolled HIV-negative males or females aged 9 to 26 years, or HIV-positive males or females of any age.
纳入排除标准: 我们纳入了无语言限制的随机对照试验 (RCTs)。我们考虑了是否纳入了 9 至 26 岁的 HIV 阴性男性或女性,或任何年龄的 HIV 阳性男性或女性的研究。
Data collection and analysis:We used methods recommended by Cochrane. We use the term 'control' to refer to comparator products containing an adjuvant or active vaccine and 'placebo' to refer to products that contain no adjuvant or active vaccine. Most primary outcomes in this review were clinical outcomes. However, for comparisons comparing dose schedules, the included RCTs were designed to measure antibody responses (i.e. immunogenicity) as the primary outcome, rather than clinical outcomes, since it is unethical to collect cervical samples from girls under 16 years of age. We analysed immunogenicity outcomes (i.e. geometric mean titres) with ratios of means, clinical outcomes (e.g. cancer and intraepithelial neoplasia) with risk ratios or rate ratios and, for serious adverse events and deaths, we calculated odds ratios. We rated the certainty of evidence with GRADE.
资料收集与分析:我们使用了 Cochrane 推荐的方法。我们使用术语“对照”来指代含有佐剂或活性疫苗的对照商品,使用术语“安慰剂”来指代不含佐剂或活性疫苗的商品。本综述中的大多数主要结局是临床结局。然而,为了比较剂量方案,纳入的 RCT 旨在测量抗体反应(即免疫原性)作为主要结局,而不是临床结局,因为从 16 岁以下女孩收集宫颈样本是不道德的。我们分析了免疫原性结局(即几何平均滴度)与均值比,临床结局(例如癌症和上皮内瘤变)与风险比或比率比,对于严重不良事件和死亡,我们计算了比值比。我们用GRADE评价证据质量。
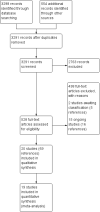
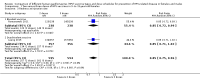
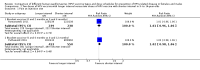
Main results: 主要结果:We included 20 RCTs with 31,940 participants. The length of follow-up in the included studies ranged from seven months to five years. Two doses versus three doses of HPV vaccine in 9- to 15-year-old females Antibody responses after two-dose and three-dose HPV vaccine schedules were similar after up to five years of follow-up (4 RCTs, moderate- to high-certainty evidence). No RCTs collected clinical outcome data. Evidence about serious adverse events in studies comparing dose schedules was of very low-certainty owing to imprecision and indirectness (three doses 35/1159; two doses 36/1158; 4 RCTs). One death was reported in the three-dose group (1/898) and none in the two-dose group (0/899) (low-certainty evidence). Interval between doses of HPV vaccine in 9- to 14-year-old females and males Antibody responses were stronger with a longer interval (6 or 12 months) between the first two doses of HPV vaccine than a shorter interval (2 or 6 months) at up to three years of follow-up (4 RCTs, moderate- to high-certainty evidence). No RCTs collected data about clinical outcomes. Evidence about serious adverse events in studies comparing intervals was of very low-certainty, owing to imprecision and indirectness. No deaths were reported in any of the studies (0/1898, 3 RCTs, low-certainty evidence). HPV vaccination of 10- to 26-year-old males In one RCT there was moderate-certainty evidence that quadrivalent HPV vaccine, compared with control, reduced the incidence of external genital lesions (control 36 per 3081 person-years; quadrivalent 6 per 3173 person-years; rate ratio 0.16, 95% CI 0.07 to 0.38; 6254 person-years) and anogenital warts (control 28 per 2814 person-years; quadrivalent 3 per 2831 person-years; rate ratio 0.11, 95% CI 0.03 to 0.38; 5645 person-years). The quadrivalent vaccine resulted in more injection-site adverse events, such as pain or redness, than control (537 versus 601 per 1000; risk ratio (RR) 1.12, 95% CI 1.06 to 1.18, 3895 participants, high-certainty evidence). There was very low-certainty evidence from two RCTs about serious adverse events with quadrivalent vaccine (control 12/2588; quadrivalent 8/2574), and about deaths (control 11/2591; quadrivalent 3/2582), owing to imprecision and indirectness. Nonavalent versus quadrivalent vaccine in 9- to 26-year-old females and males Three RCTs were included; one in females aged 9- to 15-years (n = 600), one in females aged 16- to 26-years (n = 14,215), and one in males aged 16- to 26-years (n = 500). The RCT in 16- to 26-year-old females reported clinical outcomes. There was little to no difference in the incidence of the combined outcome of high-grade cervical epithelial neoplasia, adenocarcinoma in situ, or cervical cancer between the HPV vaccines (quadrivalent 325/6882, nonavalent 326/6871; OR 1.00, 95% CI 0.85 to 1.16; 13,753 participants; high-certainty evidence). The other two RCTs did not collect data about clinical outcomes. There were slightly more local adverse events with the nonavalent vaccine (905 per 1000) than the quadrivalent vaccine (846 per 1000) (RR 1.07, 95% CI 1.05 to 1.08; 3 RCTs, 15,863 participants; high-certainty evidence). Comparative evidence about serious adverse events in the three RCTs (nonavalent 243/8234, quadrivalent 192/7629; OR 0.60, 95% CI 0.14 to 2.61) was of low certainty, owing to imprecision and indirectness. HPV vaccination for people living with HIV Seven RCTs reported on HPV vaccines in people with HIV, with two small trials that collected data about clinical outcomes. Antibody responses were higher following vaccination with either bivalent or quadrivalent HPV vaccine than with control, and these responses could be demonstrated to have been maintained for up to 24 months in children living with HIV (low-certainty evidence). The evidence about clinical outcomes and harms for HPV vaccines in people with HIV is very uncertain (low- to very low-certainty evidence), owing to imprecision and indirectness.
我们纳入了 20 项 RCT,涉及 31,940 名参与者。纳入研究的随访时间从 7 个月到 5 年不等。9 至 15 岁女性接种两剂和三剂 HPV 疫苗 经过长达五年的随访,两剂和三剂 HPV 疫苗接种方案后的抗体反应相似(4 项随机对照试验,中等至高质量证据)。没有 RCT 收集临床结局数据。由于不精确性和间接性(三剂 35/1159;两剂 36/1158;4 项随机对照试验),在比较剂量方案的研究中,关于严重不良事件的证据质量极低。三剂组报告了 1 例死亡 (1/898),两剂组报告了 1 例死亡 (0/899)(低质量证据)。9 至 14 岁女性和男性的 HPV 疫苗接种间隔 在长达三年的随访中,前两剂 HPV 疫苗之间的间隔较长(6 或 12 个月)比较短的间隔(2 或 6 个月)抗体反应更强(4 项 RCT,中等至高质量证据)。没有 RCT 收集有关临床结局的数据。由于不精确性和间接性,在比较间隔的研究中,关于严重不良事件的证据质量极低。所有研究均未报告死亡病例(0/1898,3项随机对照试验,低质量证据)。10 至 26 岁男性的 HPV 疫苗接种在一项随机对照试验中,有中等质量证据表明,与对照组相比,四价 HPV 疫苗降低了外生殖器病变的发生率(对照组 36/3081 人年;四价疫苗/3173 人年;比率比 0.16,95% CI 0.07 至 0.38;6254 人年)和肛门生殖器疣(对照组 28/2814 人年;四价 3/2831 人年;比率比 0.11, 95% CI [0.03, 0.38];5645 人年)。 与对照组相比,四价疫苗导致更多的注射部位不良事件,如疼痛或发红(每1000人中有537人对601人;风险比(RR)1.12,95%CI 1.06至1.18,3895名受试者,高质量证据)。来自两项随机对照试验的极低质量证据关于四价疫苗(对照 12/2588;四价 8/2574)和死亡(对照 11/2591;四价 3/2582),由于不精确性和间接性。九价疫苗与四价疫苗在 9 至 26 岁女性和男性中的疗效纳入了 3 项 RCT;1 例在 9 至 15 岁的女性中 (n = 600),1 例在 16 至 26 岁的女性中 (n = 14,215),1 例在 16 至 26 岁的男性中 (n = 500)。针对 16 至 26 岁女性的 RCT 报告了临床结局。HPV 疫苗之间高级别宫颈上皮瘤变、原位腺癌或宫颈癌的联合结局发生率几乎没有差异(四价 325/6882,九价 326/6871;OR 1.00,95% CI 0.85 至 1.16;13,753 名参与者;高质量证据)。其他 2 项 RCT 未收集有关临床结局的数据。九价疫苗(每 1000 人中有 905 人)的局部不良事件略多于四价疫苗(每 1000 人中有 846 人)(RR 1.07,95% CI 1.05 至 1.08;3 项随机对照试验,15,863 名参与者;高质量证据)。关于三项 RCT 中严重不良事件的比较证据(九价 243/8234,四价 192/7629;OR 0.60,95% CI 0.14 至 2.61)由于不精确性和间接性而具有低质量。HIV 感染者的 HPV 疫苗接种 七项随机对照试验报告了 HIV 感染者的 HPV 疫苗,其中两项小型试验收集了有关临床结果的数据。 接种二价或四价 HPV 疫苗后的抗体反应高于对照,并且可以证明这些反应在 HIV 感染儿童中维持了长达 24 个月(低质量证据)。由于不精确性和间接性,关于 HIV 感染者 HPV 疫苗的临床结局和危害的证据非常不确定(低到极低质量证据)。
Authors' conclusions:The immunogenicity of two-dose and three-dose HPV vaccine schedules, measured using antibody responses in young females, is comparable. The quadrivalent vaccine probably reduces external genital lesions and anogenital warts in males compared with control. The nonavalent and quadrivalent vaccines offer similar protection against a combined outcome of cervical, vaginal, and vulval precancer lesions or cancer. In people living with HIV, both the bivalent and quadrivalent HPV vaccines result in high antibody responses. For all comparisons of alternative HPV vaccine schedules, the certainty of the body of evidence about serious adverse events reported during the study periods was low or very low, either because the number of events was low, or the evidence was indirect, or both. Post-marketing surveillance is needed to continue monitoring harms that might be associated with HPV vaccines in the population, and this evidence will be incorporated in future updates of this review. Long-term observational studies are needed to determine the effectiveness of reduced-dose schedules against HPV-related cancer endpoints, and whether adopting these schedules improves vaccine coverage rates.
Antecedentes: La aceptación de la vacuna contra el virus del papiloma humano (VPH) sigue siendo baja en muchos países, aunque las vacunas bivalentes y cuadrivalentes contra el VPH administradas en un calendario de tres dosis son efectivas para prevenir las lesiones precancerosas del cuello uterino en las mujeres. Los calendarios de vacunación más sencillos, como los que incluyen menos dosis, podrían reducir las barreras a la vacunación, al igual que los calendarios que incluyen a los hombres.
Objetivos: Evaluar la eficacia, la inmunogenicidad y los efectos perjudiciales de diferentes calendarios de dosis y diferentes tipos de vacunas contra el VPH en mujeres y hombres. MÉTODOS DE BÚSQUEDA: Se realizaron búsquedas electrónicas el 27 de septiembre 2018 en Ovid MEDLINE, el Registro Cochrane Central de Ensayos Controlados (CENTRAL) (en la Biblioteca Cochrane) y Ovid Embase. También se realizaron búsquedas en la International Clinical Trials Registry Platform de la OMS y en ClinicalTrials.gov (ambas el 27 de septiembre 2018), en sitios web de fabricantes de vacunas y se verificaron las listas de referencias de un índice de estudios sobre el VPH y otras revisiones sistemáticas pertinentes. CRITERIOS DE SELECCIÓN: Se incluyeron ensayos controlados aleatorizados (ECA) sin restricciones de idioma. Se consideraron los estudios cuando habían reclutado a hombres o mujeres con pruebas negativas para el VIH de 9 a 26 años de edad, o a hombres o mujeres con pruebas positivas para el VIH de cualquier edad. OBTENCIÓN Y ANÁLISIS DE LOS DATOS: Se siguieron los métodos recomendados por Cochrane. Se utilizó el término "control" para hacer referencia a los productos de comparación que contienen un adyuvante o vacuna activa y "placebo" para hacer referencia a los productos que no contienen un adyuvante ni vacuna activa. La mayoría de los resultados primarios de esta revisión fueron resultados clínicos. Sin embargo, para las comparaciones de los calendarios de dosis, los ECA incluidos se diseñaron para medir las respuestas de los anticuerpos (es decir, la inmunogenicidad) como resultado primario, en lugar de los resultados clínicos, debido a que no es ético recoger muestras del cuello uterino de niñas menores de 16 años de edad. Se analizaron los resultados de inmunogenicidad (es decir, títulos de la media geométrica) con los cocientes de medias, los resultados clínicos (p.ej. cáncer y neoplasia intraepitelial) con los cocientes de riesgos o los cocientes de tasas y, para los eventos adversos graves y las muertes, se calcularon los odds‐ratios. La certeza de la evidencia se evaluó con los criterios GRADE.
Resultados principales: Se incluyeron 20 ECA con 31 940 participantes. La duración del seguimiento en los estudios incluidos varió de siete meses a cinco años. Dos dosis frente a tres dosis de la vacuna contra el VPH en mujeres de 9 a 15 años de edad Las respuestas de los anticuerpos después de los calendarios de dos y tres dosis de la vacuna contra el VPH fueron similares después de hasta cinco años de seguimiento (4 ECA, evidencia de certeza moderada a alta). Ningún ECA recopiló datos de los resultados clínicos. La evidencia acerca de los eventos adversos graves en los estudios que compararon los calendarios de dosis fue de certeza muy baja debido a la imprecisión y a la falta de direccionalidad (tres dosis 35/1159; dos dosis 36/1158; 4 ECA). Se informó una muerte en el grupo de tres dosis (1/898) y ninguna en el grupo de dos dosis (0/899) (evidencia de certeza baja). Intervalo entre las dosis de la vacuna contra el VPH en mujeres y hombres de 9 a 14 años de edad Las respuestas de los anticuerpos fueron más significativas con un intervalo más largo (6 o 12 meses) entre las dos primeras dosis de la vacuna contra el VPH que con un intervalo más corto (2 o 6 meses) al momento del seguimiento de hasta tres años (4 ECA, evidencia de certeza moderada a alta). Ningún ECA recopiló datos sobre los resultados clínicos. La evidencia acerca de los eventos adversos graves en los estudios que compararon los intervalos fue de certeza muy baja, debido a la imprecisión y a la falta de direccionalidad. No se informaron muertes en ninguno de los estudios (0/1898, 3 ECA, evidencia de certeza baja). Vacunación contra el VPH en hombres de 10 a 26 años de edad En un ECA hubo evidencia de certeza moderada de que la vacuna cuadrivalente contra el VPH, en comparación con el control, redujo la incidencia de lesiones genitales externas (control 36 por 3081 personas‐año; cuadrivalente 6 por 3173 personas‐año; cociente de tasas 0,16; IC del 95%: 0,07 a 0,38; 6254 personas‐año) y verrugas anogenitales (control 28 por 2814 personas‐año; cuadrivalente 3 por 2831 años‐persona; cociente de tasas 0,11; IC del 95%: 0,03 a 0,38; 5645 años‐persona). La vacuna cuadrivalente produjo más eventos adversos relacionados con el sitio de la inyección, como dolor o enrojecimiento, que el control (537 frente a 601 por 1000; cociente de riesgos [CR] 1,12; IC del 95%: 1,06 a 1,18; 3895 participantes, evidencia de certeza alta). Hubo evidencia de certeza muy baja de dos ECA acerca de eventos adversos graves con la vacuna cuadrivalente (control 12/2588; cuadrivalente 8/2574), y acerca de las muertes (control 11/2591; cuadrivalente 3/2582), debido a la imprecisión y la falta de direccionalidad. Vacuna nonavalente frente a cuadrivalente en mujeres y hombres de 9 a 26 años de edad Se incluyeron tres ECA; uno en mujeres de 9 a 15 años de edad (n = 600), uno en mujeres de 16 a 26 años de edad (n = 14 215) y uno en hombres de 16 a 26 años de edad (n = 500). El ECA en mujeres de 16 a 26 años informó de los resultados clínicos. Hubo poca o ninguna diferencia en la incidencia del resultado combinado de neoplasia epitelial de cuello de útero de grado alto, adenocarcinoma in situ o cáncer de cuello de útero entre las vacunas contra el VPH (cuadrivalente 325/6882, nonavalente 326/6871; OR 1,00; IC del 95%: 0,85 a 1,16; 13 753 participantes; evidencia de certeza alta). Los otros dos ECA no recopilaron datos sobre los resultados clínicos. Hubo un número ligeramente mayor de eventos adversos locales con la vacuna nonavalente (905 por 1000) que con la vacuna cuadrivalente (846 por 1000) (CR 1,07; IC del 95%: 1,05 a 1,08; 3 ECA, 15 863 participantes; evidencia de certeza alta). La evidencia comparativa acerca de los eventos adversos graves en los tres ECA (nonavalente 243/8234, cuadrivalente 192/7629; OR 0,60; IC del 95%: 0,14 a 2,61) fue de certeza baja, debido a la imprecisión y a la falta de direccionalidad. Vacunación contra el VPH para las personas que conviven con el VIH Siete ECA informaron sobre las vacunas contra el VPH en personas con VIH, y dos ensayos pequeños recopilaron datos sobre los resultados clínicos. Las respuestas de los anticuerpos fueron más altas después de la vacunación con la vacuna bivalente o cuadrivalente contra el VPH que con el control, y se pudo demostrar que estas respuestas se mantuvieron hasta 24 meses en niños que convivían con el VIH (evidencia de certeza baja). La evidencia acerca de los resultados clínicos y los efectos perjudiciales de las vacunas contra el VPH en las personas con VIH es muy incierta (evidencia de certeza baja a muy baja), debido a la imprecisión y a la falta de direccionalidad.
Conclusiones de los autores: Es similar la inmunogenicidad de los calendarios de dos y tres dosis de la vacuna contra el VPH, medida con las respuestas de los anticuerpos en mujeres jóvenes. La vacuna cuadrivalente probablemente reduce las lesiones genitales externas y las verrugas anogenitales en los hombres en comparación con el control. Las vacunas nonavalentes y cuadrivalentes ofrecen una protección similar en cuanto a un resultado combinado de lesiones precancerosas o cáncer de cuello de útero, vaginal y vulvar. En los individuos que conviven con el VIH, tanto las vacunas bivalentes como las cuadrivalentes contra el VPH producen respuestas altas de los anticuerpos. Para todas las comparaciones de los calendarios alternativos de la vacuna contra el VPH, la certeza del conjunto de evidencia sobre los eventos adversos graves notificados durante los períodos de estudio fue baja o muy baja, debido a que el número de eventos fue escaso, o a que la evidencia fue indirecta, o ambos. La vigilancia posterior a la comercialización es necesaria para continuar con el control de los efectos perjudiciales que podrían estar asociados con las vacunas contra el VPH en la población, y esta evidencia se incorporará en las actualizaciones futuras de esta revisión. Se necesitan estudios observacionales a largo plazo para determinar la efectividad de los calendarios de dosis reducidas con respecto a las variables de evaluación del cáncer relacionado con el VPH, y si la adopción de estos calendarios mejora las tasas de cobertura de la vacuna.
Background:Uptake of human papillomavirus (HPV) vaccine remains low in many countries, although the bivalent and quadrivalent HPV vaccines given as a three-dose schedule are effective in the prevention of precancerous lesions of the cervix in women. Simpler immunisation schedules, such as those with fewer doses, might reduce barriers to vaccination, as may programmes that include males.
Objectives:To evaluate the efficacy, immunogenicity, and harms of different dose schedules and different types of HPV vaccines in females and males.
Search methods:We conducted electronic searches on 27 September 2018 in Ovid MEDLINE, the Cochrane Central Register of Controlled Trials (CENTRAL) (in the Cochrane Library), and Ovid Embase. We also searched the WHO International Clinical Trials Registry Platform, and ClinicalTrials.gov (both 27 September 2018), vaccine manufacturer websites, and checked reference lists from an index of HPV studies and other relevant systematic reviews.
Selection criteria:We included randomised controlled trials (RCTs) with no language restriction. We considered studies if they enrolled HIV-negative males or females aged 9 to 26 years, or HIV-positive males or females of any age.
Data collection and analysis:We used methods recommended by Cochrane. We use the term 'control' to refer to comparator products containing an adjuvant or active vaccine and 'placebo' to refer to products that contain no adjuvant or active vaccine. Most primary outcomes in this review were clinical outcomes. However, for comparisons comparing dose schedules, the included RCTs were designed to measure antibody responses (i.e. immunogenicity) as the primary outcome, rather than clinical outcomes, since it is unethical to collect cervical samples from girls under 16 years of age. We analysed immunogenicity outcomes (i.e. geometric mean titres) with ratios of means, clinical outcomes (e.g. cancer and intraepithelial neoplasia) with risk ratios or rate ratios and, for serious adverse events and deaths, we calculated odds ratios. We rated the certainty of evidence with GRADE.
Main results:We included 20 RCTs with 31,940 participants. The length of follow-up in the included studies ranged from seven months to five years. Two doses versus three doses of HPV vaccine in 9- to 15-year-old females Antibody responses after two-dose and three-dose HPV vaccine schedules were similar after up to five years of follow-up (4 RCTs, moderate- to high-certainty evidence). No RCTs collected clinical outcome data. Evidence about serious adverse events in studies comparing dose schedules was of very low-certainty owing to imprecision and indirectness (three doses 35/1159; two doses 36/1158; 4 RCTs). One death was reported in the three-dose group (1/898) and none in the two-dose group (0/899) (low-certainty evidence). Interval between doses of HPV vaccine in 9- to 14-year-old females and males Antibody responses were stronger with a longer interval (6 or 12 months) between the first two doses of HPV vaccine than a shorter interval (2 or 6 months) at up to three years of follow-up (4 RCTs, moderate- to high-certainty evidence). No RCTs collected data about clinical outcomes. Evidence about serious adverse events in studies comparing intervals was of very low-certainty, owing to imprecision and indirectness. No deaths were reported in any of the studies (0/1898, 3 RCTs, low-certainty evidence). HPV vaccination of 10- to 26-year-old males In one RCT there was moderate-certainty evidence that quadrivalent HPV vaccine, compared with control, reduced the incidence of external genital lesions (control 36 per 3081 person-years; quadrivalent 6 per 3173 person-years; rate ratio 0.16, 95% CI 0.07 to 0.38; 6254 person-years) and anogenital warts (control 28 per 2814 person-years; quadrivalent 3 per 2831 person-years; rate ratio 0.11, 95% CI 0.03 to 0.38; 5645 person-years). The quadrivalent vaccine resulted in more injection-site adverse events, such as pain or redness, than control (537 versus 601 per 1000; risk ratio (RR) 1.12, 95% CI 1.06 to 1.18, 3895 participants, high-certainty evidence). There was very low-certainty evidence from two RCTs about serious adverse events with quadrivalent vaccine (control 12/2588; quadrivalent 8/2574), and about deaths (control 11/2591; quadrivalent 3/2582), owing to imprecision and indirectness. Nonavalent versus quadrivalent vaccine in 9- to 26-year-old females and males Three RCTs were included; one in females aged 9- to 15-years (n = 600), one in females aged 16- to 26-years (n = 14,215), and one in males aged 16- to 26-years (n = 500). The RCT in 16- to 26-year-old females reported clinical outcomes. There was little to no difference in the incidence of the combined outcome of high-grade cervical epithelial neoplasia, adenocarcinoma in situ, or cervical cancer between the HPV vaccines (quadrivalent 325/6882, nonavalent 326/6871; OR 1.00, 95% CI 0.85 to 1.16; 13,753 participants; high-certainty evidence). The other two RCTs did not collect data about clinical outcomes. There were slightly more local adverse events with the nonavalent vaccine (905 per 1000) than the quadrivalent vaccine (846 per 1000) (RR 1.07, 95% CI 1.05 to 1.08; 3 RCTs, 15,863 participants; high-certainty evidence). Comparative evidence about serious adverse events in the three RCTs (nonavalent 243/8234, quadrivalent 192/7629; OR 0.60, 95% CI 0.14 to 2.61) was of low certainty, owing to imprecision and indirectness. HPV vaccination for people living with HIV Seven RCTs reported on HPV vaccines in people with HIV, with two small trials that collected data about clinical outcomes. Antibody responses were higher following vaccination with either bivalent or quadrivalent HPV vaccine than with control, and these responses could be demonstrated to have been maintained for up to 24 months in children living with HIV (low-certainty evidence). The evidence about clinical outcomes and harms for HPV vaccines in people with HIV is very uncertain (low- to very low-certainty evidence), owing to imprecision and indirectness.
Authors' conclusions:The immunogenicity of two-dose and three-dose HPV vaccine schedules, measured using antibody responses in young females, is comparable. The quadrivalent vaccine probably reduces external genital lesions and anogenital warts in males compared with control. The nonavalent and quadrivalent vaccines offer similar protection against a combined outcome of cervical, vaginal, and vulval precancer lesions or cancer. In people living with HIV, both the bivalent and quadrivalent HPV vaccines result in high antibody responses. For all comparisons of alternative HPV vaccine schedules, the certainty of the body of evidence about serious adverse events reported during the study periods was low or very low, either because the number of events was low, or the evidence was indirect, or both. Post-marketing surveillance is needed to continue monitoring harms that might be associated with HPV vaccines in the population, and this evidence will be incorporated in future updates of this review. Long-term observational studies are needed to determine the effectiveness of reduced-dose schedules against HPV-related cancer endpoints, and whether adopting these schedules improves vaccine coverage rates.
Copyright © 2019 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
Conflict of interest statement
None of the authors have a conflict of interest in relation to this review.
Cochrane Response, which is an evidence consultancy operated by The Cochrane Collaboration, was commissioned to perform part of this review for the WHO Initiative for Vaccine Research.
Hanna Bergman: HB works for Cochrane Response, an evidence services unit operated by the Cochrane Collaboration, and was paid by Cochrane Response for contributing to this review.
Brian S Buckley: BSB works for Cochrane Response, an evidence services unit operated by the Cochrane Collaboration, and was paid by Cochrane Response for contributing to this review.
Gemma Villanueva: GV works for Cochrane Response, an evidence services unit operated by the Cochrane Collaboration, and was paid by Cochrane Response for contributing to this review.
Jennifer Petkovic: JP works for Cochrane Response, an evidence services unit operated by the Cochrane Collaboration, and was paid by Cochrane Response for contributing to this review.
Chantelle Garritty: CG works as a consultant for Cochrane Response, an evidence services unit operated by Cochrane, and was paid by Cochrane Response for contributing to this review.
Vittoria Lutje: VL works as an independent consultant conducting literature searches for various research groups. None of them has any potential relevance to the submitted work.
Alina Ximena Riveros‐Balta: AXRB is an employee of the WHO Initiative for Vaccine Research, which commissioned the review.
Nicola Low: NL was the principal author of the original systematic review of alternative HPV vaccination schedules (D'Addario 2017), which was commissioned by the WHO Initiative for Vaccine Research.
Nicholas Henschke: NH works for Cochrane Response, an evidence services unit operated by Cochrane, and was paid by Cochrane Response for contributing to this review.
Figures



















































Comment in
-
British Gynaecological Cancer Society (BGCS) vulval cancer guidelines: An update on recommendations for practice 2023.Eur J Obstet Gynecol Reprod Biol2.14区2024 JanMorrison J, Baldwin P, Hanna L, Andreou A, Buckley L, Durrant L, Edey K, Faruqi A, Fotopoulou C, Ganesan R, Hillaby K, Taylor A.DOI: 10.1016/j.ejogrb.2023.11.013PMID: 38043220
Similar articles
-
Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors.Cochrane Database Syst Rev8.82区2018 May 9ReviewArbyn M, Xu L, Simoens C, Martin-Hirsch PP.DOI: 10.1002/14651858.CD009069.pub3PMID: 29740819
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev8.82区2022 Feb 1Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, Moraleda C, Rogers L, Daniels K, Green P.DOI: 10.1002/14651858.CD014217PMID: 36321557
-
Efficacy, immunogenicity, and safety of a quadrivalent HPV vaccine in men: results of an open-label, long-term extension of a randomised, placebo-controlled, phase 3 trial.Lancet Infect Dis36.41区2022 MarClinical TrialGoldstone SE, Giuliano AR, Palefsky JM, Lazcano-Ponce E, Penny ME, Cabello RE, Moreira ED Jr, Baraldi E, Jessen H, Ferenczy A, Kurman R, Ronnett BM, Stoler MH, Bautista O, Das R, Group T, Luxembourg A, Zhou HJ, Saah A.DOI: 10.1016/S1473-3099(21)00327-3PMID: 34780705
-
Sequential inactivated (IPV) and live oral (OPV) poliovirus vaccines for preventing poliomyelitis.Cochrane Database Syst Rev8.82区2019 Dec 5Ciapponi A, Bardach A, Rey Ares L, Glujovsky D, Cafferata ML, Cesaroni S, Bhatti A.DOI: 10.1002/14651858.CD011260.pub2PMID: 31801180
-
Efficacy and safety of COVID-19 vaccines.Cochrane Database Syst Rev8.82区2022 Dec 7ReviewGraña C, Ghosn L, Evrenoglou T, Jarde A, Minozzi S, Bergman H, Buckley BS, Probyn K, Villanueva G, Henschke N, Bonnet H, Assi R, Menon S, Marti M, Devane D, Mallon P, Lelievre JD, Askie LM, Kredo T, Ferrand G, Davidson M, Riveros C, Tovey D, Meerpohl JJ, Grasselli G, Rada G, Hróbjartsson A, Ravaud P, Chaimani A, Boutron I.DOI: 10.1002/14651858.CD015477PMID: 36473651
Cited by
-
Comparison of the safety and persistence of immunogenicity of bivalent HPV16/18 vaccine in healthy 9-14-year-old and 18-26-year-old Chinese females: A randomized, double-blind, non-inferiority clinical trial.Vaccine4.53区2023 Oct 26Li J, Shi LW, Li K, Huang LR, Li JB, Dong YL, Li W, Ji M, Yang Q, Zhou LY, Yuan L, Yan XM, Chen JJ, Jiang ZW, Qi YY, Li RC, Li YP, Xia JL, Yu BW, Mo ZJ, Li CG.DOI: 10.1016/j.vaccine.2023.10.041PMID: 39492306
-
HPV Vaccination Coverage in Brazil's State of Paraná: Spatial Distribution and Advances in Public Health.Vaccines (Basel)5.23区2024 Sep 29Pelloso FC, Pazin DC, Silva LL, Carvalho MDB, Borghesan DHP, Consolaro MEL, Santos LD, Ribeiro HF, Stevanato KP, Marques VD, Camparoto CW, Pujals C, Pedroso RB, Pelloso SM.DOI: 10.3390/vaccines12101118PMID: 39460285
-
Early adoption of innovation in HPV prevention strategies: closing the gap in cervical cancer.Ecancermedicalscience1.2N/A2024 Sep 11ReviewMahajan I, Kadam A, McCann L, Ghose A, Wakeham K, Dhillon NS, Stanway S, Boussios S, Banerjee S, Priyadarshini A, Sirohi B, Torode JS, Mitra S.DOI: 10.3332/ecancer.2024.1762PMID: 39430092
-
The importance of the quadrivalent HPV vaccine in the elimination of cervical cancer in Brazil.Rev Bras Ginecol Obstet1.0N/A2024 Sep 6Roteli-Martins CM, Maranhão AGK, Fialho SCAV, da Silva-Filho AL.DOI: 10.61622/rbgoPMID: 39380580
-
Global parental acceptance, attitudes, and knowledge regarding human papillomavirus vaccinations for their children: a systematic literature review and meta-analysis.BMC Womens Health2.43区2024 Sep 27Heyde S, Osmani V, Schauberger G, Cooney C, Klug SJ.DOI: 10.1186/s12905-024-03377-5PMID: 39334328
References
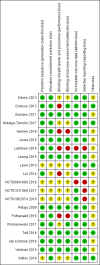
References to studies included in this review
Denny 2013 {published and unpublished data}
-
- Denny L, Hendricks B, Gordon C, Thomas F, Hezareh M, Dobbelaere, et al. Safety and immunogenicity of the HPV‐16/18 AS04‐adjuvanted vaccine in HIV‐positive women in South Africa: a partially‐blind randomised placebo controlled study. Vaccine 2013;31(48):5745‐53. - PubMed
-
- GSK 107863 (HPV‐020 PRI). Evaluation of the safety and immunogenicity of GlaxoSmithKline Biologicals' HPV vaccine 580299 (Cervarix TM) in adult human immunodeficiency virus (HIV) infected female subjects. www.gsk‐clinicalstudyregister.com/files2/gsk‐107863‐clinical‐study‐report2‐redac... (accessed 16 August 2018).
-
- GlaxoSmithKline. Evaluation of the safety and immunogenicity of GlaxoSmithKline Biologicals' HPV vaccine 580299 (Cervarix TM) in adult human immunodeficiency virus (HIV) infected female subjects. clinicaltrials.gov/ct2/show/results/NCT00586339 (accessed 16 August 2018).
Dobson 2013 {published data only (unpublished sought but not used)}
-
- Dobson SR, McNeil S, Dionne M, Dawar M, Ogilvie G, Krajden M, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA 2013;309:1793‐802. - PubMed
-
- Krajden M, Cook D, Yu A, Chow R, Mei W, McNeil S, et al. Human papillomavirus 16 (HPV 16) and HPV 18 antibody responses measured by pseudovirus neutralization and competitive Luminex assays in a two‐ versus three‐dose HPV vaccine trial. Clinical and Vaccine Immunology 2011;18(3):418‐23. - PMC - PubMed
-
- Krajden M, Cook D, Yu A, Chow R, Su Q, Mei W, et al. Assessment of HPV 16 and HPV 18 antibody responses by pseudovirus neutralization, MerckcLIA and Merck total IgG LIA immunoassays in a reduced dosage quadrivalent HPV vaccine trial. Vaccine 2014;32(5):624‐30. - PubMed
-
- Ogilvie G, Sauvageau C, Dionne M, McNeil S, Krajden M, Money D, et al. Immunogenicity of 2 vs 3 doses of the quadrivalent human papillomavirus vaccine in girls aged 9 to 13 years after 60 months. JAMA 2017;317:1687‐8. - PubMed
Giuliano 2011 {published and unpublished data}
-
- Merck Sharp, Dohme. An investigational vaccine in reducing the incidence of anogenital warts in young men. clinicaltrials.gov/ct2/show/results/NCT00090285 (accessed 16 August 2018).
-
- Palefsky JM, Giuliano AR, Goldstone S, Moreira ED, Aranda C, Jessen H, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. New England Journal of Medicine 2011;365:1576‐85. - PubMed
Hidalgo‐Tenorio 2017 {published data only (unpublished sought but not used)}
-
- Hidalgo‐Tenorio C, Ramirez‐Taboada J, Gil‐Anguita C, Esquivias J, Omar‐Mohamed‐Balgahata M, SamPedro A, et al. Safety and immunogenicity of the quadrivalent human papillomavirus (qHPV) vaccine in HIV‐positive Spanish men who have sex with men (MSM). AIDS Research and Therapy 2017;14:34. - PMC - PubMed
Iversen 2016 {published and unpublished data}
-
- Iversen OE, Miranda MJ, Ulied A, Soerdal T, Lazarus E, Chokephaibulkit K, et al. Immunogenicity of the 9‐valent HPV vaccine using 2‐dose regimens in girls and boys vs a 3‐dose regimen in women. JAMA 2016;316:2411‐21. - PubMed
-
- Iversen OE, Miranda MJ, Ulied A, Soerdal T, Lazarus E, Chokephaibulkit K, et al. Immunogenicity of the 9‐valent HPV vaccine using 2‐dose regimens in girls and boys vs a 3‐dose regimen in women. Obstetrical and Gynecological Survey 2017;72:412‐3. - PubMed
-
- Merck Sharp, Dohme. Phase III study of a 2‐dose regimen of a multivalent human papillomavirus (HPV) vaccine (V503), administered to 9 to 14 year‐olds and compared to young women, 16 to 26 years old (V503‐010). clinicaltrials.gov/ct2/show/results/NCT01984697 (accessed 16 August 2018).
Joura 2015 {published and unpublished data}
-
- Bouchard C, Vuocolo S, Merck. Effect of the 9vHPV vaccine on abnormal cytology and genital procedures related to HPV31/33/45/52/58. Journal of Lower Genital Tract Disease 2014;18(5 Suppl 1):S9.
-
- Chen YH, Gesser R, Luxembourg A. A seamless phase IIB/III adaptive outcome trial: design rationale and implementation challenges. Clinical Trials Journal 2015;12(1):84‐90. - PubMed
-
- Guevara A, Cabello R, Woelber L, Moreira ED Jr, Joura E, Reich O, et al. Antibody persistence and evidence of immune memory at 5 years following administration of the 9‐valent HPV vaccine. Vaccine 2017;35:5050‐7. - PubMed
-
- Huh WK, Joura EA, Giuliano AR, Iversen OE, Andrade RP, Ault KA, et al. Final efficacy, immunogenicity, and safety analyses of a nine‐valent human papillomavirus vaccine in women aged 16‐26 years: a randomised, double‐blind trial. Lancet 2017;390:2143‐59. - PubMed
-
- Joura E, Garland S, Giuliano A, Bautista O, Chen J, Moeller E, et al. End of study efficacy for vulvovaginal disease of a novel 9‐valent HPV L1 virus‐like particle vaccine in 16‐26 year old women. Journal of Lower Genital Tract Disease 2015;19(3 Suppl 1):S10.
Lehtinen 2018 {published and unpublished data}
-
- GSK 106636 (HPV‐040 PRI). Evaluation of the effectiveness of two vaccination strategies using GlaxoSmithKline Biologicals’ HPV vaccine GSK580299 administered in healthy adolescents. www.gsk‐clinicalstudyregister.com/study/106636?search=study&search_terms=106636#csr (accessed 25 August 2018).
-
- Lehtinen M, Apter D, Baussano I, Eriksson T, Natunen K, Paavonen J, et al. Characteristics of a cluster‐randomized phase IV human papillomavirus vaccination effectiveness trial. Vaccine 2015;33(10):1284‐90. - PubMed
-
- Lehtinen M, Eriksson T, Apter D, Hokkanen M, Natunen K, Paavonen J, et al. Safety of the human papillomavirus (HPV)‐16/18 AS04‐adjuvanted vaccine in adolescents aged 12–15 years: interim analysis of a large community‐randomized controlled trial. Human Vaccines & Immunotherapeutics 2016;12(12):3177‐85. - PMC - PubMed
-
- Lehtinen M, Soderlund‐Strand A, Vanska S, Luostarinen T, Eriksson T, Natunen K, et al. Impact of gender‐neutral or girls‐only vaccination against human papillomavirus ‐ results of a community‐randomized clinical trial (I). International Journal of Cancer 2018;142(5):949‐58. - PubMed
-
- NCT00534638. Effectiveness, safety and immunogenicity of GSK Biologicals' HPV vaccine GSK580299 (Cervarix TM) administered in healthy adolescents. clinicaltrials.gov/ct2/show/results/NCT00534638 (accessed 25 August 2018).
Leung 2015 {published and unpublished data}
-
- GSK 115411 (HPV‐071 PRI). Immunogenicity and safety study of GlaxoSmithKline Biologicals' HPV‐16/18 L1 AS04 vaccine and Merck's Gardasil® vaccine when administered according to alternative 2‐dose schedules in 9‐14 year old females. www.gsk‐clinicalstudyregister.com/files2/gsk‐115411‐clinical‐study‐report‐redact... (accessed 16 August 2018).
-
- GlaxoSmithKline. Immunogenicity and safety study of GlaxoSmithKline Biologicals' HPV‐16/18 L1 AS04 vaccine and Merck's Gardasil® vaccine when administered according to alternative 2‐dose schedules in 9‐14 year old females. clinicaltrials.gov/ct2/show/results/NCT01462357 (accessed 16 August 2018).
-
- Leung TF, Liu AP, Lim FS, Thollot F, Oh HM, Lee BW, et al. Comparative immunogenicity and safety of human papillomavirus (HPV)‐16/18 AS04‐adjuvanted vaccine and HPV‐6/11/16/18 vaccine administered according to 2‐and 3‐dose schedules in girls aged 9‐14 years: results to month 12 from a randomized trial. Human Vaccines & Immunotherapeutics 2015;11:1689‐702. - PMC - PubMed
-
- Leung TF, Liu AP, Lim FS, Thollot F, Oh HML, Lee BW, et al. Comparative immunogenicity and safety of human papillomavirus (HPV)‐16/18 AS04‐adjuvanted vaccine and 4vHPV vaccine administered according to two‐ or three‐dose schedules in girls aged 9‐14 years: results to month 36 from a randomized trial. Vaccine 2018;36:98‐106. - PubMed
Levin 2010 {published data only (unpublished sought but not used)}
-
- Weinberg A, Song L‐Y, Saah A, Brown M, Moscicki AB, Meyer WA, et al. Humoral, mucosal, and cell‐mediated immunity against vaccine and nonvaccine genotypes after administration of quadrivalent human papillomavirus vaccine to HIV‐infected children. Journal of Infectious Diseases 2012;206(8):1309‐18. - PMC - PubMed
Lin 2014 {published and unpublished data}
-
- Merck Sharp, Dohme. Randomized trial of alternative HPV vaccination schedules in males in a university setting. clinicaltrials.gov/ct2/show/results/NCT01184079 (accessed 16 August 2018).
NCT00941889 2016 {unpublished data only}
-
- NCT00941889. The effect of HPV vaccination on recurrence rates in HIV patients with condylomata. clinicaltrials.gov/ct2/show/results/NCT00941889?view=results (accessed 27 August 2018).
NCT01031069 2017 {unpublished data only}
-
- GlaxoSmithKline. Safety and immunogenicity of Cervarix™ in human immunodeficiency virus infected females. www.gsk‐clinicalstudyregister.com/files2/109823‐clinical‐study‐result‐summary.pdf (accessed 27 August 2018).
-
- NCT01031069. Evaluation of safety and immunogenicity of a human papillomavirus (HPV) vaccine in human immunodeficiency virus (HIV) infected females. clinicaltrials.gov/ct2/show/NCT01031069 (accessed on 27 August 2018).
NCT01862874 2018 {published data only}
-
- NCT01862874. Efficacy and tolerability study of V501 in Japanese males (V501‐122). clinicaltrials.gov/ct2/show/NCT01862874 (accessed 27 August 2018).
Petaja 2009 {published and unpublished data}
-
- GSK 580299/011 (HPV‐011). A phase I/II, observer‐blind, randomized, controlled study to assess the immunogenicity and safety of GlaxoSmithKline Biologicals' HPV‐16/18 L1 VLP AS04 vaccine administered intramuscularly according to a 0, 1, 6 month schedule in healthy male subjects aged 10‐18 years. www.gsk‐clinicalstudyregister.com/files2/gsk‐580299‐011‐clinical‐study‐report‐re... (accessed 16 August 2018).
-
- Petäjä T, Keränen H, Karppa T, Kawa A, Lantela S, Siitari‐Mattila M, et al. Immunogenicity and safety of human papillomavirus (HPV)‐16/18 AS04‐adjuvanted vaccine in healthy boys aged 10‐18 years. Journal of Adolescent Health 2009;44(1):33‐40. - PubMed
Puthanakit 2016 {published and unpublished data}
-
- GSK 114700 (HPV‐070 PRI). Immunogenicity and safety study of GlaxoSmithKline Biologicals' HPV‐16/18 L1 AS04 vaccine when administered according to alternative 2‐dose schedules in 9‐14 year old females. www.gsk‐clinicalstudyregister.com/files2/gsk‐114700‐clinical‐study‐report‐redact... (accessed 16 August 2018).
-
- GlaxoSmithKline. Immunogenicity and safety study of GlaxoSmithKline Biologicals' HPV‐16/18 L1 AS04 vaccine when administered according to alternative 2‐dose schedules in 9‐14 year old females. clinicaltrials.gov/ct2/show/results/NCT01381575 (accessed 16 August 2018).
-
- Puthanakit T, Huang LM, Chiu CH, Tang RB, Schwarz TF, Esposito S, et al. Randomized open trial comparing 2‐dose regimens of the human papillomavirus 16/18 AS04‐adjuvanted vaccine in girls aged 9‐14 years versus a 3‐dose regimen in women aged 15‐25 years. Journal of Infectious Diseases 2016;214:525‐36. - PMC - PubMed
Romanowski 2011 {published and unpublished data}
-
- GSK 110659 (HPV‐048 PRI). Evaluation of the safety and immunogenicity of GSK Biologicals' HPV vaccine 580299 when administered in healthy females aged 9‐25 years using an alternative schedule and an alternative dosing as compared to the standard schedule and dosing. www.gsk‐clinicalstudyregister.com/files2/gsk‐110659‐clinical‐study‐report‐redact... (accessed 16 August 2018).
-
- GlaxoSmithKline. Evaluation of the safety and immunogenicity of GSK Biologicals' HPV vaccine 580299 when administered in healthy females aged 9‐25 years using an alternative schedule and an alternative dosing as compared to the standard schedule and dosing. clinicaltrials.gov/ct2/show/results/NCT00541970 (accessed 16 August 2018).
-
- Romanowski B, Schwarz TF, Ferguson L, Peters K, Dionne M, Behre U, et al. Sustained immunogenicity of the HPV‐16/18 AS04‐adjuvanted vaccine administered as a two‐dose schedule in adolescent girls: five‐year clinical data and modelling predictions from a randomized study. Human Vaccines & Immunotherapeutics 2016;12:20‐9. - PMC - PubMed
-
- Romanowski B, Schwarz TF, Ferguson LM, Ferguson M, Peters K, Dionne M, et al. Immune response to the HPV‐16/18 AS04‐adjuvanted vaccine administered as a 2‐dose or 3‐dose schedule up to 4 years after vaccination: results from a randomized study. Human Vaccines & Immunotherapeutics 2014;10:1155‐65. - PMC - PubMed
-
- Romanowski B, Schwarz TF, Ferguson LM, Peters K, Dionne M, Schulze K, et al. Immunogenicity and safety of the HPV‐16/18 AS04‐adjuvanted vaccine administered as a 2‐dose schedule compared with the licensed 3‐dose schedule: results from a randomized study. Human Vaccines 2011;7:1374‐86. - PMC - PubMed
Toft 2014 {published data only (unpublished sought but not used)}
-
- Faust H, Toft L, Sehr P, Müller M, Bonde J, Forslund O, et al. Human papillomavirus neutralizing and cross‐reactive antibodies induced in HIV‐positive subjects after vaccination with quadrivalent and bivalent HPV vaccines. Vaccine 2016;34(13):1559‐65. - PubMed
-
- Toft L, Storgaard M, Muller M, Sehr P, Bonde J, Tolstrup M, et al. Immunogenicity and reactogenicity of Cervarix versus Gardasil in HIV‐infected adults: an RCT. Topics in Antiviral Medicine 2014;22(e‐1):174‐5.
-
- Toft L, Storgaard M, Müller M, Sehr P, Bonde J, Tolstrup M, et al. Comparison of the immunogenicity and reactogenicity of Cervarix and Gardasil human papillomavirus vaccines in HIV‐infected adults: a randomized, double‐blind clinical trial. Journal of Infectious Diseases 2014;209(8):1165‐73. - PubMed
-
- Toft L, Tolstrup M, Müller M, Sehr P, Bonde J, Storgaard M, et al. Comparison of the immunogenicity of Cervarix® and Gardasil® human papillomavirus vaccines for oncogenic non‐vaccine serotypes HPV‐31, HPV‐33, and HPV‐45 in HIV‐infected adults. Human Vaccines & Immunotherapeutics 2014;10(5):1147‐54. - PMC - PubMed
van Damme 2016 {published data only (unpublished sought but not used)}
-
- Damme P, Meijer CJ, Kieninger D, Schuyleman A, Thomas S, Luxembourg A, et al. A phase III clinical study to compare the immunogenicity and safety of the 9‐valent and quadrivalent HPV vaccines in men. Vaccine 2016;34:4205‐12. - PubMed
Vesikari 2015 {published and unpublished data}
-
- Merck Sharp, Dohme. A randomized, double‐blinded, controlled with GARDASIL (human papillomavirus vaccine [types 6, 11, 16, 18] (recombinant, adsorbed)), Phase III clinical trial to study the immunogenicity and tolerability of V503 (9‐valent human papillomavirus (HPV) vaccine) in preadolescent and adolescent girls (9‐ to 15‐year‐old). clinicaltrials.gov/ct2/show/results/NCT01304498 (accessed 16 August 2018).
-
- Vesikari T, Brodszki N, Damme P, Diez‐Domingo J, Icardi G, Petersen LK, et al. A randomized, double‐blind, phase III study of the immunogenicity and safety of a 9‐valent human papillomavirus L1 virus‐like particle vaccine (V503) versus Gardasil® in 9–15‐year‐old girls. Pediatric Infectious Disease Journal 2015;34:992‐8. - PubMed
Wilkin 2018 {published and unpublished data}
-
- Cranston R, Yang M, Paczuski P, Cespedes M, Chiao E, Webster‐Cyriaque J, et al. Baseline data of a Phase 3 trial of the quadrivalent HPV vaccine in HIV+ males and females: ACTG 5298. Topics in Antiviral Medicine 2014;22:364.
-
- NCT01461096. Quadrivalent HPV vaccine to prevent anal HPV in HIV‐infected men and women. clinicaltrials.gov/ct2/show/results/NCT01461096 (accessed 27 August 2018).
-
- Wilkin TJ, Chen H, Cespedes M, Paczuski P, Godfrey C, Chiao E, et al. ACTG A5298: a phase 3 trial of the quadrivalent HPV vaccine in older HIV+ adults. Topics in Antiviral Medicine 2018;24(E‐1):65‐6.
References to studies excluded from this review
Beachler 2016 {published data only}
Bhatia 2016 {published data only}
-
- Bhatia R, Kavanagh K, Cubie HA, Serrano I, Wennington H, Hopkins M, et al. Use of HPV testing for cervical screening in vaccinated women – insights from the SHEVa (Scottish HPV Prevalence in Vaccinated Women) study. International Journal of Cancer 2016;138(12):2922‐31. - PubMed
Bianchi 2016 {published data only}
-
- Bianchi S, Boveri S, Igidbashian S, Amendola A, Urbinati AM, Frati ER, et al. Chlamydia trachomatis infection and HPV/Chlamydia trachomatis coinfection among HPV‐vaccinated young women at the beginning of their sexual activity. Archives of Gynecology and Obstetrics 2016;294(6):1227‐33. - PubMed
Brown 2012 {published data only}
Canfell 2017 {published data only}
-
- Canfell K, Caruana M, Gebski V, Darlington‐Brown J, Heley S, Brotherton J, et al. Cervical screening with primary HPV testing or cytology in a population of women in which those aged 33 years or younger had previously been offered HPV vaccination: results of the Compass pilot randomised trial. PLOS Medicine 2017;14(9):e1002388. - PMC - PubMed
Carozzi 2016 {published data only}
-
- Carozzi FM, Ocello C, Burroni E, Faust H, Zappa M, Paci E, et al. Effectivenessof HPV vaccination in women reaching screening age in Italy. Journal of Clinical Virology 2016;84:74‐81. - PubMed
Choudhury 2016 {published data only}
Esposito 2011 {published data only}
-
- Esposito S, Birlutiu V, Jarcuska P, Perino A, Man SC, Vladareanu R, et al. Immunogenicity and safety of human papillomavirus‐16/18 AS04‐adjuvanted vaccine administered according to an alternative dosing schedule compared with the standard dosing schedule in healthy women aged 15 to 25 years: results from a randomized study. Pediatric Infectious Disease Journal 2011;30(3):e49‐55. - PubMed
Flagg 2018 {published data only}
Garland 2016 {published data only}
-
- Garland SM, Paavonen J, Jaisamrarn U, Naud P, Salmeron J, Chow SN, et al. Prior human papillomavirus‐16/18 AS04‐adjuvanted vaccination prevents recurrent high grade cervical intraepithelial neoplasia after definitive surgical therapy: post‐hoc analysis from a randomized controlled trial. International Journal of Cancer 2016;139(12):2812‐26. - PMC - PubMed
Gilca 2015 {published data only}
-
- Gilca V, Sauvageau C, Boulianne N, Serres G, Crajden M, Ouakki M, et al. The effect of a booster dose of quadrivalent or bivalent HPV vaccine when administered to girls previously vaccinated with two doses of quadrivalent HPV vaccine. Human Vaccines & Immunotherapeutics 2015;11(3):732‐8. - PMC - PubMed
Hamsikova 2017 {published data only}
-
- Hamsikova E, Smahelova J, Ludvikova V, Salakova M, Rychla J, Skrenkova J, et al. The prevalence of HPV infections in HPV‐vaccinated women from the general population. Apmis 2017;125(6):585‐95. - PubMed
Harari 2016 {published data only}
Haskins‐Coulter 2017 {published data only}
Lamontagne 2013 {published data only}
-
- Lamontagne DS, Thiem VD, Huong VM, Tang Y, Neuzil KM. Immunogenicity of quadrivalent HPV vaccine among girls 11 to 13 years of age vaccinated using alternative dosing schedules: results 29 to 32 months after third dose. Journal of Infectious Diseases 2013;208(8):1325‐34. - PubMed
Lehtinen 2017 {published data only}
-
- Lehtinen M, Lagheden C, Luostarinen T, Eriksson T, Apter D, Harjula K, et al. Ten‐year follow‐up of human papillomavirus vaccine efficacy against the most stringent cervical neoplasia end‐point‐registry‐based follow‐up of three cohorts from randomized trials. BMJ Open 2017;7(8):e015867. - PMC - PubMed
Luxembourg 2017 {published data only}
-
- Luxembourg A, Kjaer SK, Nygard M, Ellison MC, Group T, Marshall JB, et al. Design of a long‐term follow‐up effectiveness, immunogenicity and safety study of women who received the 9‐valent human papillomavirus vaccine. Contemporary Clinical Trials 2017;52:54‐61. - PubMed
Money 2016 {published data only}
-
- Money DM, Moses E, Blitz S, Vandriel SM, Lipsky N, Walmsley SL, et al. HIV viral suppression results in higher antibody responses in HIV‐positive women vaccinated with the quadrivalent human papillomavirus vaccine. Vaccine 2016;34(40):4799‐806. - PubMed
Neuzil 2011 {published data only}
-
- Neuzil KM, Thiem VD, Janmohamed A, Huong VM, Tang Y, Diep NT, et al. Immunogenicity and reactogenicity of alternative schedules of HPV vaccine in Vietnam: a cluster randomized noninferiority trial. JAMA 2011;305(14):1424‐31. - PubMed
Wheeler 2016 {published data only}
-
- Wheeler CM, Skinner SR, Rosario‐Raymundo MR, Garland SM, Chatterjee A, Lazcano‐Ponce E, et al. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04‐adjuvanted vaccine in women older than 25 years: 7‐year follow‐up of the phase 3, double‐blind, randomised controlled VIVIANE study. Lancet Infectious Diseases 2016;16(10):1154‐68. - PubMed
Zhu 2017 {published data only}
Zimmerman 2010 {published data only}
-
- Zimmerman RK, Nowalk MP, Lin CJ, Fox DE, Ko FS, Wettick E, et al. Randomized trial of an alternate human papillomavirus vaccine administration schedule in college‐aged women. Journal of Women's Health 2010;19(8):1441‐7. - PubMed
References to studies awaiting assessment
Li 2012 {published data only (unpublished sought but not used)}
-
- Huang T, Liu Y, Li Y, Liao Y, Shou Q, Zheng M, et al. Evaluation on the persistence of anti‐HPV immune responses to the quadrivalent HPV vaccine in Chinese females and males: Up to 3.5 years of follow‐up. Vaccine 2018;36(11):1368‐1374. - PubMed
-
- Li R, Li Y, Radley D, Liu Y, Huang T, Sings HL, et al. Safety and immunogenicity of a vaccine targeting human papillomavirus types 6, 11, 16 and 18: a randomized, double‐blind, placebo‐controlled trial in Chinese males and females. Vaccine 2012;30(28):4284‐91. - PubMed
Reisinger 2007 {published data only (unpublished sought but not used)}
-
- Ferris D, Samakoses R, Block SL, Lazcano‐Ponce E, Restrepo JA, Reisinger KS, et al. Long‐term study of a quadrivalent human papillomavirus vaccine. Pediatrics 2014;134(3):e657‐65. - PubMed
-
- Ferris DG, Samakoses R, Block SL, Lazcano‐Ponce E, Restrepo JA, Mehlsen J, et al. 4‐Valent Human Papillomavirus (4vHPV) Vaccine in Preadolescents and Adolescents After 10 Years. Pediatrics 2017;140(6):pii: e20163947. - PubMed
-
- Reisinger KS, Block SL, Lazcano‐Ponce E, Samakoses R, Esser MT, Erick J, et al. Safety and persistent immunogenicity of a quadrivalent human papillomavirus types 6, 11, 16, 18L1 virus‐like particle vaccine in preadolescents and adolescents: a randomized controlled trial. Pediatr Infect Dis J 2007;26(3):201‐9. - PubMed
References to ongoing studies
NCT01735006 {published data only}
-
- NCT01735006. Efficacy and immunogenicity study of recombinant human papillomavirus bivalent (type 16/18) vaccine. clinicaltrials.gov/ct2/show/NCT01735006 (accessed 27 August 2018).
NCT01824537 {published data only}
-
- NCT01824537. Transmission reduction and prevention with HPV vaccination (TRAP‐HPV) study (TRAP‐HPV). clinicaltrials.gov/ct2/show/NCT01824537 (accessed 27 August 2018).
NCT02009800 {published data only}
-
- NCT02009800. ICI‐VPH: impact of HPV immunisation schedules against HPV (ICI‐VPH). clinicaltrials.gov/ct2/show/NCT02009800 (accessed 27 August 2018).
NCT02087384 {published data only}
-
- NCT02087384. HPV (human papilloma virus) vaccination after treatment of anal intraepithelial neoplasia (AIN) (VACCAIN‐P). clinicaltrials.gov/ct2/show/NCT02087384 (accessed 27 August 2018).
NCT02405520 {published data only}
-
- NCT02405520. Safety and immunogenicity study of the recombinant human papillomavirus virus type 6/11 bivalent vaccine. clinicaltrials.gov/ct2/show/NCT02405520 (accessed on 27 August 2018).
NCT02562508 {published data only}
-
- NCT02562508. A bridging study of a recombinant human papillomavirus 16/18 bivalent vaccine in preadolescent girls. clinicaltrials.gov/ct2/show/NCT02562508 (accessed 27 August 2018).
NCT02567955 {published data only}
-
- NCT02567955. Immunogenicity and safety of Gardasil‐9 and Cervarix. clinicaltrials.gov/ct2/show/NCT02567955 (accessed on 27 August 2018).
NCT02710851 {published data only}
-
- NCT02710851. Immunogenicity study of the recombinant human papillomavirus virus type 6/11 bivalent vaccine. clinicaltrials.gov/ct2/show/NCT02710851 (accessed on 27 August 2018).
NCT02733068 {published data only}
-
- NCT02733068. A phase III study of human papillomavirus (HPV)‐16/18 vaccine. clinicaltrials.gov/ct2/show/NCT02733068 (accessed 27 August 2018).
NCT02740777 {published data only}
-
- NCT02740777. Evaluating the 2‐dose immunization schedule of human papillomavirus (HPV)‐16/18 in adolescent females. clinicaltrials.gov/ct2/show/NCT02740777 (accessed 27 August 2018).
NCT02750202 {published data only}
-
- NCT02750202. Effectiveness study of human papilloma virus (HPV) vaccines to prevent recurrence of genital warts (TheraVACCS). clinicaltrials.gov/ct2/show/NCT02750202 (accessed on 27 August 2018).
NCT02834637 {published data only}
-
- NCT02834637. A dose reduction immunobridging and safety study of two HPV vaccines in Tanzanian girls (DoRIS). clinicaltrials.gov/ct2/show/NCT02834637 (accessed 27 August 2018).
NCT02888418 {published data only}
-
- NCT02888418. Safety and immunogenicity study of human papilloma virus vaccine in women aged 9 to 30 and men aged 9 to 17. clinicaltrials.gov/ct2/show/NCT02888418 (accessed 27 August 2018).
NCT03180034 {published data only}
-
- NCT03180034. Scientific evaluation of one or two doses of the bivalent or nonavalent prophylactic HPV vaccines. https://clinicaltrials.gov/ct2/show/NCT03180034 (accessed on 27 August 2018) 2017.
NCT03296397 {published data only}
-
- NCT03296397. Efficacy of quadrivalent HPV vaccine to prevent relapses of genital warts after initial therapeutic response (CONDYVAC). clinicaltrials.gov/ct2/show/NCT03296397 (accessed 27 August 2018).
Additional references
Alemany 2014
-
- Alemany L, Saunier M, Tinoco L, Quiros B, Alvarado‐Cabrero I, Alejo M, et al on behalf of the HPV VVAP study group. Large contribution of human papillomavirus in vaginal neoplastic lesions: a worldwide study in 597 samples. European Journal of Cancer 2014;50:2846‐54. - PubMed
Arbyn 2018
Block 2006
-
- Block SL, Nolan T, Sattler C, Barr E, Giacoletti KE, Marchant CD, et al. Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus‐like particle vaccine in male and female adolescents and young adult women. Pediatrics 2006;118(5):2135‐45. - PubMed
Bogaards 2015
Bonhoeffer 2002
-
- Bonhoeffer J, Kohl K, Chen R, Duclos P, Heijbel H, Heininger U, et al: The Brighton Colloaboration. The Brighton Collaboration: addressing the need for standardized case definitions of adverse events following immunization (AEFI). Vaccine 2002;21:298‐302. - PubMed
Bosch 2002
Bouvard 2009
-
- Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, Ghissassi F, et al: WHO International Agency for Research on Cancer Monograph Working Group. A review of human carcinogens‐‐Part B: biological agents. Lancet Oncology 2009;10(4):321‐2. - PubMed
Bradburn 2007
-
- Bradburn MJ, Deeks JJ, Berlin JA, Russell Localio A. Much ado about nothing: a comparison of the performance of meta‐analytical methods with rare events. Statistics in Medicine 2007;26(1):53‐77. - PubMed
Brotherton 2018
-
- Brotherton JM, Bloem PN. Population‐based HPV vaccination programmes are safe and effective: 2017 update and the impetus for achieving better global coverage. Best Practice & Research. Clinical Obstetrics & Gynaecology 2018;47:42‐58. - PubMed
Bruni 2010
-
- Bruni L, Diaz M, Castellsagué X, Ferrer E, Bosch FX, Sanjosé S. Cervical human papillomavirus prevalence in 5 continents: meta‐analysis of 1 million women with normal cytological findings. The Journal of Infectious Diseases 2010;202(12):1789‐99. - PubMed
de Martel 2017
de Sanjose 2010
-
- Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, et al on behalf of the Retrospective International Survey and HPV Time Trends Study Group. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross‐sectional worldwide study. Lancet Oncology 2010;11:1048‐56. - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. - PubMed
Djurisic 2017
-
- Djurisic S, Jakobsen JC, Petersen SB, Kenfelt M, Gluud C. Aluminium adjuvants used in vaccines versus placebo or no intervention. Cochrane Database of Systematic Reviews 2017, Issue 9. [DOI: 10.1002/14651858.CD012805] - DOI
Donken 2015
-
- Donken R, Knol MJ, Bogaards JA, Klis FR, Meijer CJ, Melker HE. Inconclusive evidence for non‐inferior immunogenicity of two‐ compared with three‐dose HPV immunization schedules in preadolescent girls: a systematic review and meta‐analysis. Journal of Infection 2015;71:61‐73. - PubMed
Drolet 2019
-
- Drolet M, Bénard É, Pérez N, Brisson M, on behalf of the HPV Vaccination Impact Study Group. Population‐level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta‐analysis. Lancet 2019;pii: S0140‐6736(19):30298‐3. - PMC - PubMed
Gallagher 2018
-
- Gallagher KE, LaMontagne DS, Watson‐Jones D. Status of HPV vaccine introduction and barriers to country uptake. Vaccine 2018;36(32 Pt A):4761‐7. - PubMed
Gillison 2015
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 27 August 2018. McMaster University (developed by Evidence Prime), 2015.
Greer 1995
Guyatt 2011a
-
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407‐15. - PubMed
Guyatt 2011b
-
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfland M, et al. GRADE guidelines: 7. Rating the quality of evidence—inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. - PubMed
Guyatt 2011c
-
- Guyatt GH, Oxman AD, Montori M, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence — publication bias. Journal of Clinical Epidemiology 2011;64(12):1277‐82. - PubMed
Guyatt 2011d
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. - PubMed
Higgins 2011a
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
Higgins 2017
-
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
HogenEsch 2018
Huang 2011
-
- Huang HY, Andrews E, Jones J, Skovron ML, Tilson H. Pitfalls in meta‐analyses on adverse events reported from clinical trials. Pharmacoepidemiology and drug safety 2011;20:1014‐20. - PubMed
IARC 2014
-
- International Agency for Research in Cancer. Primary End‐points for Prophylactic HPV Vaccine Trials. IARC Working Group Report. World Health Organization International Agency for Research on Cancer, 2014. - PubMed
Ioannidis 2004
-
- Ioannidis JP, Evans SJ, Gøtzsche PC, O'Neill RT, Altman DG, Schulz K, et al. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Annals of Internal Medicine 2004;141(10):781‐8. - PubMed
Jefferson 2004
-
- Jefferson T, Rudin M, Pietrantonj C. Adverse events after immunisation with aluminium‐containing DTP vaccines: systematic review of the evidence. Lancet Infectious Diseases 2004;4(2):84‐90. - PubMed
Jørgensen 2018a
Jørgensen 2018b
-
- Jørgensen L, Gøtzsche PC, Jefferson T. The Cochrane HPV vaccine review was incomplete and ignored important evidence of bias. BMJ Evidence‐Based Medicine 2018;23:165‐8. - PubMed
Kreimer 2011
Kreimer 2015
Krustrup 2009
LaMontagne 2017
-
- LaMontagne DS, Bloem PJ, Brotherton JM, Gallagher KE, Badiane O, Ndiaye C. Progress in HPV vaccination in low‐ and lower‐middle‐income countries. International Journal of Gynecology & Obstetrics 2017;138:7‐14. - PubMed
Lineberry 2016
-
- Lineberry N, Berlin JA, Mansi B, Glasser S, Berkwits M, Klem C, et al. Recommendations to improve adverse event reporting in clinical trial publications: a joint pharmaceutical industry/journal editor perspective. BMJ 2016;355:i5078. - PubMed
Lundh 2017
Markowitz 2018
-
- Markowitz LE, Drolet M, Perez N, Jit M, Brisson M. Human papillomavirus vaccine effectiveness by number of doses: systematic review of data from national immunization programs. Vaccine 2018;36(32 Pt A):4806‐15. - PubMed
McCredie 2008
-
- McCredie MR, Sharples KJ, Paul C, Baranyai J, Medley G, Jones RW, Skegg DC. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncology 2008;9(5):425‐34. - PubMed
Paavonen 2007
-
- Paavonen J, Jenkins D, Bosch FX, Naud P, Salmerón J, Wheeler CM, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus‐like‐particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double‐blind, randomised controlled trial. Lancet 2007;369(9580):2161‐70. - PubMed
Piaggio 2012
-
- Piaggio G, Elbourne DR, Pocock SJ, Evans SJ, Altman DG. Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement.. JAMA 2012;308:2594‐604. - PubMed
Sankaranarayanan 2016
Schiller 2018
Schim van der Loeff 2014
-
- Schim van der Loeff MF, Mooij SH, Richel O, Vries HJ, Prins JM. HPV and anal cancer in HIV‐infected individuals: a review. Current HIV/AIDS Reports 2014;11(3):250‐62. - PubMed
Sharma 2017
-
- Sharma T, Gøtzsche P, Kuss O. The Yusuf‐Peto method was not a robust method for meta‐analyses of rare events data from antidepressant trials. Journal of Clinical Epidemiology 2017;91:129‐36. - PubMed
Smith 2011
-
- Smith MA, Lew J‐B, Walker RJ, Brotherton JM, Nickson C, Canfell K. The predicted impact of HPV vaccination on male infections and male HPV‐related cancers in Australia. Vaccine 2011;29:9112‐22. - PubMed
Stanley 2006
-
- Stanley MA. Human papillomavirus vaccines. Reviews in Medical Virology 2006;16:139‐49. - PubMed
Stanley 2014
Sturegard 2013
-
- Sturegard E, Johansson H, Ekström J, Hansson B‐G, Johnsson A, Gustafsson E, et al. Human papillomavirus typing in reporting of Condyloma. Sexually Transmitted Diseases 2013;40:123‐9. - PubMed
Syrjänen 2010
-
- Syrjänen S. The role of human papillomavirus infection in head and neck cancers. Annals of Oncology 2010;21:243‐5. - PubMed
Vardas 2011
Walling 2016
-
- Walling EB, Benzoni N, Dornfeld J, Bhandari R, Sisk BA, Garbutt J, et al. Interventions to improve HPV vaccine uptake: a systematic review. Pediatrics 2016;138(1):e20153863. - PubMed
WHO 2017
-
- World Health Organization. Human papillomavirus vaccines: WHO position paper, May 2017. Weekly Epidemiological Record 2017;92:241‐68.
References to other published versions of this review
Bergman 2017
-
- Bergman H, Henschke N, Buckley B, Villanueva G, Petkovic J, Garritty C. Protocol for an update of a systematic review and meta‐analysis of the immunogenicity and efficacy of HPV vaccines in females and males aged 9‐26 years. osf.io/qchzb (accessed 31 October 2018).
D'Addario 2017
-
- D'Addario M, Redmond S, Scott P, Egli‐Gany D, Riveros‐Balta AX, Restrepo AM, et al. Two‐dose schedules for human papillomavirus vaccine: systematic review and meta‐analysis. Vaccine 2017;35:2892‐901. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
